Chapter 3 Notes rev 101409 Democritus was a







- Slides: 23

Chapter 3 Notes (rev. 10/14/09)

Democritus was a Greek philosopher who “…was one of two founders of ancient atomist theory”. “The atomists held that there are smallest indivisible bodies from which everything else is composed, and that these move about in an infinite void space”. “The atomists held that there are two fundamentally different kinds of realities composing the natural world, atoms and void. Atoms, from the Greek adjective atomos or atomon, ‘indivisible, ’ are infinite in number and various in size and shape, and perfectly solid, with no internal gaps”. Text http: //plato. stanford. edu/entries/democritus/ image http: //reich-chemistry. wikispaces. com/file/view/demo. _atom_model. gif/97847685/demo. _atom_model. gif

• Atoms: Smallest particle of an element that retains the chemical identity of that element. • Principles of Chemical Behavior: * Lavoisier: Law of Conservation of Matter * Proust: Law of Constant Composition • a compound always contains the same elements in the same proportions by mass.

John Dalton’s: Atomic Theory of Matter • • 4 Postulates: elements are composed of atoms all atoms of an element are identical, but different from atoms in other elements atoms are neither created nor destroyed a given compound always has the same relative number and kind of atoms.

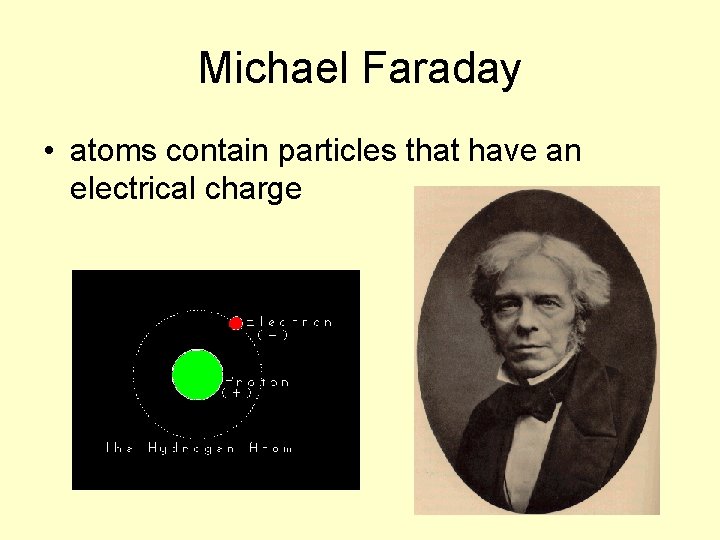
Michael Faraday • atoms contain particles that have an electrical charge

Ben Franklin • studied electricity • he determined the following: – there are 2 kinds of charge positive and negative – 2 like charges repel each other – opposites charges attract each other – excess negative charge can be discharged as static electricity

Ben Franklin • Do you remember Ben Franklin’s famous kite experiment?

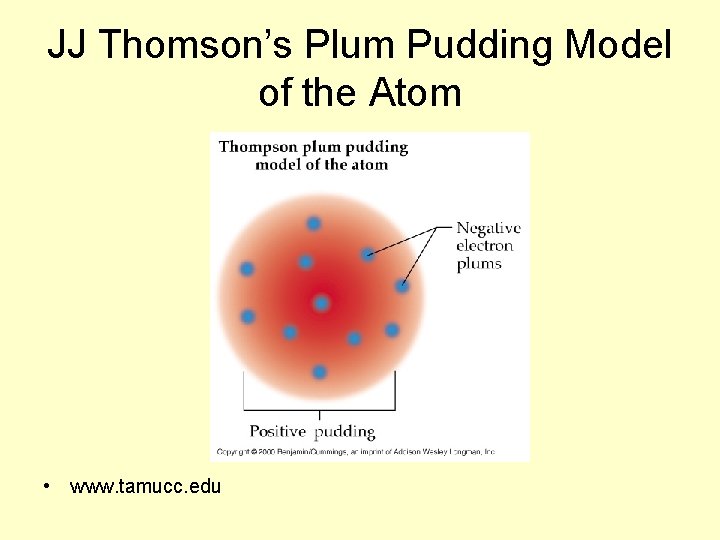
J. J. Thomson • called the negative particles electrons • determined the charge to mass ratio of an electron • The Plum Pudding Model is Thomson’s name for his model of the atom

JJ Thomson’s Plum Pudding Model of the Atom • www. tamucc. edu

Cathode Ray Tube (CRT) • negative end is the cathode • positive end is the anode A cathode ray is radiation streaming from a cathode to an anode in a CRT • it is a stream of particles • a magnet can deflect the ray • cathode ray particles have a negative charge

• http: //www. chem. uiuc. edu/clcwebsite/cathode. html

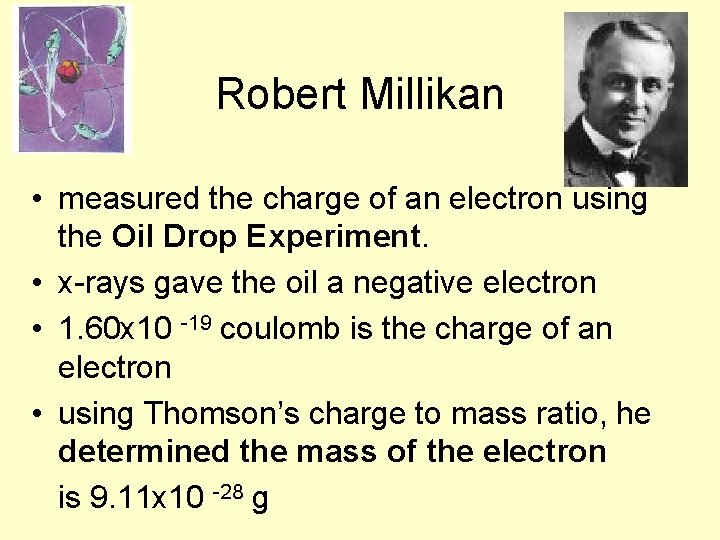
Robert Millikan • measured the charge of an electron using the Oil Drop Experiment. • x-rays gave the oil a negative electron • 1. 60 x 10 -19 coulomb is the charge of an electron • using Thomson’s charge to mass ratio, he determined the mass of the electron is 9. 11 x 10 -28 g

Robert Millikan’s Oil Drop Experiment • Robert Millikan received the Nobel Prize for his work • www. 68 pair. com

Henri Becquerel • discovered that uranium exhibits radioactivity • the chemical properties of an element change as it gives off radiation

Ernest Rutherford • alpha particles have a +2 charge • beta particles are high speed electrons • gamma rays are not composed of particles

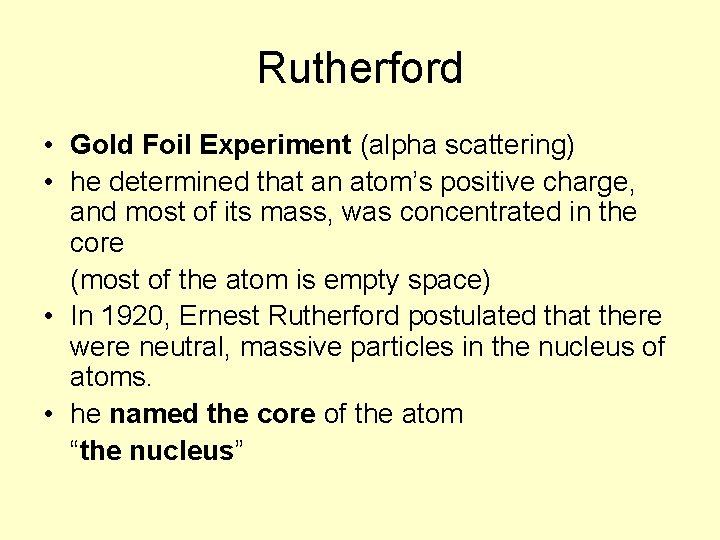
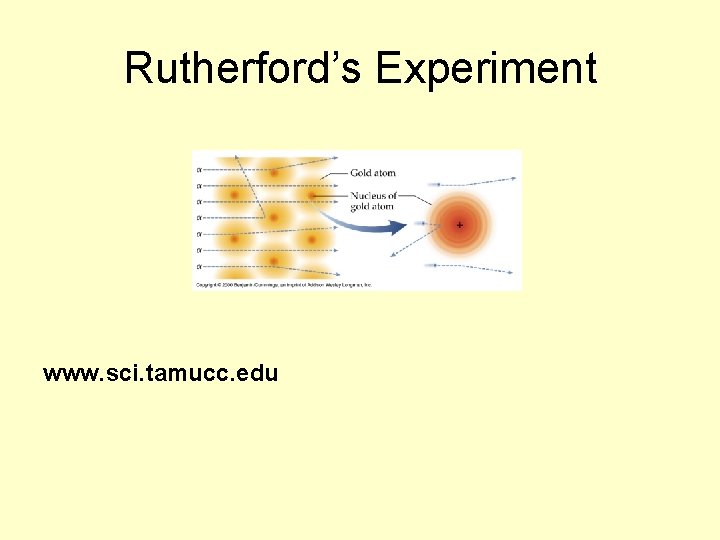
Rutherford • Gold Foil Experiment (alpha scattering) • he determined that an atom’s positive charge, and most of its mass, was concentrated in the core (most of the atom is empty space) • In 1920, Ernest Rutherford postulated that there were neutral, massive particles in the nucleus of atoms. • he named the core of the atom “the nucleus”

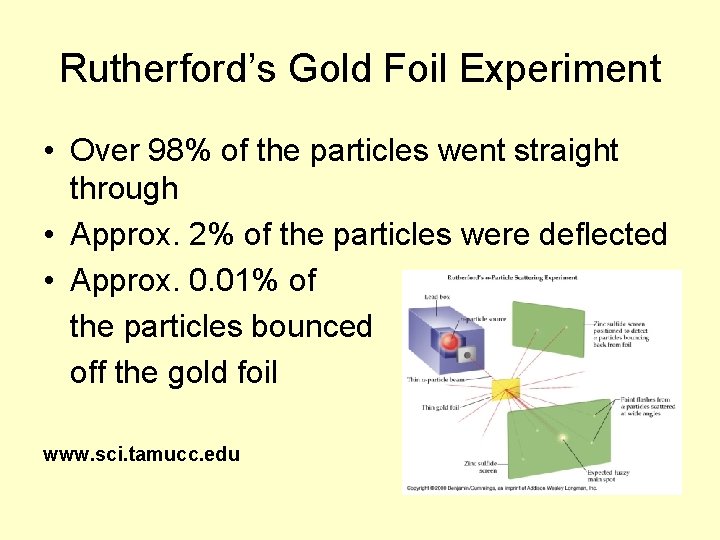
Rutherford’s Gold Foil Experiment • Over 98% of the particles went straight through • Approx. 2% of the particles were deflected • Approx. 0. 01% of the particles bounced off the gold foil www. sci. tamucc. edu

Rutherford’s Experiment www. sci. tamucc. edu

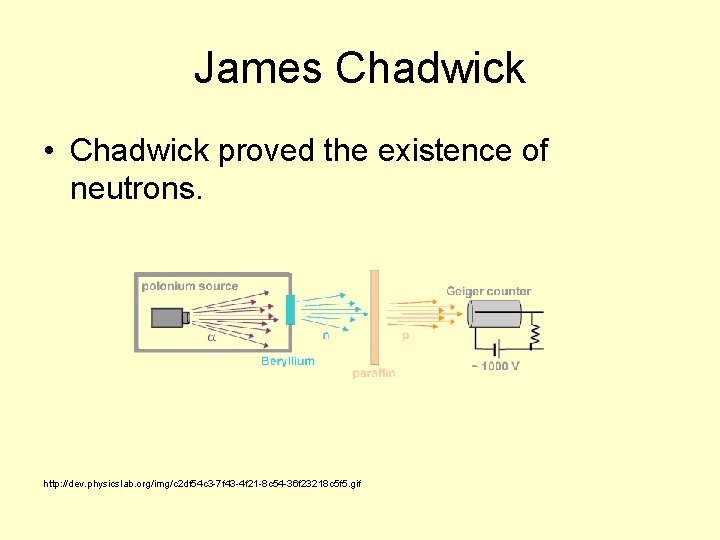
James Chadwick • Chadwick proved the existence of neutrons. http: //dev. physicslab. org/img/c 2 df 54 c 3 -7 f 43 -4 f 21 -8 c 54 -36 f 23218 c 5 f 5. gif

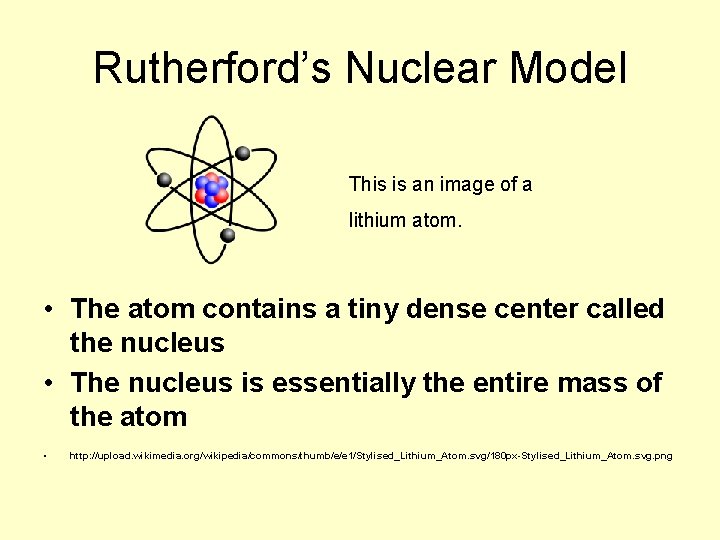
Rutherford’s Nuclear Model This is an image of a lithium atom. • The atom contains a tiny dense center called the nucleus • The nucleus is essentially the entire mass of the atom • http: //upload. wikimedia. org/wikipedia/commons/thumb/e/e 1/Stylised_Lithium_Atom. svg/180 px-Stylised_Lithium_Atom. svg. png

The Nucleus • The nucleus is positively charged • The amount of positive charge in the nucleus balances the negative charge of the electrons • The electrons move around in the empty space of the atom surrounding the nucleus

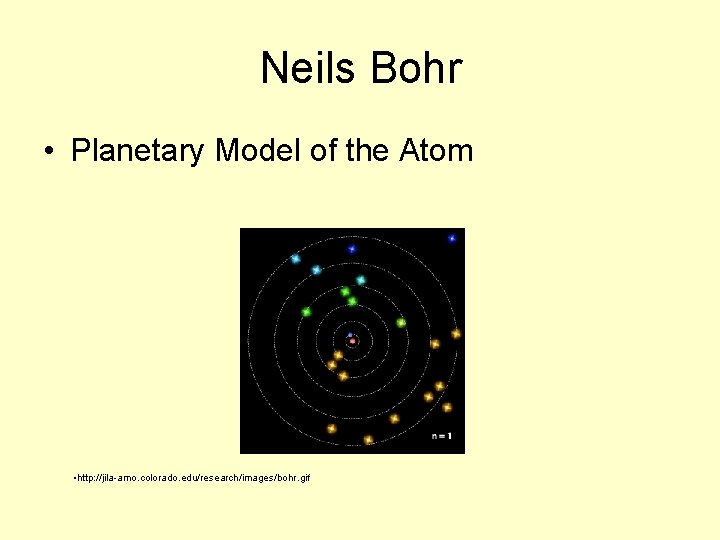
Neils Bohr • Planetary Model of the Atom • http: //jila-amo. colorado. edu/research/images/bohr. gif

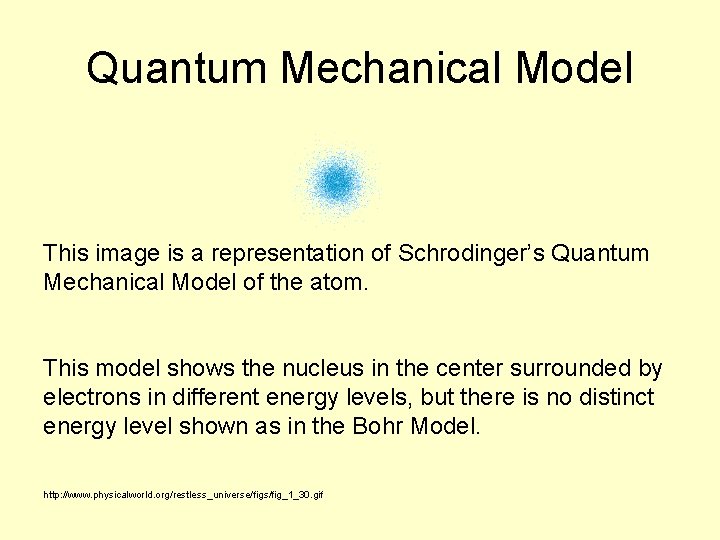
Quantum Mechanical Model This image is a representation of Schrodinger’s Quantum Mechanical Model of the atom. This model shows the nucleus in the center surrounded by electrons in different energy levels, but there is no distinct energy level shown as in the Bohr Model. http: //www. physicalworld. org/restless_universe/figs/fig_1_30. gif