Chapter 3 Molecules of Life Cengage Learning 2016











- Slides: 55

Chapter 3 Molecules of Life © Cengage Learning 2016

3. 1 Fear of Frying • Organisms consist of the same molecules – Different arrangements impact function • Trans and cis fats are good examples – Cis fats: most naturally occurring fats – Trans fats: unhealthy fats found mostly in partially hydrogenated vegetable oils – Trans fats: linked to diabetes, heart attacks, and atherosclerosis in humans © Cengage Learning 2016

Trans Fats: An Unhealthy Food O O OH OH C C H— C— H H— C— H H— C— H oleic acid H— C— H has a cis bond: C— H elaidic acid has a trans bond: H— C— H C— H H— C— H H— C— H H— C— H H— C— H HH H— C— HH © Cengage Learning 2016

3. 2 Organic Molecules • Carbon is the backbone of life and can form: – Four covalent bonds with other atoms – A variety of structures, including rings and chains – Polar and nonpolar bonds • “Organic” means that a compound consists mostly of carbon and hydrogen – These and many other elements are present in nonliving things as well, just at different proportions © Cengage Learning 2016

Modeling Organic Molecules • The structure of an organic molecule controls its function • Chemical structure can be represented in four different ways – Structural formula – Simplified carbon ring structures – Ball-and-stick models – Space-filling models © Cengage Learning 2016

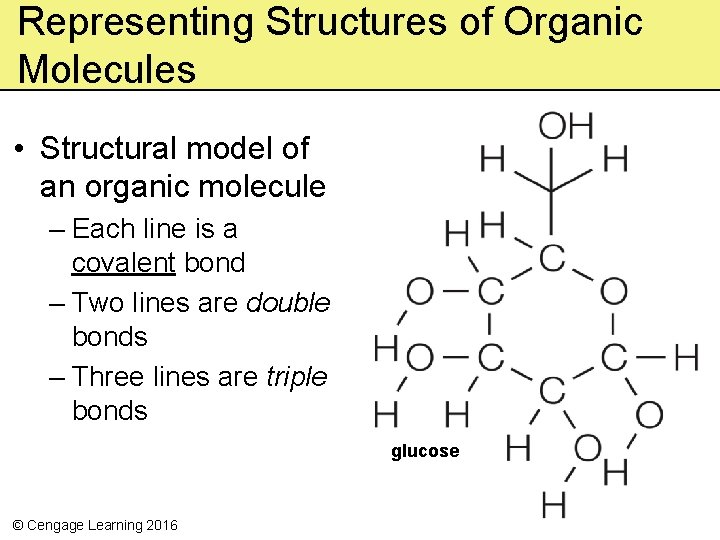
Representing Structures of Organic Molecules • Structural model of an organic molecule – Each line is a covalent bond – Two lines are double bonds – Three lines are triple bonds glucose © Cengage Learning 2016

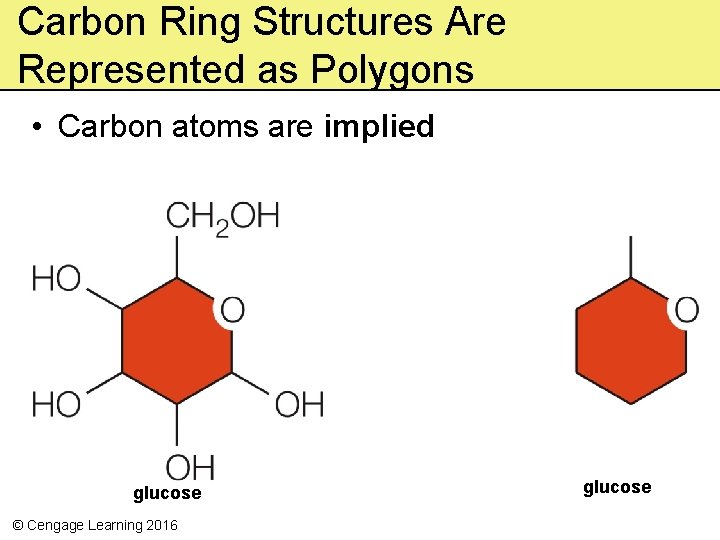
Carbon Ring Structures Are Represented as Polygons • Carbon atoms are implied glucose © Cengage Learning 2016 glucose

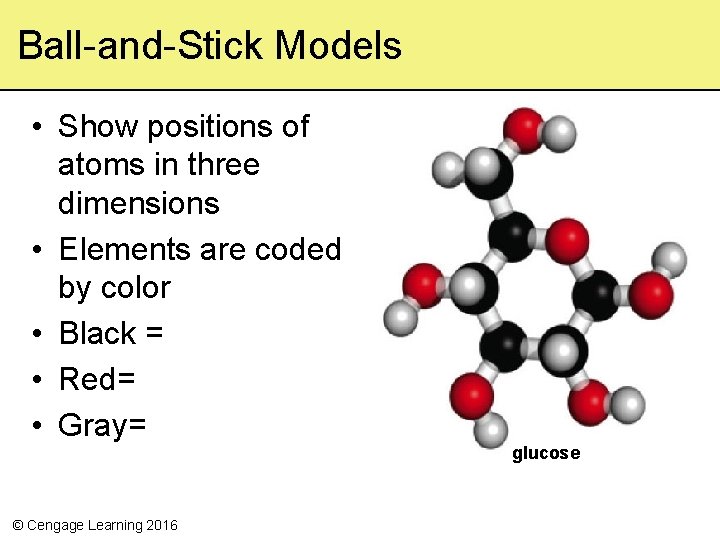
Ball-and-Stick Models • Show positions of atoms in three dimensions • Elements are coded by color • Black = • Red= • Gray= © Cengage Learning 2016 glucose

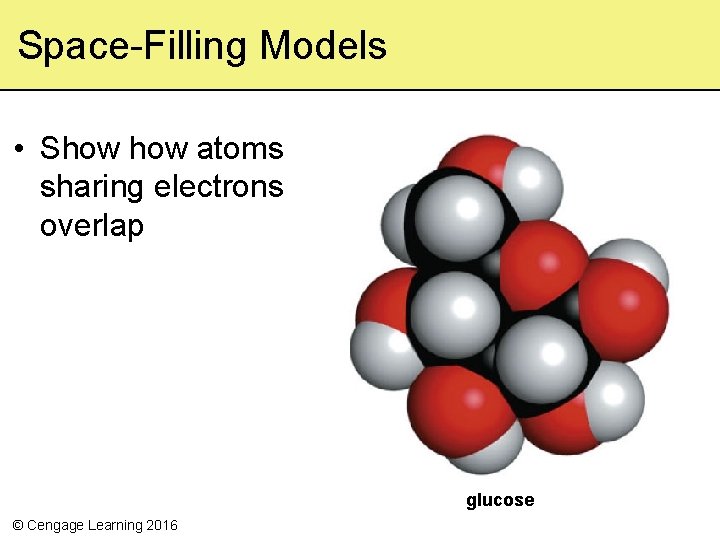
Space-Filling Models • Show atoms sharing electrons overlap glucose © Cengage Learning 2016

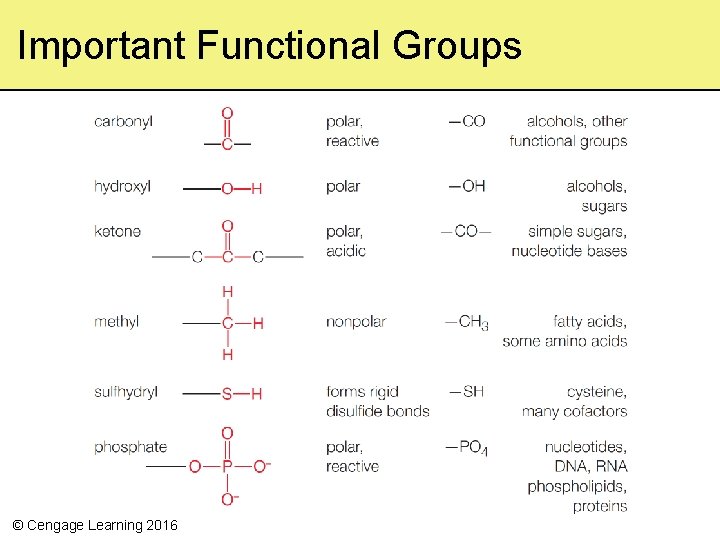
3. 3 Molecules of Life—From Structure to Function • Functional groups are important for molecules of life – Bond an atom or small molecular group to a carbon in an organic compound – Impact chemical properties such as acidity or polarity – Impart chemical behavior of molecules of life © Cengage Learning 2016

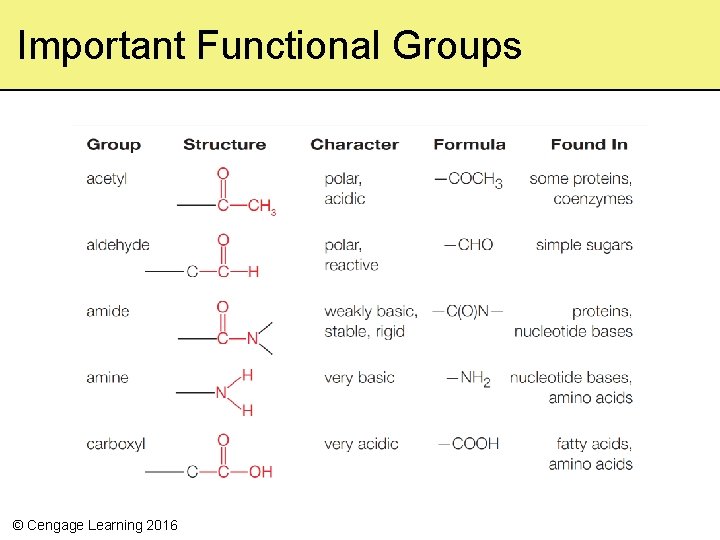
Important Functional Groups © Cengage Learning 2016

Important Functional Groups © Cengage Learning 2016

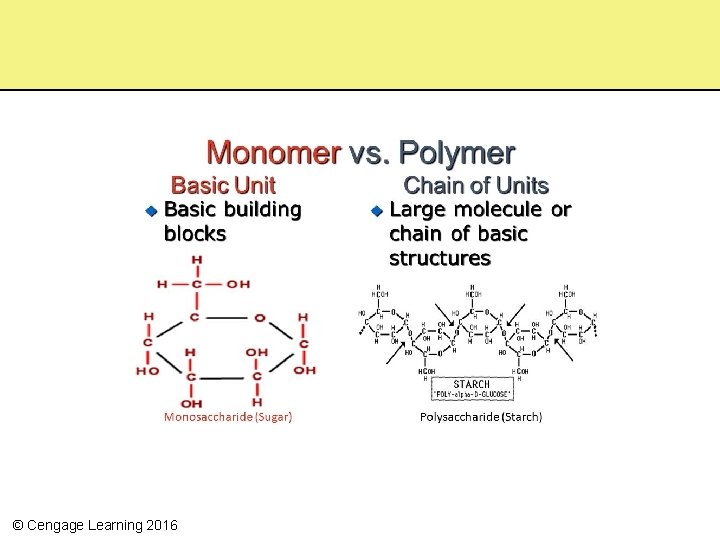
Assembling Complex Molecules • Monomers – Small organic molecules used as subunits to build larger molecules (polymers) – Simple sugars, fatty acids, amino acids, and nucleotides • Polymers – Larger molecules that are chains of monomers – May be split and used for energy – Complex carbohydrates, lipids, proteins, and nucleic acids © Cengage Learning 2016

© Cengage Learning 2016

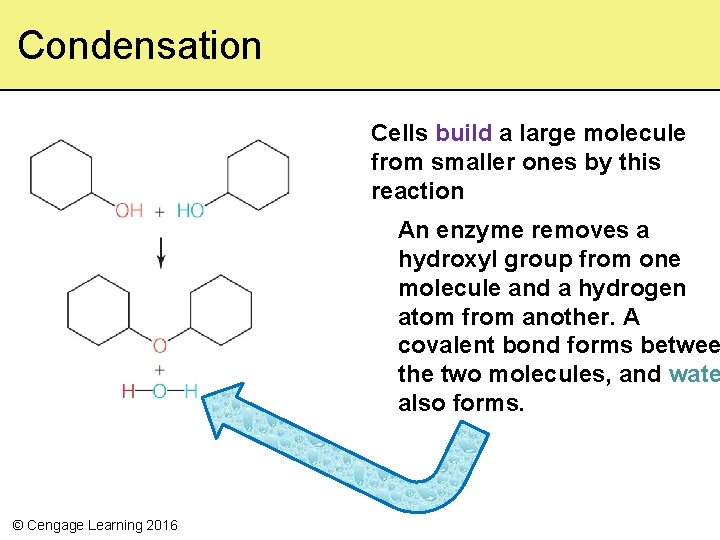
What Cells Do to Organic Compounds • Metabolism – Activities by which cells acquire and use energy to construct, rearrange, and split organic molecules – Allows cells to live, grow, and reproduce – Requires enzymes (proteins that increase the speed of reactions) – Two important reactions: condensation and hydrolysis © Cengage Learning 2016

Condensation Cells build a large molecule from smaller ones by this reaction An enzyme removes a hydroxyl group from one molecule and a hydrogen atom from another. A covalent bond forms betwee the two molecules, and wate also forms. © Cengage Learning 2016

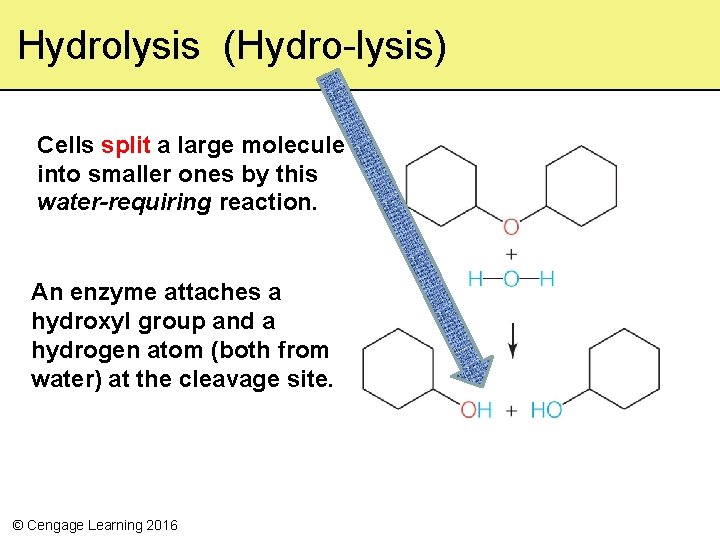
Hydrolysis (Hydro-lysis) Cells split a large molecule into smaller ones by this water-requiring reaction. An enzyme attaches a hydroxyl group and a hydrogen atom (both from water) at the cleavage site. © Cengage Learning 2016

3. 4 Carbohydrates • The most abundant biological molecules – Carbon, hydrogen, and oxygen in a 1: 2: 1 ratio – Used for structural materials, fuels, and for storing and transporting energy • Simple carbohydrates – Monosaccharides = 1 sugar – Carbon backbone with carbonyl and hydroxyl function groups – Soluble in water © Cengage Learning 2016

Carbohydrates in Biological Systems • Short chain carbohydrates – Oligosaccharides = a few monosaccharides – Disaccharides like lactose and sucrose have two sugar units • Complex carbohydrates – Polysaccharides = 100 s or 1000 s of sugars – Straight or branched chains – One or many types of monosaccharides – Three of the most common: cellulose, starch, and glycogen © Cengage Learning 2016

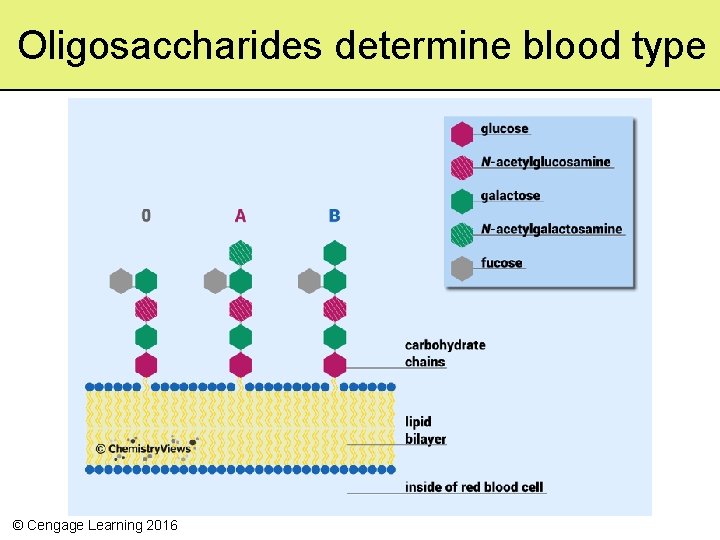
Oligosaccharides determine blood type © Cengage Learning 2016

© Cengage Learning 2016

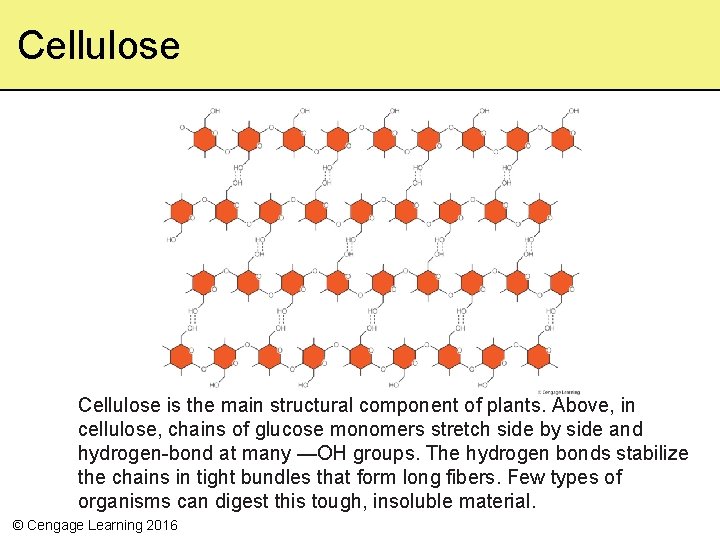
Cellulose is the main structural component of plants. Above, in cellulose, chains of glucose monomers stretch side by side and hydrogen-bond at many —OH groups. The hydrogen bonds stabilize the chains in tight bundles that form long fibers. Few types of organisms can digest this tough, insoluble material. © Cengage Learning 2016

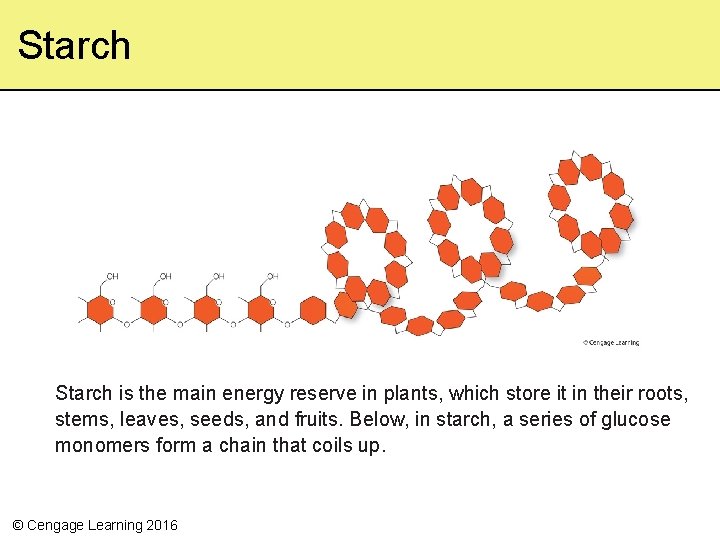
Starch is the main energy reserve in plants, which store it in their roots, stems, leaves, seeds, and fruits. Below, in starch, a series of glucose monomers form a chain that coils up. © Cengage Learning 2016

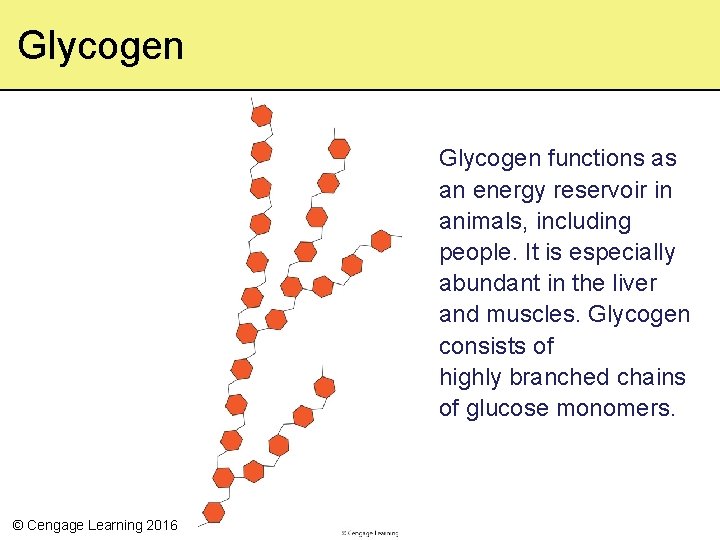
Glycogen functions as an energy reservoir in animals, including people. It is especially abundant in the liver and muscles. Glycogen consists of highly branched chains of glucose monomers. © Cengage Learning 2016

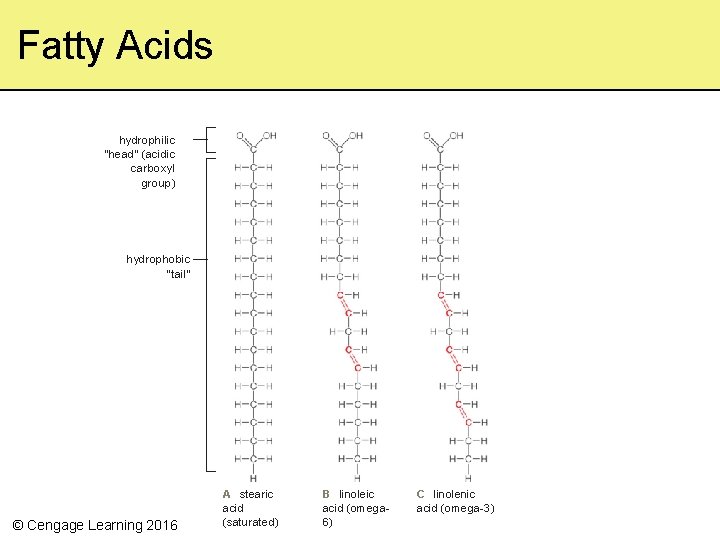
3. 5 Lipids • Fatty, oily, or waxy organic compounds – Vary in structure but always hydrophobic • Fatty acids are present in most lipids – A long hydrophobic hydrocarbon tail with a hydrophilic carboxyl head – Saturated fatty acids have straight tails with single bonds (solid fats) – Unsaturated fatty acids have crooked tails with double bonds (liquid fats EXCEPT trans fat) © Cengage Learning 2016

Fatty Acids hydrophilic “head” (acidic carboxyl group) hydrophobic “tail” © Cengage Learning 2016 A stearic acid (saturated) B linoleic acid (omega 6) C linolenic acid (omega-3)

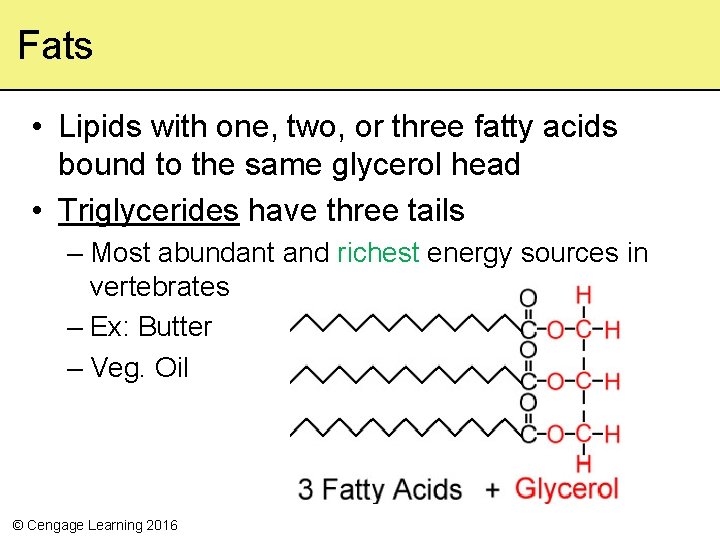
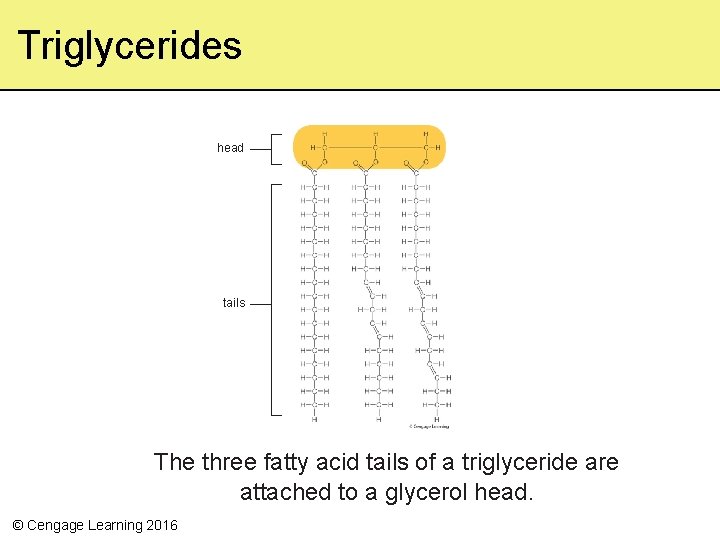
Fats • Lipids with one, two, or three fatty acids bound to the same glycerol head • Triglycerides have three tails – Most abundant and richest energy sources in vertebrates – Ex: Butter – Veg. Oil © Cengage Learning 2016

Triglycerides head tails The three fatty acid tails of a triglyceride are attached to a glycerol head. © Cengage Learning 2016

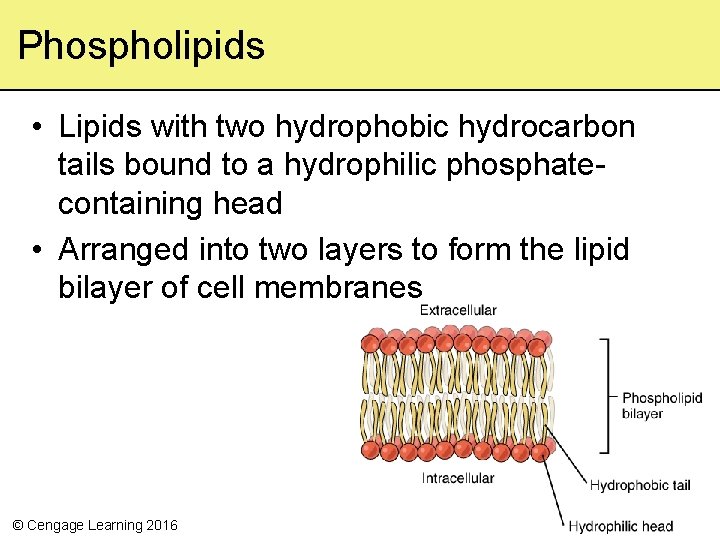
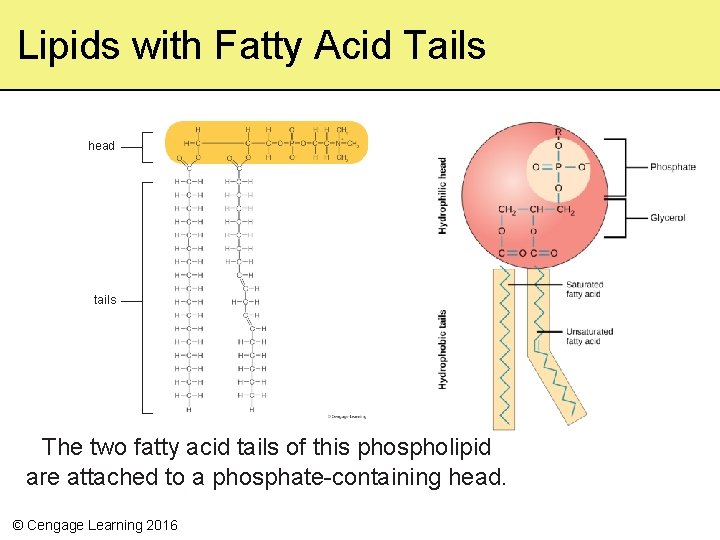
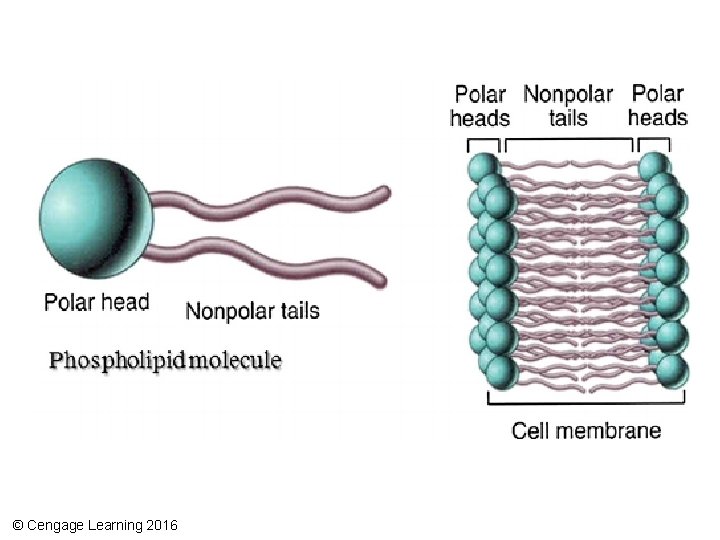
Phospholipids • Lipids with two hydrophobic hydrocarbon tails bound to a hydrophilic phosphatecontaining head • Arranged into two layers to form the lipid bilayer of cell membranes © Cengage Learning 2016

Lipids with Fatty Acid Tails head tails The two fatty acid tails of this phospholipid are attached to a phosphate-containing head. © Cengage Learning 2016

© Cengage Learning 2016

Waxes • Complex mixtures with long fatty-acid tails bonded to long-chain alcohols or carbon rings • Protective, water-repellant covering © Cengage Learning 2016

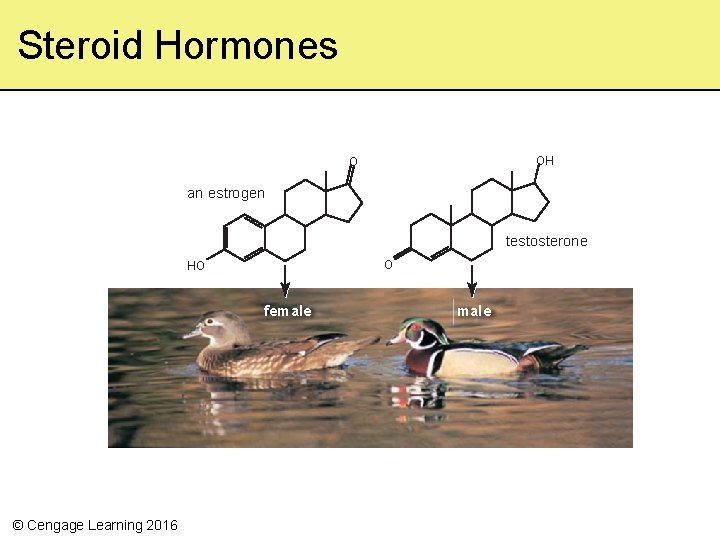
Steroids • Lipids with a rigid backbone of four carbon rings and no fatty-acid tails • Cholesterol – Most important steroid in animal tissue – Component of eukaryotic cell membranes – Remodeled into bile salts, vitamin D, and steroid hormones • The female sex hormone estrogen • The male sex hormone testosterone © Cengage Learning 2016

Steroid Hormones OH O an estrogen testosterone O HO female © Cengage Learning 2016 male

© Cengage Learning 2016

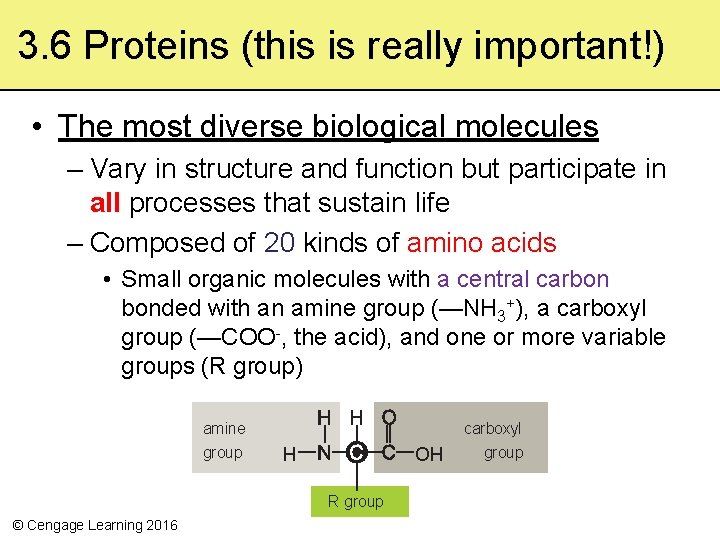
3. 6 Proteins (this is really important!) • The most diverse biological molecules – Vary in structure and function but participate in all processes that sustain life – Composed of 20 kinds of amino acids • Small organic molecules with a central carbon bonded with an amine group (—NH 3+), a carboxyl group (—COO-, the acid), and one or more variable groups (R group) amine group H OH R group © Cengage Learning 2016 carboxyl group

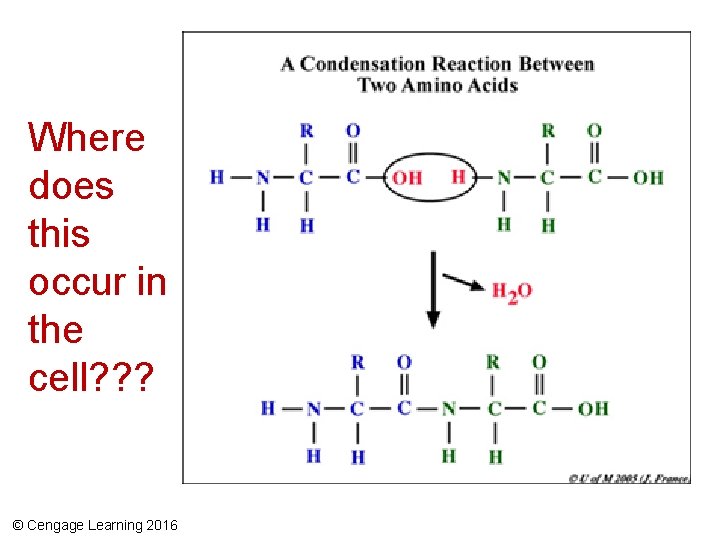
Polypeptides • Amino acid chains formed from protein synthesis • Polypeptide – A chain of amino acids – Bonded together by peptide bonds – Condensation reaction between the amine group of one amino acid and the carboxyl group of another amino acid © Cengage Learning 2016

Where does this occur in the cell? ? ? © Cengage Learning 2016

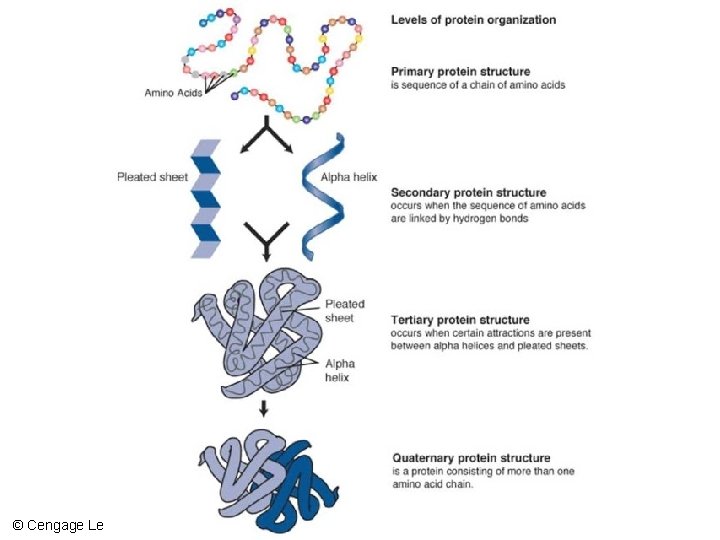
Primary and Secondary Protein Structure • Primary structure – The unique amino acid sequence of a protein • Secondary structure – The polypeptide chain folds and forms hydrogen bonds between amino acids © Cengage Learning 2016

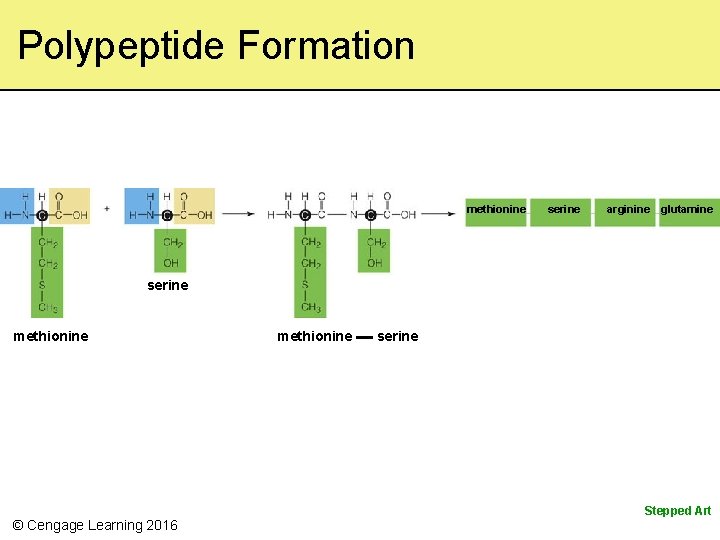
Polypeptide Formation methionine serine arginine glutamine serine methionine serine Stepped Art © Cengage Learning 2016

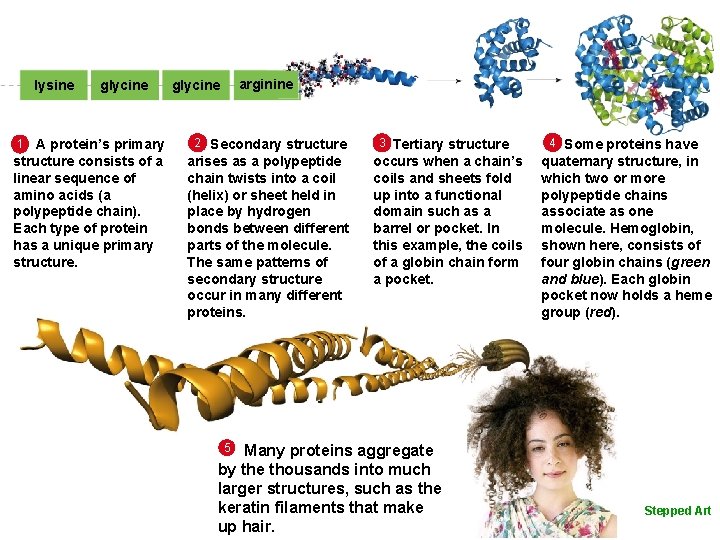
lysine glycine arginine glycine A protein’s primary structure consists of a linear sequence of amino acids (a polypeptide chain). Each type of protein has a unique primary structure. 1 2 Secondary structure arises as a polypeptide chain twists into a coil (helix) or sheet held in place by hydrogen bonds between different parts of the molecule. The same patterns of secondary structure occur in many different proteins. 3 Tertiary structure occurs when a chain’s coils and sheets fold up into a functional domain such as a barrel or pocket. In this example, the coils of a globin chain form a pocket. Many proteins aggregate by the thousands into much larger structures, such as the keratin filaments that make up hair. 4 Some proteins have quaternary structure, in which two or more polypeptide chains associate as one molecule. Hemoglobin, shown here, consists of four globin chains (green and blue). Each globin pocket now holds a heme group (red). 5 © Cengage Learning 2016 Stepped Art

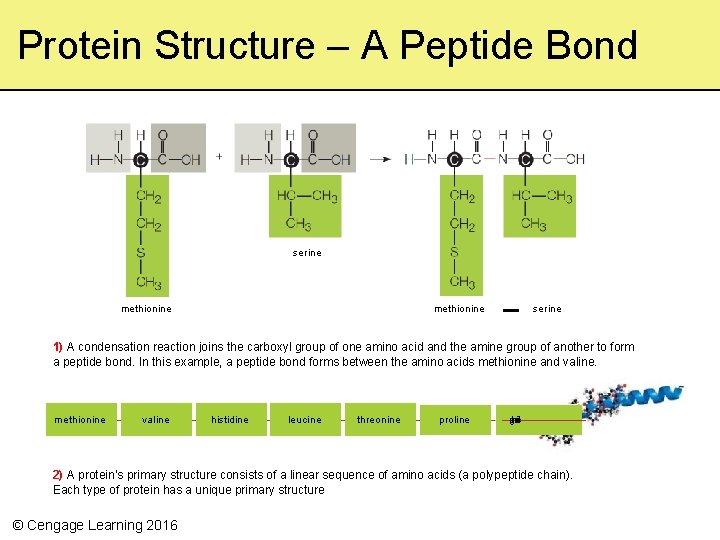
Protein Structure – A Peptide Bond serine methionine serine 1) A condensation reaction joins the carboxyl group of one amino acid and the amine group of another to form a peptide bond. In this example, a peptide bond forms between the amino acids methionine and valine. methionine valine histidine leucine threonine proline u g atlm a cdcii 2) A protein’s primary structure consists of a linear sequence of amino acids (a polypeptide chain). Each type of protein has a unique primary structure © Cengage Learning 2016

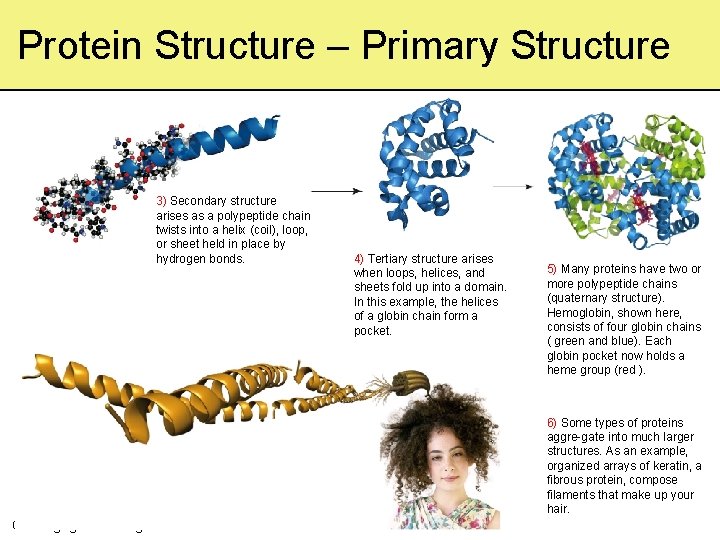
Protein Structure – Primary Structure 3) Secondary structure arises as a polypeptide chain twists into a helix (coil), loop, or sheet held in place by hydrogen bonds. 4) Tertiary structure arises when loops, helices, and sheets fold up into a domain. In this example, the helices of a globin chain form a pocket. 5) Many proteins have two or more polypeptide chains (quaternary structure). Hemoglobin, shown here, consists of four globin chains ( green and blue). Each globin pocket now holds a heme group (red ). 6) Some types of proteins aggre-gate into much larger structures. As an example, organized arrays of keratin, a fibrous protein, compose filaments that make up your hair. © Cengage Learning 2016

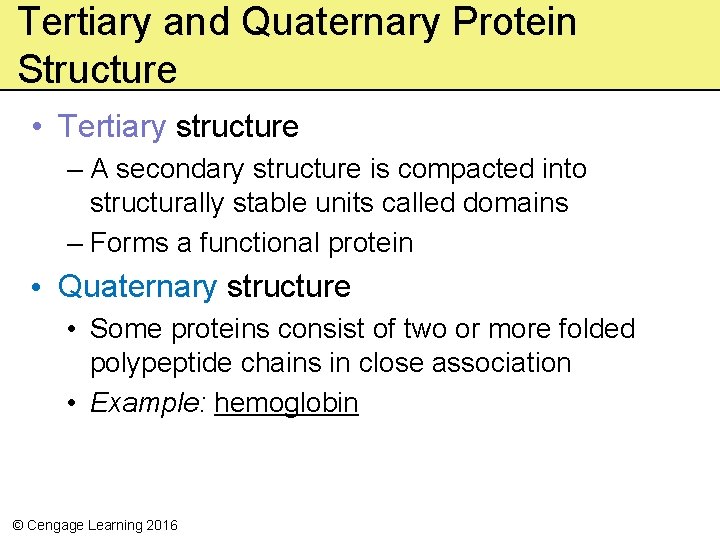
Tertiary and Quaternary Protein Structure • Tertiary structure – A secondary structure is compacted into structurally stable units called domains – Forms a functional protein • Quaternary structure • Some proteins consist of two or more folded polypeptide chains in close association • Example: hemoglobin © Cengage Learning 2016

© Cengage Learning 2016

3. 7 Why is Protein Structure So Important? • Proteins function requires a correct 3 D shape • Denatured proteins no longer have correct shapes – Heat, changes in p. H, salts, and detergents can disrupt the hydrogen bonds that maintain a protein’s shape • Once a protein’s shape unravels, so does its function © Cengage Learning 2016

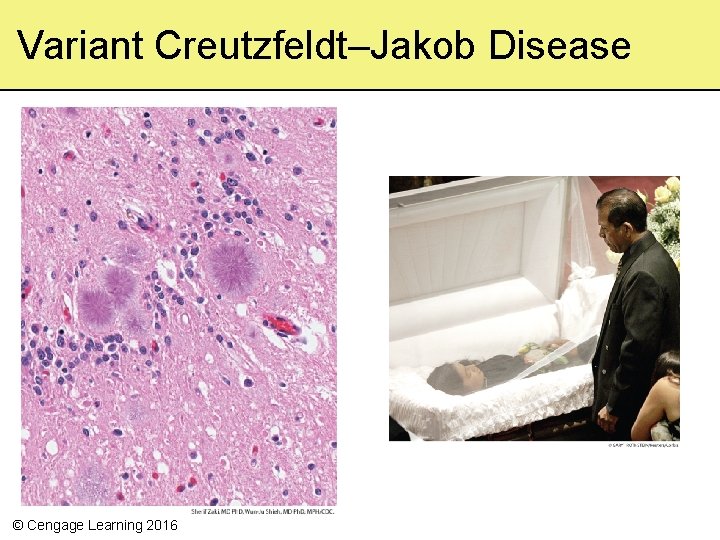
Prions • Prion diseases are caused by misfolded proteins – Mad cow disease (bovine spongiform encephalitis) – Creutzfeldt–Jakob disease in humans – Scrapie in sheep • Infectious diseases – Characterized by deterioration of mental and physical abilities – Eventually cause death © Cengage Learning 2016

Variant Creutzfeldt–Jakob Disease © Cengage Learning 2016

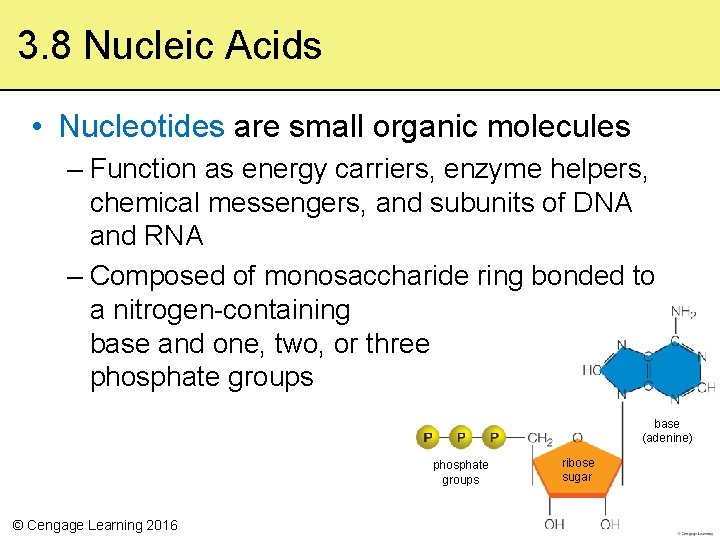
3. 8 Nucleic Acids • Nucleotides are small organic molecules – Function as energy carriers, enzyme helpers, chemical messengers, and subunits of DNA and RNA – Composed of monosaccharide ring bonded to a nitrogen-containing base and one, two, or three phosphate groups base (adenine) phosphate groups © Cengage Learning 2016 ribose sugar

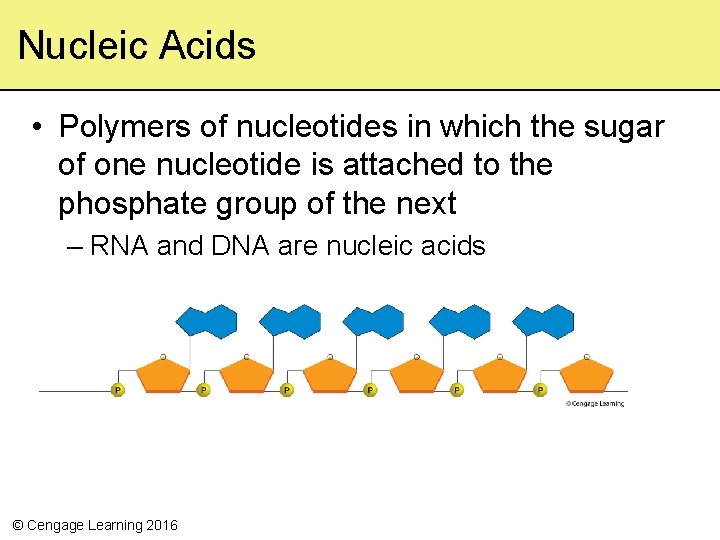
Nucleic Acids • Polymers of nucleotides in which the sugar of one nucleotide is attached to the phosphate group of the next – RNA and DNA are nucleic acids © Cengage Learning 2016

RNA and DNA • RNA (ribonucleic acid) – Contains four kinds of nucleotide monomers, including ATP – Important in protein synthesis • DNA (deoxyribonucleic acid) – Two chains of nucleotides twisted together into a double helix and held by hydrogen bonds – Contains all inherited information necessary to build an organism, coded in the order of nucleotide bases © Cengage Learning 2016

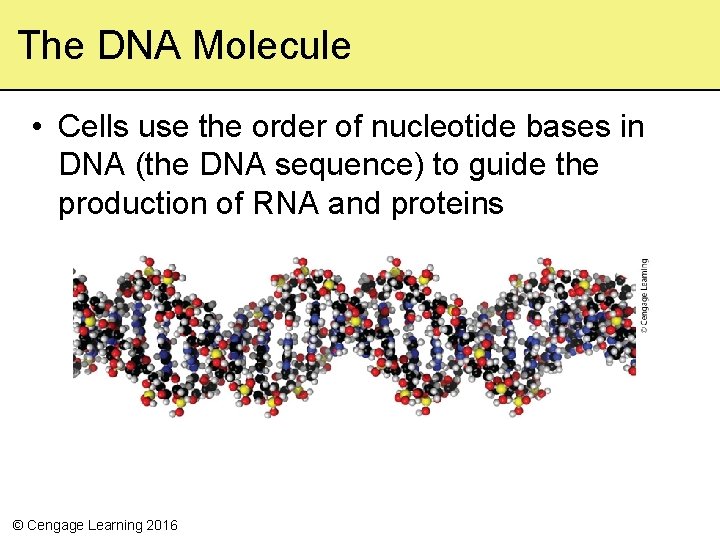
The DNA Molecule • Cells use the order of nucleotide bases in DNA (the DNA sequence) to guide the production of RNA and proteins © Cengage Learning 2016

© Cengage Learning 2016

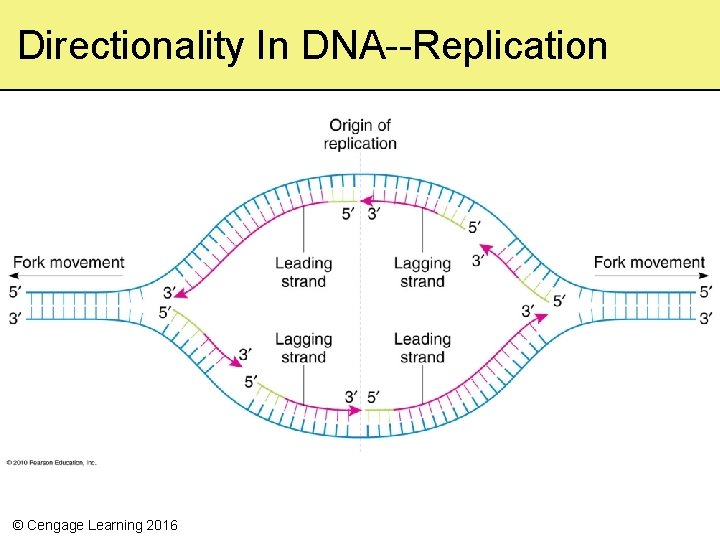
Directionality In DNA--Replication © Cengage Learning 2016

Points to Ponder • Consider trans fat foods such as red meats and dairy products. Are there dangers in limiting these foods in different age groups, such as in children? Benefits? • Why do athletes eat high carbohydrate meals before they perform? • Consider the effects of nutritional deficiencies on the body. Why is a balanced diet so critically important? © Cengage Learning 2016