Chapter 3 Methods of Studying Microorganisms 2004 Wadsworth



















- Slides: 23

Chapter 3 Methods of Studying Microorganisms © 2004 Wadsworth – Thomson Learning

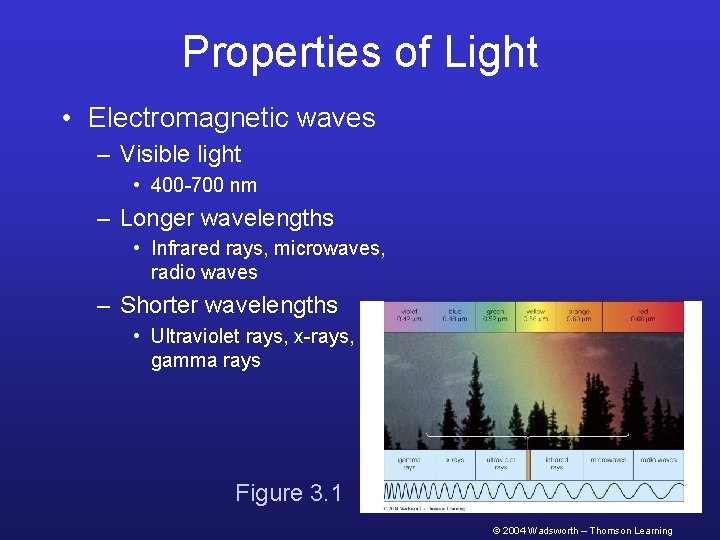
Properties of Light • Electromagnetic waves – Visible light • 400 -700 nm – Longer wavelengths • Infrared rays, microwaves, radio waves – Shorter wavelengths • Ultraviolet rays, x-rays, gamma rays Figure 3. 1 © 2004 Wadsworth – Thomson Learning

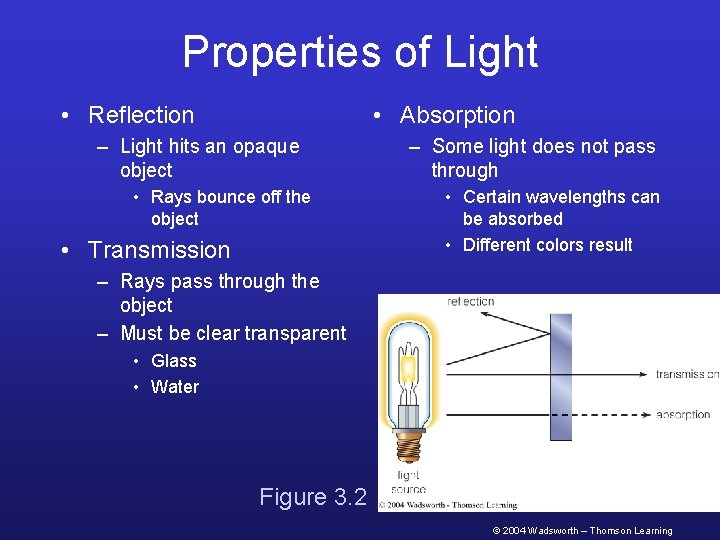
Properties of Light • Reflection • Absorption – Light hits an opaque object • Rays bounce off the object • Transmission – Some light does not pass through • Certain wavelengths can be absorbed • Different colors result – Rays pass through the object – Must be clear transparent • Glass • Water Figure 3. 2 © 2004 Wadsworth – Thomson Learning

Properties of Light • Diffraction – Light rays bend when they pass near an opaque object • Refraction – Bending of light Figure 3. 3 • Object of different density • Slows down • Bending of rays – Refractive index • Determines the speed of light through a medium Figure 3. 4 © 2004 Wadsworth – Thomson Learning

Microscopy • Magnification – Enlargement of image – Convex lens • Refracts light of image • Contrast – Absorption varies light intensity – Specimen absorbs light • Resolution – Distinguish between two points – Resolving power • Closest and yet distinguish • Size of lens • Wavelength of light • Refractive index Figure 3. 5 © 2004 Wadsworth – Thomson Learning

Microscopy • Compound microscope – Light source – Condenser • Direct light through object – Stage mount • Holds the specimen – Objective lenses • Various magnifying powers – Ocular lens • Additional magnification • Total magnification – Objective lens X ocular lens Figure 3. 6 © 2004 Wadsworth – Thomson Learning

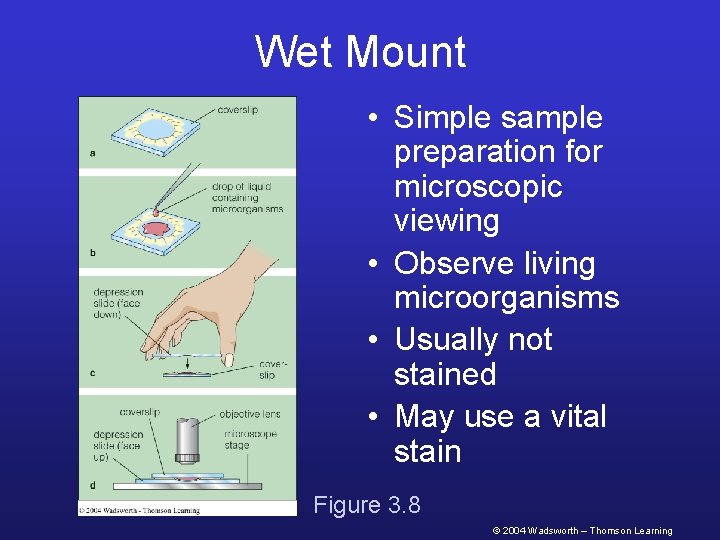
Wet Mount • Simple sample preparation for microscopic viewing • Observe living microorganisms • Usually not stained • May use a vital stain Figure 3. 8 © 2004 Wadsworth – Thomson Learning

Stains • Increase contrast • Require fixation of sample – Heat fixation • Coagulates proteins and causes to stick to slide – Chemical fixation • Types of dyes – Acidic: safranin, acid fuchsin, crystal violet, methylene blue – Basic: eosin, basic fuchsin, congo red • Simple stains – Make cells visible with one dye • Differential stains – Distinguish between types of microorganisms © 2004 Wadsworth – Thomson Learning

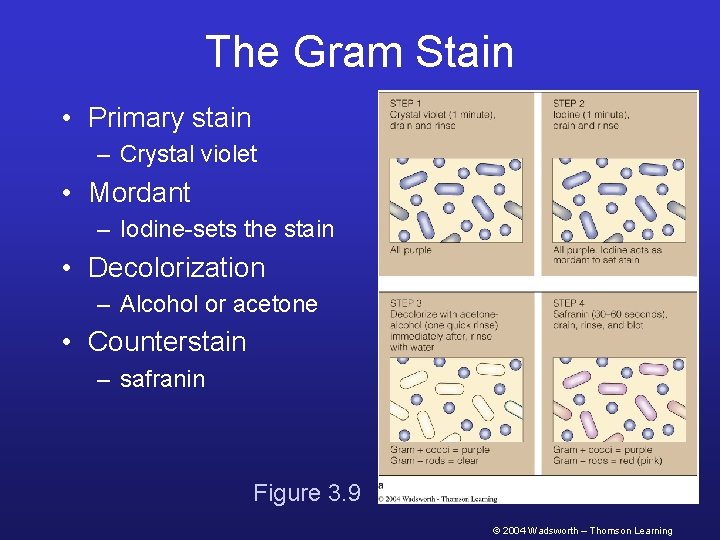
The Gram Stain • Primary stain – Crystal violet • Mordant – Iodine-sets the stain • Decolorization – Alcohol or acetone • Counterstain – safranin Figure 3. 9 © 2004 Wadsworth – Thomson Learning

Other Light Microscopes • Phase contrast microscopy – Rings in objective and condenser • Increase contrast of certain parts of specimen – Cellular movement and internal structure • Darkfield microscopy – Light is scattered off of object • Only light entering objectives is from specimen – Viewing surface structures • Nomarsky microscopy – Prisms in objective and condenser – Living organisms in animal tissues • Fluorescence microscopy – Fluorescent material illuminated by UV light © 2004 Wadsworth – Thomson Learning

Scanning microscopes • Concentrating on small field of view • Confocal microscopy – Same object viewed simultaneously from opposite sides • Illuminating microscope – Focused on very small area • Receiving microscope with photodetector – Connected to computer – Computer generates image – Three-dimensional • Multiple scans and different depths © 2004 Wadsworth – Thomson Learning

Electron microscopes • Electrons instead of light rays • Much greater magnification • Transmission electron microscope (TEM) Figure 3. 15 b – Electrons pass through specimen – Captured on photographic film – Ultra-thin specimen • Scanning electron microscope (SEM) – Electrons hit specimen and cause secondary electrons to eject from it – Captures the surface only Figure 3. 18 a © 2004 Wadsworth – Thomson Learning

Sample preparation for EM • Freeze fracturing • Shadow casting Figure 3. 16 Figure 3. 17 © 2004 Wadsworth – Thomson Learning

Viewing atoms and molecules • Scanned-proximity probe microscopes – Scanning tunneling microscope • View surfaces that conduct electricity – Metals – Semi-conducting materials – Atomic force microscope • • • Biologically important molecules Attractive and repulsive forces Diamond probe detects forces Laser beam detects bending of beam Resolution of 10 pm: 1/100 th of nm © 2004 Wadsworth – Thomson Learning

Culturing microorganisms Figure 3. 21 • Transfer of microorganism – Sterilize transfer loop – Dip loop into broth culture – Streak onto solid medium © 2004 Wadsworth – Thomson Learning

Sterilization • Eliminating all microorganisms • Culture media must be sterilized • Heat sterilization Figure 3. 20 – Moist heat • Autoclave • 121 o. C for 20 minutes – Dry heat • 170 o. C for 90 minutes • Filtration – Membrane filters • Chemicals © 2004 Wadsworth – Thomson Learning

Pure culture • Streak plate method – Streak inoculum onto one portion of the plate – Sterilize the loop – Streak through the first inoculum and spread into second section – Repeat several times – Incubate – Observe isolated colonies Figure 3. 21 © 2004 Wadsworth – Thomson Learning

Pure culture • Pour plate method – Make serial dilutions of bacterial suspension – Mix diluted sample with melted agar – Pour into plate – Incubate – Observe for isolated Figure 3. 22 colonies © 2004 Wadsworth – Thomson Learning

Culture media • Defined media – Produced from pure chemicals • Complex media – Extracts of natural sources • Beef, blood, milk, protein, yeast, soybeans • Precise composition not known • Selective media – Contents select for specific microorganism • Differential media – Identification of microorganisms © 2004 Wadsworth – Thomson Learning

Growth conditions • Temperature – Incubators – Water baths • p. H – Growth medium at optimal p. H – Buffers maintain p. H over period of growth © 2004 Wadsworth – Thomson Learning

Growth conditions • Oxygen – Strict aerobes • Require oxygen – Strict anaerobes • Oxygen is toxic – Facultative anaerobes • Use oxygen when available • Can grow without oxygen – Aerotolerant anaerobes • Can’t use oxygen but not toxic – Microaerophilic • Need low concentrations of oxygen © 2004 Wadsworth – Thomson Learning

Oxygen culturing conditions • Culturing – Anaerobic chambers • All oxygen is replaced with other gas – Shaking machines • Increase oxygen in the media – Candle jars • Not anaerobic but reduces available oxygen Figure 3. 25 © 2004 Wadsworth – Thomson Learning

Preserving cultures • Cold storage – Short-term: refrigeration slows growth • Must continually transfer – Long-term: freezing • Add substance to reduce freeze-killing – Glycerol, skim milk, dimethyl sulfoxide (DMSO) – Lyophilization • Long term—freeze drying • Frozen and dried under vacuum © 2004 Wadsworth – Thomson Learning