CHAPTER 3 METALS AND NON METALS CLASS MADE







- Slides: 23

CHAPTER - 3 METALS AND NON METALS CLASS MADE BY SCHOOL : - X : - MANAS MAHAJAN : - K. V. GANESHKHIND PUNE-7

1 a) Physical properties of metals : • • • Metals are solids. (except mercury) Metals are hard. (except Lithium, Potassium, Sodium) Metals have metallic lustre. (shine) Metals are malleable. (can be beaten into thin sheets) Metals are ductile. (can be drawn into wires) Metals have high melting points. (Gallium and Ceasium have low melting points. They melt in the palm of the hand) Metals have high boiling points. Metals are good conductors of heat. ( Best conductors are silver and copper. Poor conductors are Lead and Mercury) Metals are good conductors of electricity. ( Best conductors are Silver and Copper) Metals are sonorus. (produce sound when beaten)

b) Physical properties of non metals : • Non metals may be solids, liquids or gases. (Solids – Carbon, Sulphur, Phosphorus etc. Liquid – Bromine, Gases – Oxygen, Hydrogen, Nitrogen etc. ) • Non metals are soft. (except diamond which is the hardest natural substance) • Non metals do not have lustre. ( except iodine cryatals) • Non metals are not malleable. • Non metals are not ductile. • Non metals which are solids and liquids have low melting points. • Non metals which are solids and liquids have low boiling points. • Non metals are bad conductors of heat. • Non metals are bad conductors of electricity. (except graphite) • Non metals are not sonorus.

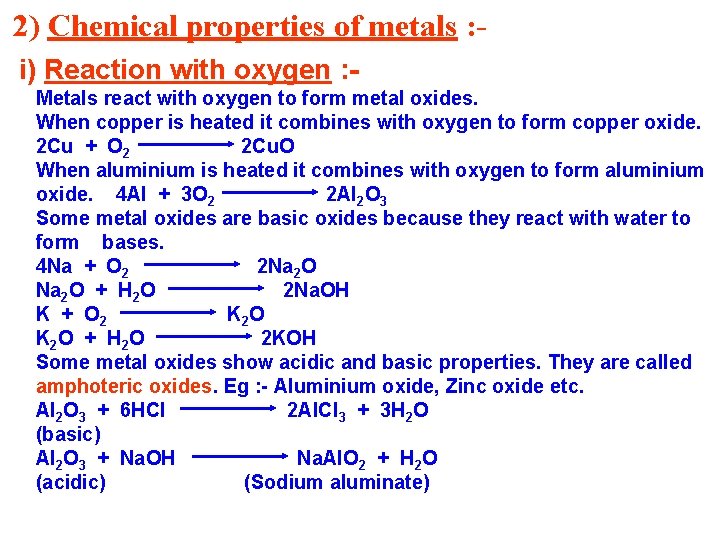
2) Chemical properties of metals : i) Reaction with oxygen : Metals react with oxygen to form metal oxides. When copper is heated it combines with oxygen to form copper oxide. 2 Cu + O 2 2 Cu. O When aluminium is heated it combines with oxygen to form aluminium oxide. 4 Al + 3 O 2 2 Al 2 O 3 Some metal oxides are basic oxides because they react with water to form bases. 4 Na + O 2 2 Na 2 O + H 2 O 2 Na. OH K + O 2 K 2 O + H 2 O 2 KOH Some metal oxides show acidic and basic properties. They are called amphoteric oxides. Eg : - Aluminium oxide, Zinc oxide etc. Al 2 O 3 + 6 HCl 2 Al. Cl 3 + 3 H 2 O (basic) Al 2 O 3 + Na. OH Na. Al. O 2 + H 2 O (acidic) (Sodium aluminate)

The reactivity of different metals with oxygen is different : • Metals like potassium and sodium react vigorously with oxygen and catch fire if kept in open. Hence they are stored in kerosene to prevent burning. • If magnesium is heated, it burns with a bright flame. • If iron is heated it glows brightly. • If copper is heated it does not burn but forms a black coating of copper oxide. • Silver and gold does not react with oxygen even at high temperature. • Some metals like magnesium, aluminium, zinc, lead etc. forms an oxide layer over it which prevents further oxidation. They are called self protecting metals.

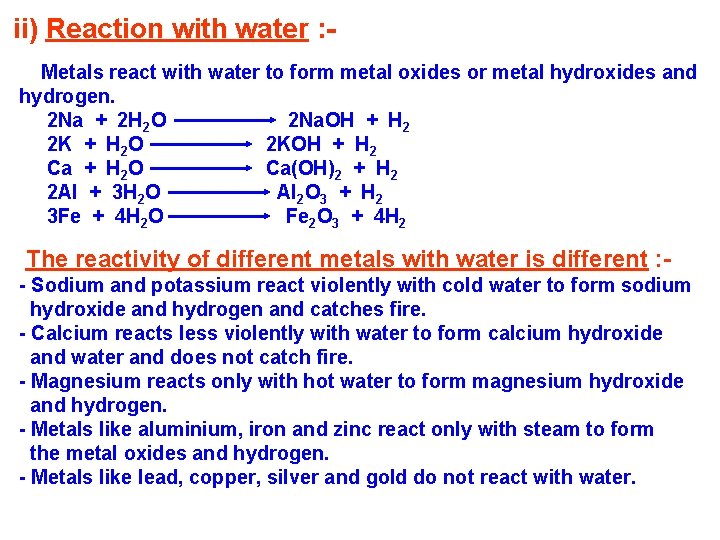
ii) Reaction with water : Metals react with water to form metal oxides or metal hydroxides and hydrogen. 2 Na + 2 H 2 O 2 Na. OH + H 2 2 K + H 2 O 2 KOH + H 2 Ca + H 2 O Ca(OH)2 + H 2 2 Al + 3 H 2 O Al 2 O 3 + H 2 3 Fe + 4 H 2 O Fe 2 O 3 + 4 H 2 The reactivity of different metals with water is different : - Sodium and potassium react violently with cold water to form sodium hydroxide and hydrogen and catches fire. - Calcium reacts less violently with water to form calcium hydroxide and water and does not catch fire. - Magnesium reacts only with hot water to form magnesium hydroxide and hydrogen. - Metals like aluminium, iron and zinc react only with steam to form the metal oxides and hydrogen. - Metals like lead, copper, silver and gold do not react with water.

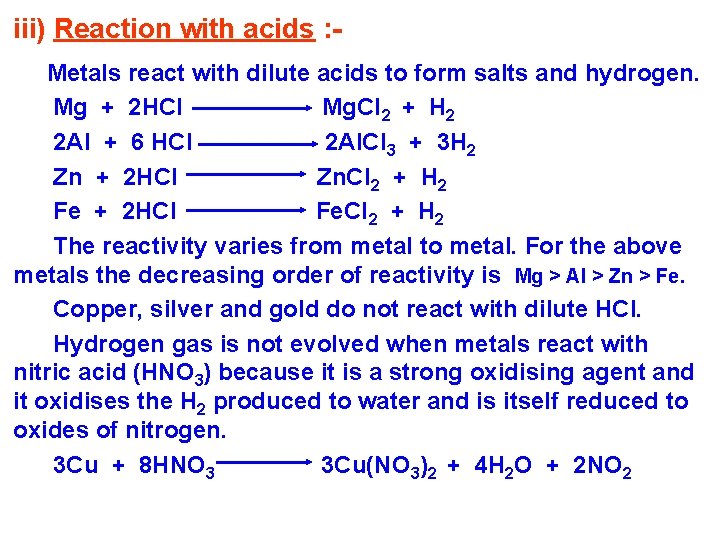
iii) Reaction with acids : Metals react with dilute acids to form salts and hydrogen. Mg + 2 HCl Mg. Cl 2 + H 2 2 Al + 6 HCl 2 Al. Cl 3 + 3 H 2 Zn + 2 HCl Zn. Cl 2 + H 2 Fe + 2 HCl Fe. Cl 2 + H 2 The reactivity varies from metal to metal. For the above metals the decreasing order of reactivity is Mg > Al > Zn > Fe. Copper, silver and gold do not react with dilute HCl. Hydrogen gas is not evolved when metals react with nitric acid (HNO 3) because it is a strong oxidising agent and it oxidises the H 2 produced to water and is itself reduced to oxides of nitrogen. 3 Cu + 8 HNO 3 3 Cu(NO 3)2 + 4 H 2 O + 2 NO 2

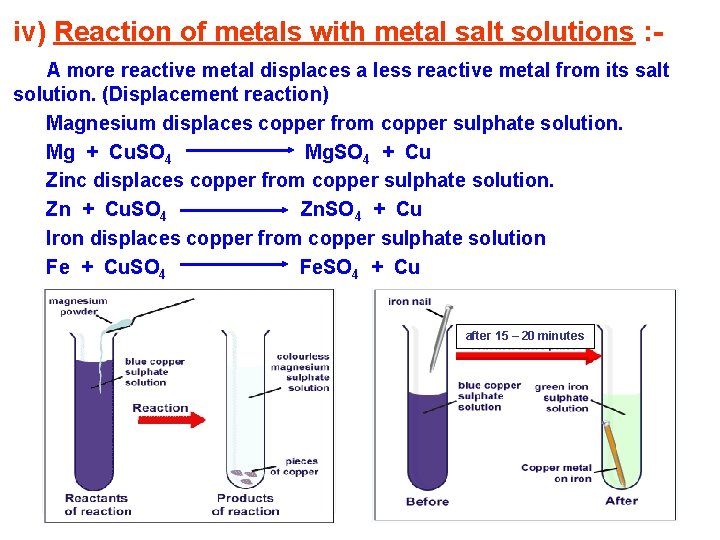
iv) Reaction of metals with metal salt solutions : A more reactive metal displaces a less reactive metal from its salt solution. (Displacement reaction) Magnesium displaces copper from copper sulphate solution. Mg + Cu. SO 4 Mg. SO 4 + Cu Zinc displaces copper from copper sulphate solution. Zn + Cu. SO 4 Zn. SO 4 + Cu Iron displaces copper from copper sulphate solution Fe + Cu. SO 4 Fe. SO 4 + Cu after 15 – 20 minutes

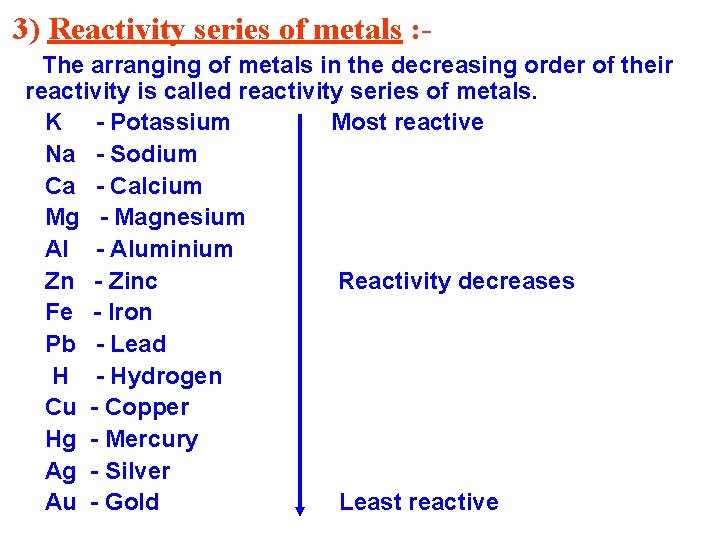
3) Reactivity series of metals : The arranging of metals in the decreasing order of their reactivity is called reactivity series of metals. K - Potassium Most reactive Na - Sodium Ca - Calcium Mg - Magnesium Al - Aluminium Zn - Zinc Reactivity decreases Fe - Iron Pb - Lead H - Hydrogen Cu - Copper Hg - Mercury Ag - Silver Au - Gold Least reactive

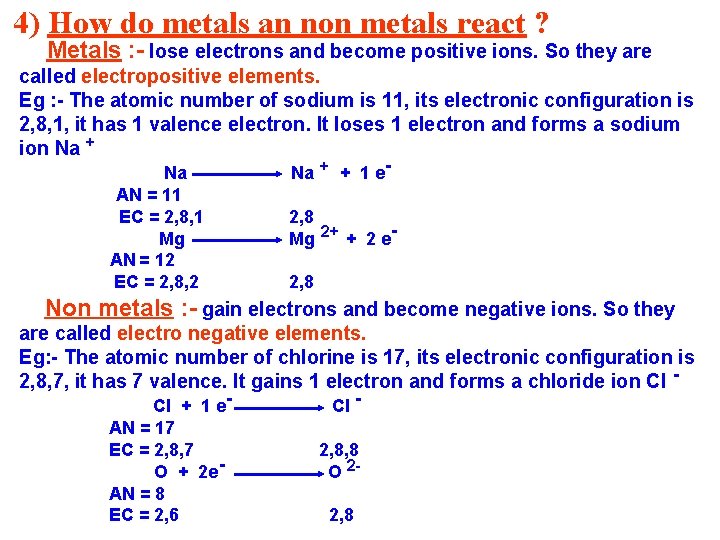
4) How do metals an non metals react ? Metals : - lose electrons and become positive ions. So they are called electropositive elements. Eg : - The atomic number of sodium is 11, its electronic configuration is 2, 8, 1, it has 1 valence electron. It loses 1 electron and forms a sodium ion Na + Na AN = 11 EC = 2, 8, 1 Mg AN = 12 EC = 2, 8, 2 Na + + 1 e- 2, 8 Mg 2+ + 2 e 2, 8 Non metals : - gain electrons and become negative ions. So they are called electro negative elements. Eg: - The atomic number of chlorine is 17, its electronic configuration is 2, 8, 7, it has 7 valence. It gains 1 electron and forms a chloride ion Cl Cl + 1 e. AN = 17 EC = 2, 8, 7 O + 2 e. AN = 8 EC = 2, 6 Cl - 2, 8, 8 O 22, 8

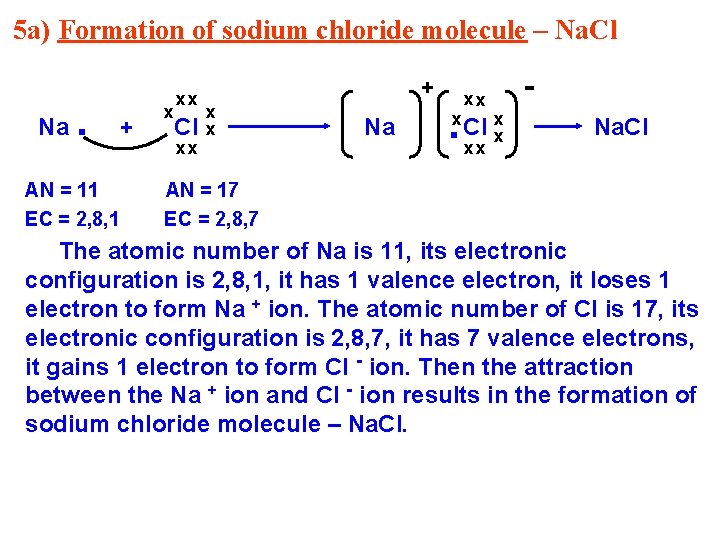
5 a) Formation of sodium chloride molecule – Na. Cl Na . AN = 11 EC = 2, 8, 1 + x xx Cl xx + x x Na xx . x x. Clx xx Na. Cl AN = 17 EC = 2, 8, 7 The atomic number of Na is 11, its electronic configuration is 2, 8, 1, it has 1 valence electron, it loses 1 electron to form Na + ion. The atomic number of Cl is 17, its electronic configuration is 2, 8, 7, it has 7 valence electrons, it gains 1 electron to form Cl - ion. Then the attraction between the Na + ion and Cl - ion results in the formation of sodium chloride molecule – Na. Cl.

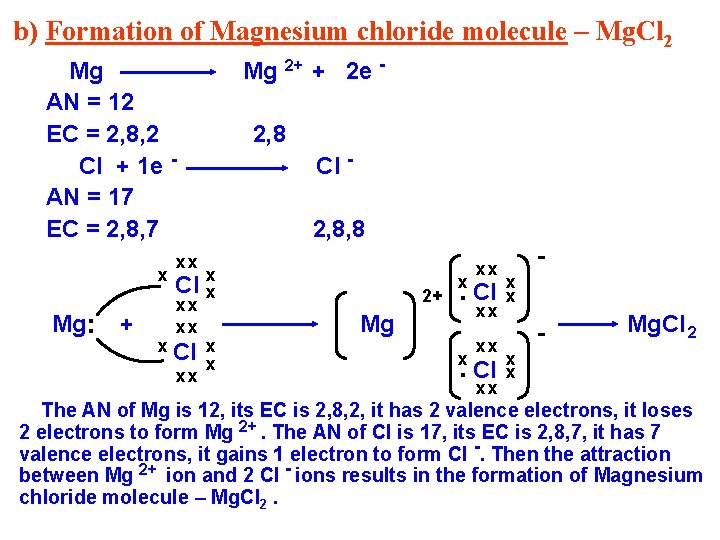
b) Formation of Magnesium chloride molecule – Mg. Cl 2 Mg 2+ + 2 e - Mg AN = 12 EC = 2, 8, 2 Cl + 1 e AN = 17 EC = 2, 8, 7 Mg. . x + x 2, 8 Cl 2, 8, 8 xx Cl xx xx xx Cl xx x x 2+ Mg . Cl x xx xx x x - Mg. Cl 2 x x The AN of Mg is 12, its EC is 2, 8, 2, it has 2 valence electrons, it loses 2 electrons to form Mg 2+. The AN of Cl is 17, its EC is 2, 8, 7, it has 7 valence electrons, it gains 1 electron to form Cl -. Then the attraction between Mg 2+ ion and 2 Cl - ions results in the formation of Magnesium chloride molecule – Mg. Cl 2.

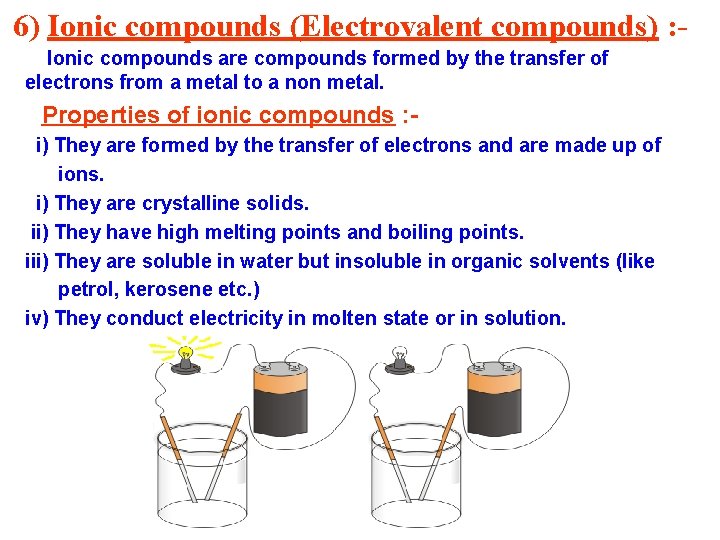
6) Ionic compounds (Electrovalent compounds) : Ionic compounds are compounds formed by the transfer of electrons from a metal to a non metal. Properties of ionic compounds : i) They are formed by the transfer of electrons and are made up of ions. i) They are crystalline solids. ii) They have high melting points and boiling points. iii) They are soluble in water but insoluble in organic solvents (like petrol, kerosene etc. ) iv) They conduct electricity in molten state or in solution.

7 a) Occurence of metals : Some metals like gold, silver, platinum etc are found in the free state in the earth’s crust because they are least reactive. Most metals are found as oxides, carbonates, sulphides, halides etc. Minerals : - are elements or compounds which occur naturally inside the earth’s crust. Ore : - is a mineral from which metals can be extracted profitably. Gangue : - is the impurities present in the ore like rock particles, sand particles, clay particles etc. b) Extraction of metals from their ores : Metals are extracted from their ores in three main steps. They are : i) Concentration of the ore (Enrichment of the ore). ii) Reduction to the metal. iii) Refining (Purification of the metal). Concentration of the ore : - is the removal of gangue (impurities) from the ore by different methods.

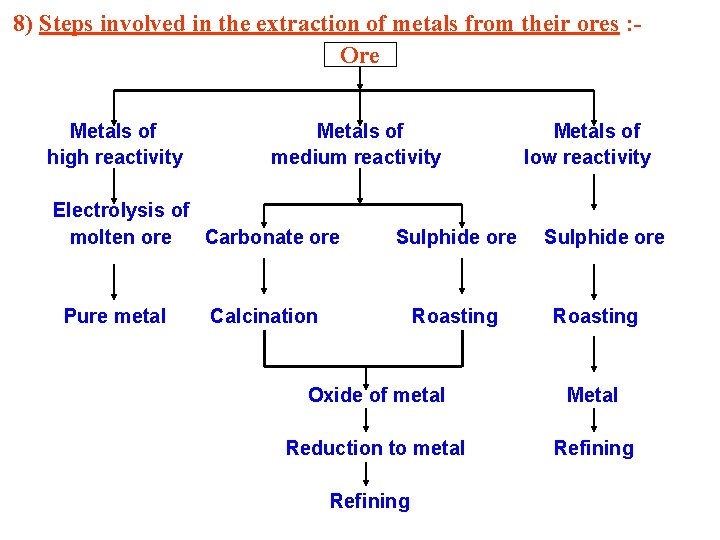
8) Steps involved in the extraction of metals from their ores : Ore Metals of high reactivity Metals of medium reactivity Electrolysis of molten ore Carbonate ore Pure metal Sulphide ore Calcination Roasting Metals of low reactivity Sulphide ore Roasting Oxide of metal Metal Reduction to metal Refining

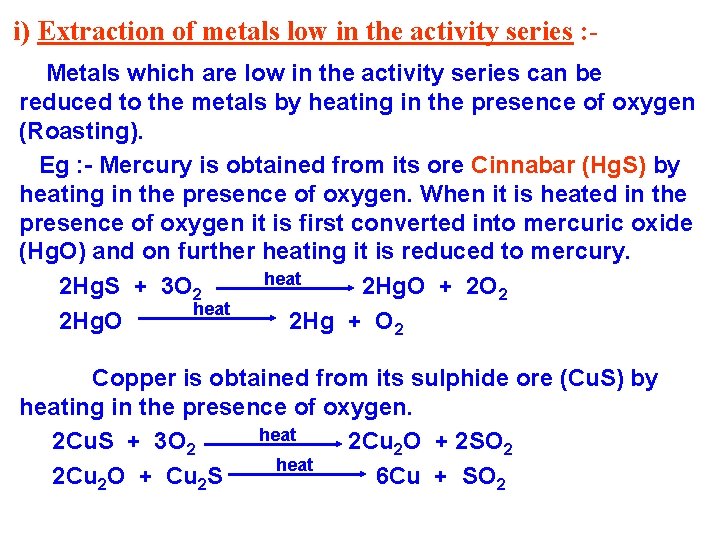
i) Extraction of metals low in the activity series : Metals which are low in the activity series can be reduced to the metals by heating in the presence of oxygen (Roasting). Eg : - Mercury is obtained from its ore Cinnabar (Hg. S) by heating in the presence of oxygen. When it is heated in the presence of oxygen it is first converted into mercuric oxide (Hg. O) and on further heating it is reduced to mercury. heat 2 Hg. S + 3 O 2 2 Hg. O + 2 O 2 heat 2 Hg. O 2 Hg + O 2 Copper is obtained from its sulphide ore (Cu. S) by heating in the presence of oxygen. heat 2 Cu. S + 3 O 2 2 Cu 2 O + 2 SO 2 heat 2 Cu 2 O + Cu 2 S 6 Cu + SO 2

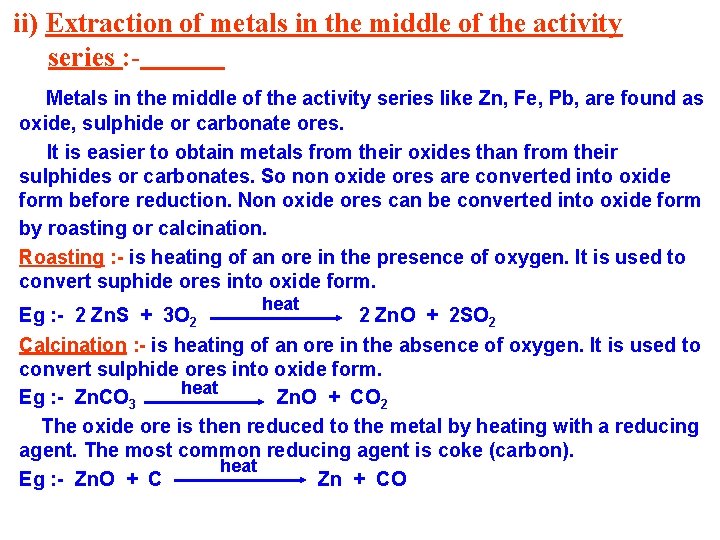
ii) Extraction of metals in the middle of the activity series : Metals in the middle of the activity series like Zn, Fe, Pb, are found as oxide, sulphide or carbonate ores. It is easier to obtain metals from their oxides than from their sulphides or carbonates. So non oxide ores are converted into oxide form before reduction. Non oxide ores can be converted into oxide form by roasting or calcination. Roasting : - is heating of an ore in the presence of oxygen. It is used to convert suphide ores into oxide form. heat Eg : - 2 Zn. S + 3 O 2 2 Zn. O + 2 SO 2 Calcination : - is heating of an ore in the absence of oxygen. It is used to convert sulphide ores into oxide form. heat Eg : - Zn. CO 3 Zn. O + CO 2 The oxide ore is then reduced to the metal by heating with a reducing agent. The most common reducing agent is coke (carbon). heat Eg : - Zn. O + C Zn + CO

Thermit reactions : Sometimes reactive metals like Na, Ca, Al etc. are used as reducing agents to obtain metals from their oxides. Eg : - 3 Mn. O 2 + 4 Al Mn + 3 Al 2 O 3 + Heat (Manganese) dioxide) The reaction between metal oxides and aluminium is highly exothermic and the metals are obtained in molten state. Such reactions are called thermit reactions. The reaction between iron oxide and aluminium produces molten iron. This reaction is used to join rail tracks, broken machine parts etc. Fe 2 O 3 + 2 Al Al 2 O 3 + 2 Fe + Heat

GENERAL

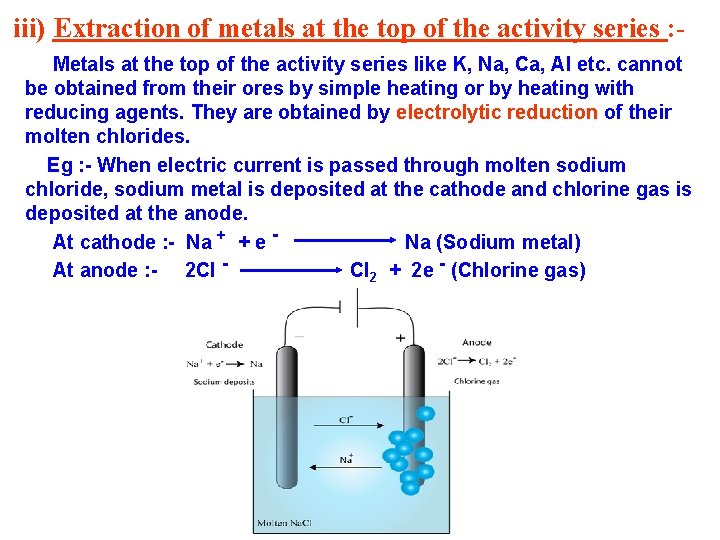
iii) Extraction of metals at the top of the activity series : Metals at the top of the activity series like K, Na, Ca, Al etc. cannot be obtained from their ores by simple heating or by heating with reducing agents. They are obtained by electrolytic reduction of their molten chlorides. Eg : - When electric current is passed through molten sodium chloride, sodium metal is deposited at the cathode and chlorine gas is deposited at the anode. At cathode : - Na + + e Na (Sodium metal) At anode : - 2 Cl Cl 2 + 2 e - (Chlorine gas)

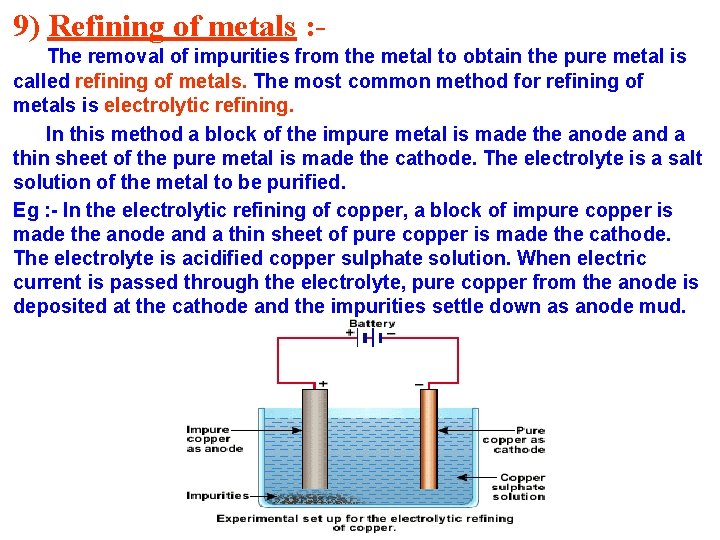
9) Refining of metals : The removal of impurities from the metal to obtain the pure metal is called refining of metals. The most common method for refining of metals is electrolytic refining. In this method a block of the impure metal is made the anode and a thin sheet of the pure metal is made the cathode. The electrolyte is a salt solution of the metal to be purified. Eg : - In the electrolytic refining of copper, a block of impure copper is made the anode and a thin sheet of pure copper is made the cathode. The electrolyte is acidified copper sulphate solution. When electric current is passed through the electrolyte, pure copper from the anode is deposited at the cathode and the impurities settle down as anode mud.

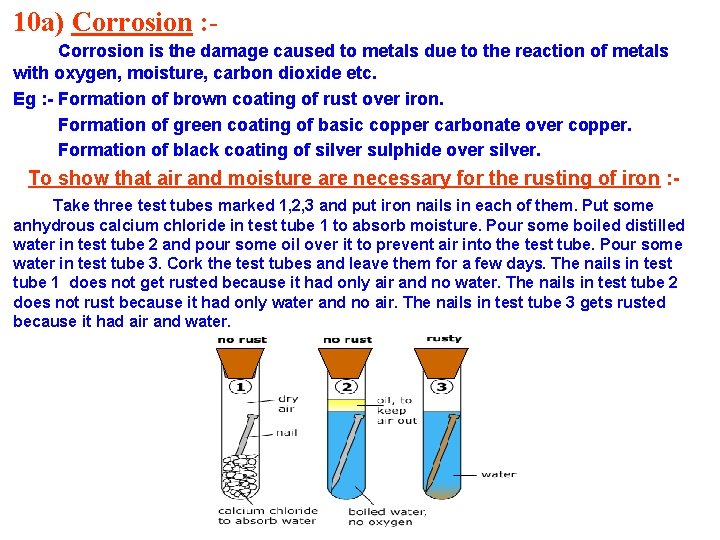
10 a) Corrosion : Corrosion is the damage caused to metals due to the reaction of metals with oxygen, moisture, carbon dioxide etc. Eg : - Formation of brown coating of rust over iron. Formation of green coating of basic copper carbonate over copper. Formation of black coating of silver sulphide over silver. To show that air and moisture are necessary for the rusting of iron : Take three test tubes marked 1, 2, 3 and put iron nails in each of them. Put some anhydrous calcium chloride in test tube 1 to absorb moisture. Pour some boiled distilled water in test tube 2 and pour some oil over it to prevent air into the test tube. Pour some water in test tube 3. Cork the test tubes and leave them for a few days. The nails in test tube 1 does not get rusted because it had only air and no water. The nails in test tube 2 does not rust because it had only water and no air. The nails in test tube 3 gets rusted because it had air and water.

b) Prevention of corrosion : Corrosion of metals can be prevented by : i) Applying oil or grease. ii) Applying paint. iii) By galvanisation. (Coating with zinc) iv) By tinning. (Coating with tin) v) By electroplating. (Coating a less reactive metal like chromium) vi) By alloying. (Making alloys) c) Alloy : An alloy is a homogeneous mixture of a metal with other metals or non metal. Eg : - Steel – iron, carbon Stainless steel – iron, carbon, cobalt, nickel Brass – copper, zinc Bronze – copper, tin Solder – Lead, tin (used for welding electrical wires together) If one of the metals in an alloy is mercury, it is called an amalgam.