Chapter 3 MatterProperties and Changes Chemistry 3 1



















- Slides: 31

Chapter 3: Matter-Properties and Changes Chemistry

3. 1 Properties of Matter � Substances (Pure Substance) ◦ Matter that has a uniform and unchanging composition ◦ Table Salt, Water � Physical Property- a characteristic that can be observed and measured without changing the samples ◦ Hard, soft, shiny, dull, brittle, flexible heavy, light, density, freezing point, boiling point, etc.

Physical Properties � Extensive Properties- dependent upon amount of substance ◦ Length, volume � Intensive Property- independent of amount of substance. ◦ Density (at constant pressure and volume), appearance

Chemical Property � Chemical Property- ability of a substance to combine with or change into one or more substances ◦ Iron forms rust with air, gold’s inability to combine with most other substances

Practice Problems: Identify each as physical or chemical properties a) Silver tarnishes when it comes in contact with hydrogen sulfide in the air b) A sheet of copper can be pounded into a bowl c) Barium melts at 725 o. C d) Helium does not react with any other element e) A bar of lead is more easily bent than is a bar of aluminum of the same size f) Potassium metal is kept submerged in oil to prevent contact with oxygen or water

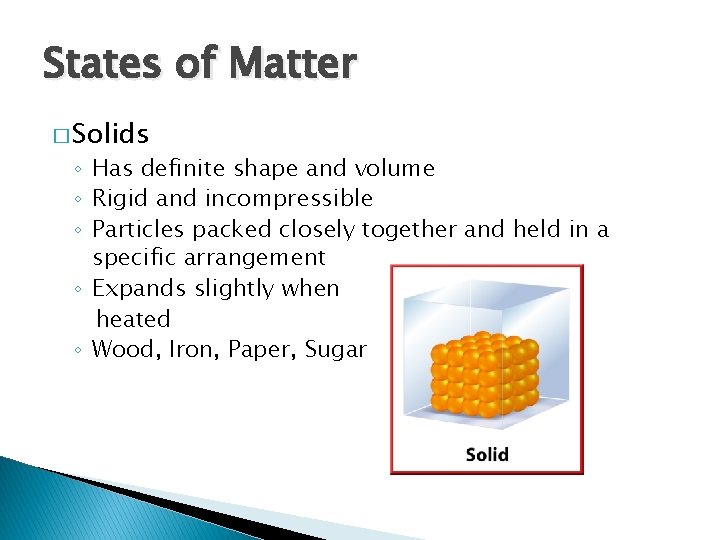
States of Matter � Solids ◦ Has definite shape and volume ◦ Rigid and incompressible ◦ Particles packed closely together and held in a specific arrangement ◦ Expands slightly when heated ◦ Wood, Iron, Paper, Sugar

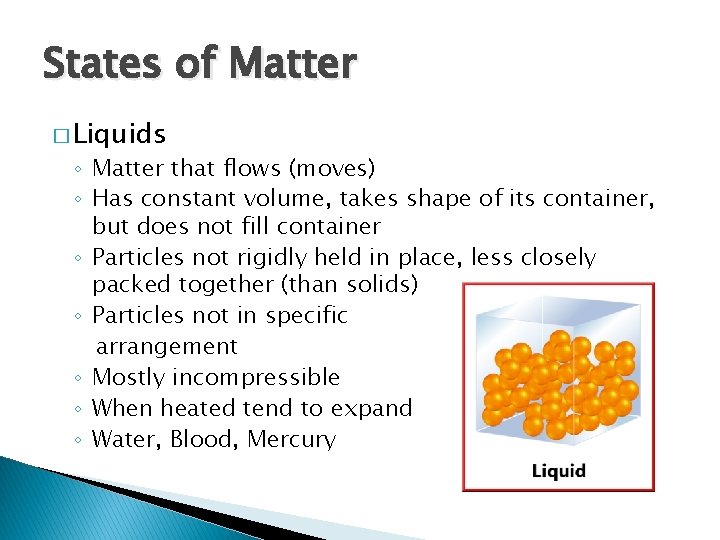
States of Matter � Liquids ◦ Matter that flows (moves) ◦ Has constant volume, takes shape of its container, but does not fill container ◦ Particles not rigidly held in place, less closely packed together (than solids) ◦ Particles not in specific arrangement ◦ Mostly incompressible ◦ When heated tend to expand ◦ Water, Blood, Mercury

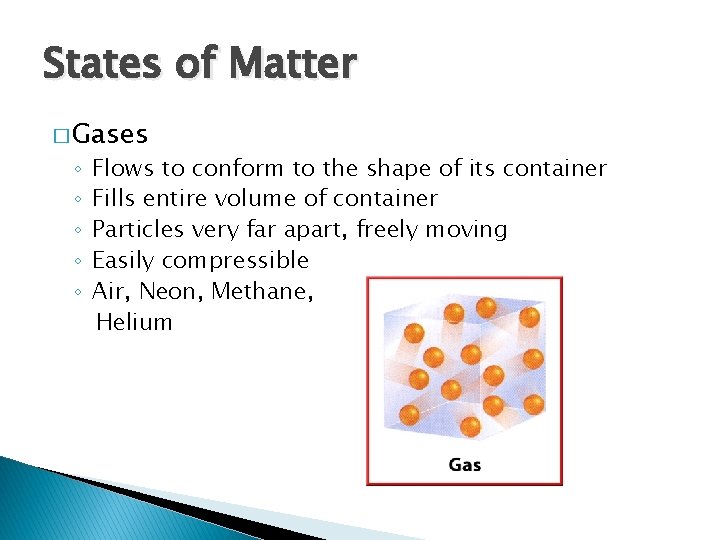
States of Matter � Gases ◦ ◦ ◦ Flows to conform to the shape of its container Fills entire volume of container Particles very far apart, freely moving Easily compressible Air, Neon, Methane, Helium

Gas vs. Vapor � Gas… something naturally in gaseous state at room temperature ◦ Air, Helium � Vapor… something that is normally a solid or liquid at room temperature ◦ Steam (At room temperature water is a vapor)

Practice Problems: � Identify each of the following as a property of a solid, liquid, or gas ◦ ◦ ◦ Flows and takes the shape of its container Compressible Made of particles held in a specific arrangement Has a definite volume Always occupies the entire space of its container Has definite volume but flows

3. 2 Changes in Matter � Physical Changes- alters a substance without changing its composition (what it is) ◦ Examples: liquid-water, solid-ice, gas-steam ◦ Terms like…bend, grind, crumple, split, crush, boil, freeze, vaporize, melt, etc

Changes in Matter � Chemical Changes- process that involves one or more substance changing into new substance…Chemical reaction � New Substance has different properties form old substances ◦ Example: iron reacts with air to form iron oxide (rust), striking a match, battery to steel wool ◦ Terms like…explode, rust, oxidize, corrode, tarnish, ferment, burn, or rot

Chemical Reactions � Staring substances called reactants, new substances called products � Chemical Equation-shows relationship between reactants and products in chemical reaction ◦ Example: iron + oxygen → rust (reactants) (product)

Practice Problems: Identify as Chemical or Physical Change a) Moisture in the air forms beads of water on a cold window pane b) An electric current changes water into hydrogen and oxygen c) yeast cells in bread dough makes carbon dioxide and ethanol from sugar d) olive oil, vinegar, salt, and pepper are shaken together to make salad dressing e) Molten bronze is poured into a mold and solidifies to form a figurine f) A reactant decomposes to form 2 products

Law of Conservation of Mass � Law of Conservation of Mass-during a chemical reaction mass is neither lost or gained � Mass Reactants � Example: = Mass Products A thin strip of iron with a mass of 15. 72 g is placed into a solution containing 21. 12 g of copper (II) sulfate and copper begins to form. After a while, the reaction stops because all of the copper (II) sulfate has reacted. The iron strip if found to have a mass of 8. 33 g. The mass of copper formed is found to be 8. 41 g. What mass of iron (II) sulfate as been formed in the reaction?

Practice Problem 1) A sealed glass tube contains 2. 25 g of copper and 3. 32 g of sulfur. The mass of the tube and its contents is 18. 48 g. Upon heating, a reaction forms copper (II) sulfide (Cu. S). All of the copper reacts, but only 1. 14 g of sulfur reacts. Predict what the mass of the tube and its contents will be after the reaction is completed. Explain your reasoning.

Practice Problem 2) A reaction between sodium hydroxide and hydrogen chloride gas produces sodium chloride and water. A reaction o 22. 85 g of sodium hydroxide with 20. 82 g of hydrogen chloride gives off 10. 29 g of water. What mass of sodium chloride is formed in the reaction?

3. 3 Mixtures of Matter � Mixture: a combination of two or more substances where the pure substance retain chemical properties (Physically Combined) ◦ Heterogeneous mixture- does not blend smoothly (individual substance remain distinct) �Sand water, oil and water, pizza, muddy water, smoky air ◦ Homogeneous mixture- constant composition throughout, has single phase �Salt-water, gasoline, cough suppressant, pure air

Homogeneous Mixture � Solutions- homogeneous mixture � The atoms/molecules of two (or more) substances are completely mingled with one another � Alloy: homogeneous mixture of tow or more metals, or of metals and nonmetals ◦ Solid solutions

Practice Problems: Identify as homogeneous or heterogeneous � 70% isopropyl rubbing alcohol � A pile of rusty iron filings � Concrete � Saltwater � Gasoline � Bread

Separating Mixtures � Physical means separation- separation by physical ◦ Sand-iron: separate using a magnet � Filtration- uses a porous barrier (filter paper) to separate solid from liquid ◦ Sand-water: separate using filter paper � Distillation: uses differences in boiling points ◦ Table salt-water: boiling out the water

Separation of Mixtures � Crystallization: results in the formation of pure solid particles of a substance from solution containing the dissolved substance ◦ Rock Candy � Chromatography- separates the components of a mixture (called the mobile phase) on the basis of the tendency of each to travel across a surface (called stationary phase) ◦ Ink separation

3. 4 Elements and Compounds � Matter: classified as pure substance or mixture (homogeneous or heterogeneous) � Pure substance: ◦ Element ◦ Compound

Elements � Element: pure substance that cannot be separated into simpler substances by physical or chemical means ◦ Example: Copper, Oxygen, and Gold ◦ Have unique name and symbol ◦ 91 element occur naturally on Earth, 110 altogether

Periodic Table � Dmitri Mendeleev (1869) ◦ Organized (know ones at time) the element into rows and columns based on their similarities ◦ Considered to be innovative, due to fact that his chart could account for elements that were not yet discovered � Vertical Columns: called groups or families (have similar properties) � Horizontal Rows: called periods (repeat the properties of the elements above them)

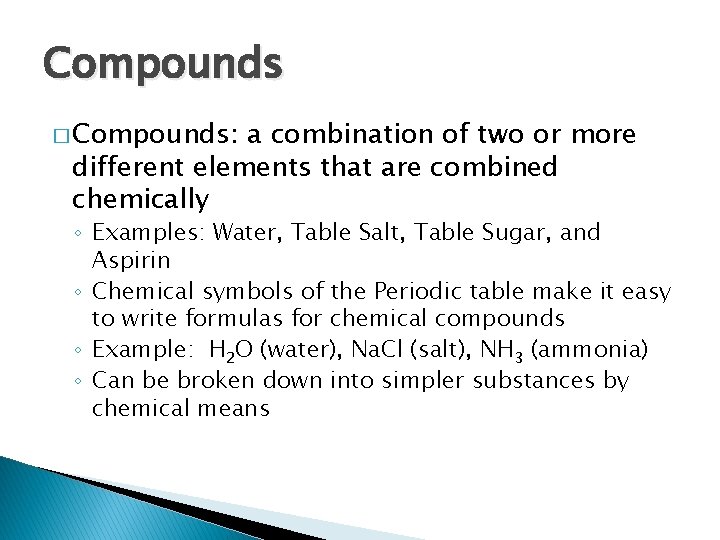
Compounds � Compounds: a combination of two or more different elements that are combined chemically ◦ Examples: Water, Table Salt, Table Sugar, and Aspirin ◦ Chemical symbols of the Periodic table make it easy to write formulas for chemical compounds ◦ Example: H 2 O (water), Na. Cl (salt), NH 3 (ammonia) ◦ Can be broken down into simpler substances by chemical means

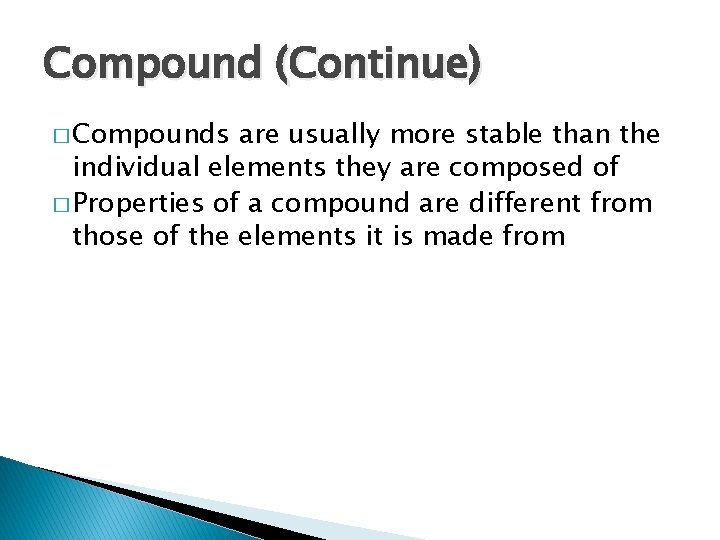
Compound (Continue) � Compounds are usually more stable than the individual elements they are composed of � Properties of a compound are different from those of the elements it is made from

Practice Problems � Identify each of the following as an example of an element or compound ◦ ◦ ◦ ◦ Sucrose (table sugar) The helium in a balloon Baking soda A diamond Aluminum foil The substances listed on the periodic table Calcium chloride pellets used to melt ice

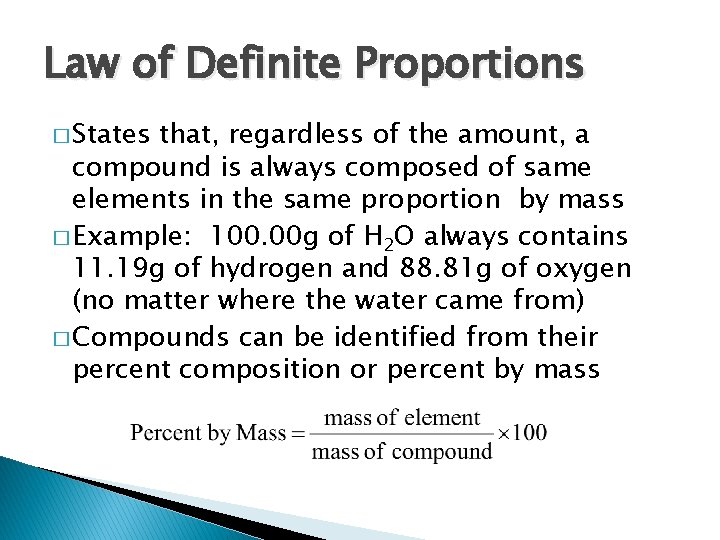
Law of Definite Proportions � States that, regardless of the amount, a compound is always composed of same elements in the same proportion by mass � Example: 100. 00 g of H 2 O always contains 11. 19 g of hydrogen and 88. 81 g of oxygen (no matter where the water came from) � Compounds can be identified from their percent composition or percent by mass

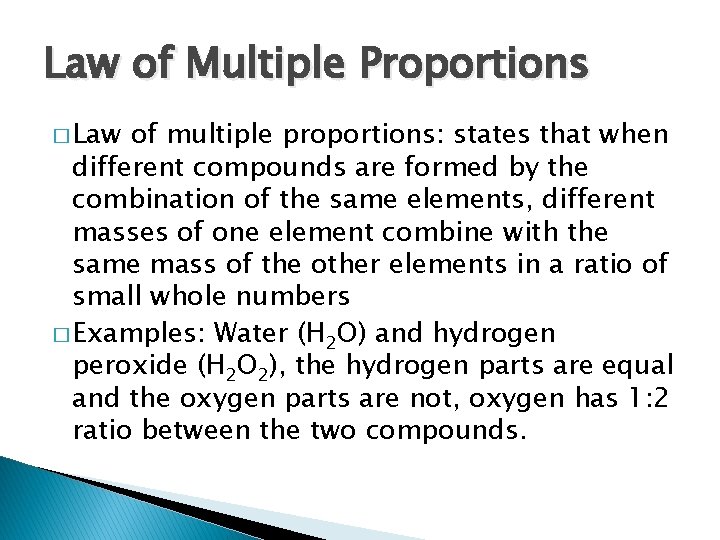
Law of Multiple Proportions � Law of multiple proportions: states that when different compounds are formed by the combination of the same elements, different masses of one element combine with the same mass of the other elements in a ratio of small whole numbers � Examples: Water (H 2 O) and hydrogen peroxide (H 2 O 2), the hydrogen parts are equal and the oxygen parts are not, oxygen has 1: 2 ratio between the two compounds.

Practice Problems �A 134. 50 g sample of aspirin is made up of 6. 03 g of hydrogen 80. 70 g of carbon, and 47. 77 g of oxygen. What is the percent by mass of each element in aspirin? � A 2. 89 g sample of sulfur reacts with 5. 72 g of copper to form a black compound. What is the percentage composition of the compound?