Chapter 3 Matter and Minerals What is Matter







- Slides: 28

Chapter 3: Matter and Minerals

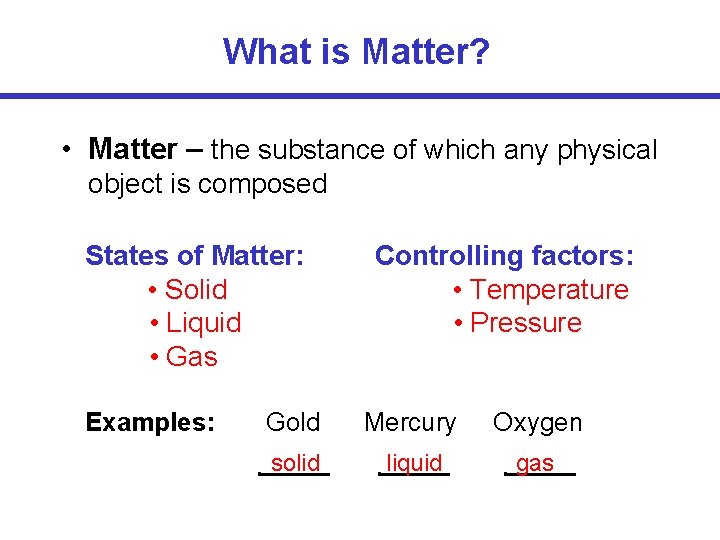
What is Matter? • Matter – the substance of which any physical object is composed States of Matter: • Solid • Liquid • Gas Examples: Controlling factors: • Temperature • Pressure Gold Mercury Oxygen solid liquid gas

The stuff that makes up all matter • The make-up of solid matter on Earth: Atoms Elements Compounds Minerals Rocks (smallest) • Elements: – fundamental building blocks – smallest matter that can’t be broken down (largest)

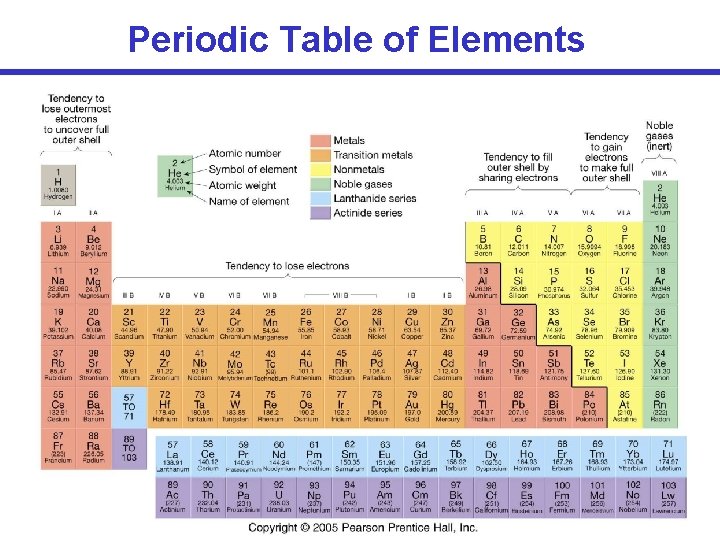
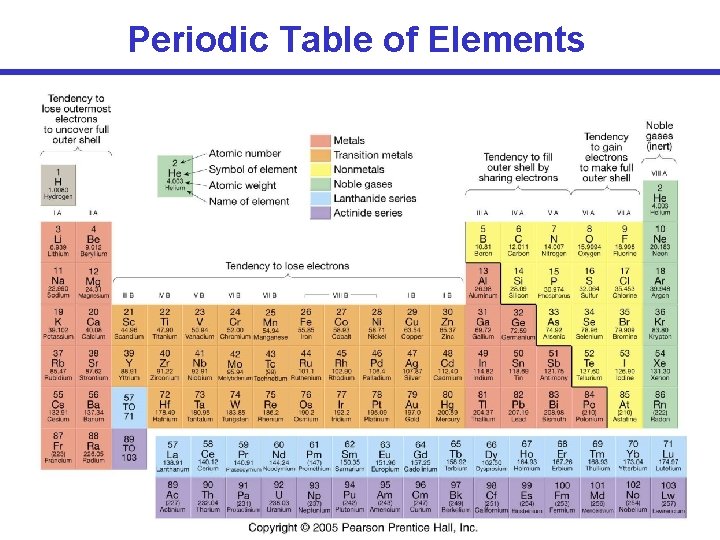
Periodic Table of Elements

The stuff that makes up all matter • The make-up of solid matter on Earth: Atoms Elements Compounds Minerals Rocks (smallest) (largest) • Atoms: – the stuff that builds elements – the smallest particle that uniquely defines an element

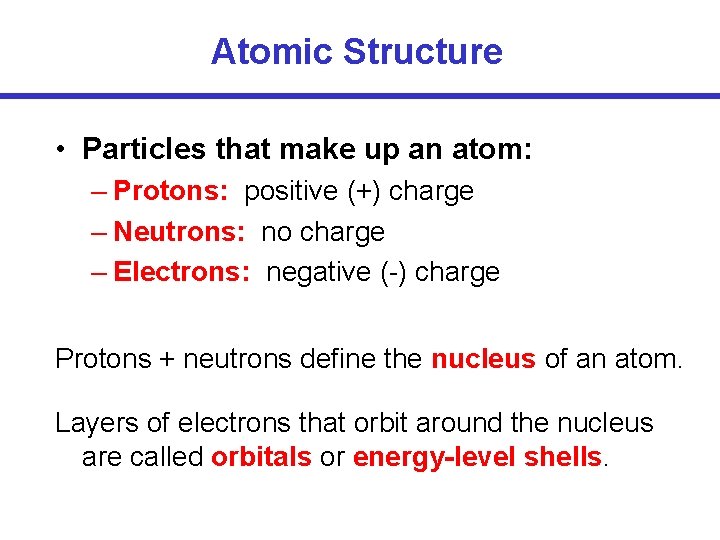
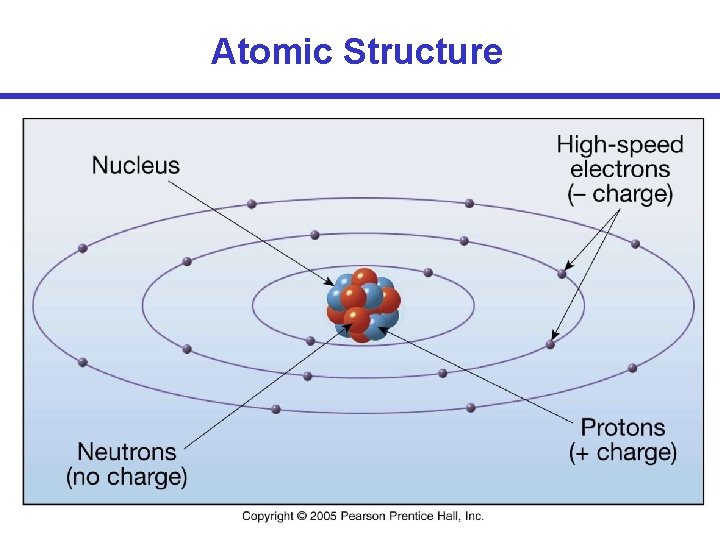
Atomic Structure • Particles that make up an atom: – Protons: positive (+) charge – Neutrons: no charge – Electrons: negative (-) charge Protons + neutrons define the nucleus of an atom. Layers of electrons that orbit around the nucleus are called orbitals or energy-level shells.

Atomic Structure

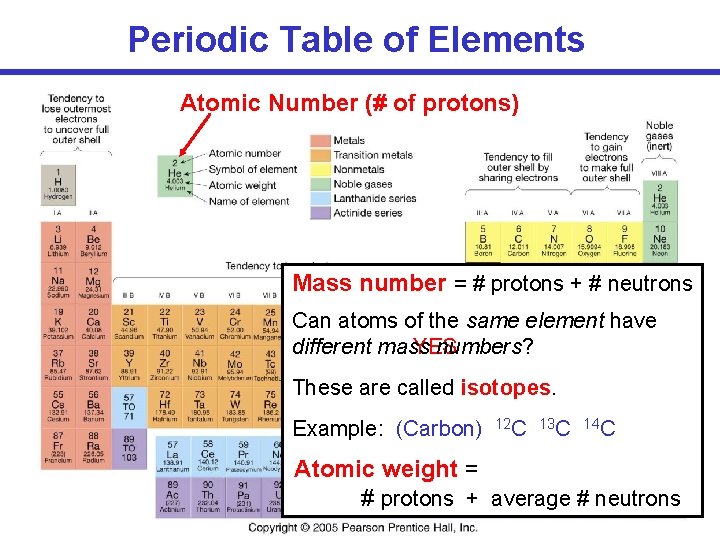
Periodic Table of Elements Atomic Number (# of protons) Mass number = # protons + # neutrons Can atoms of the same element have different mass YES numbers? These are called isotopes. Example: (Carbon) 12 C 13 C 14 C Atomic weight = # protons + average # neutrons

Atomic Structure • Atoms of the same element: • have the same number of protons (i. e. , same atomic number) • can have different numbers of neutrons (referred to as isotopes) • can have different numbers of electrons • Ion – an atom that has gained or lost an electron

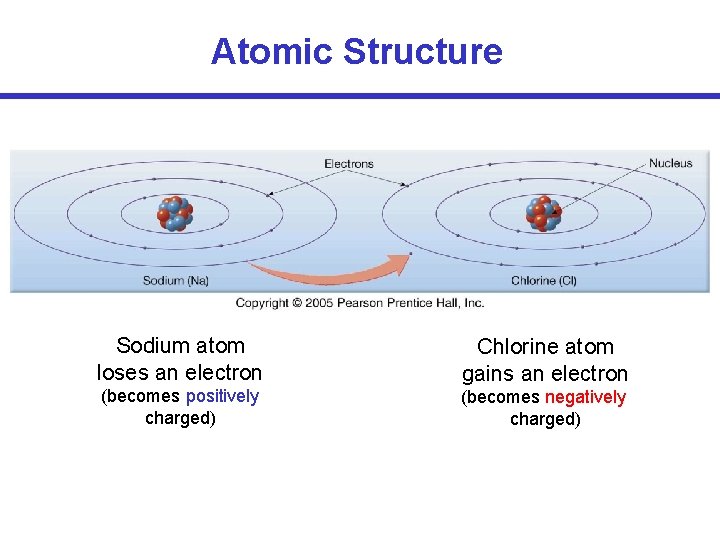
Atomic Structure Sodium atom loses an electron Chlorine atom gains an electron (becomes positively charged) (becomes negatively charged)

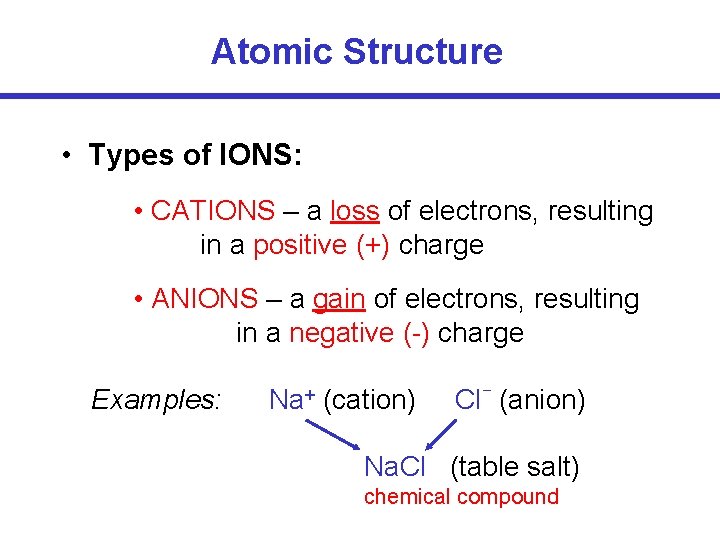
Atomic Structure • Types of IONS: • CATIONS – a loss of electrons, resulting in a positive (+) charge • ANIONS – a gain of electrons, resulting in a negative (-) charge Examples: Na+ (cation) – Cl (anion) Na. Cl (table salt) chemical compound

Compounds • Definition: – A chemical compound consists of elements that combine in a specific ratio. Examples: Na. Cl H 2 O • The smallest quantity of a compound is called a molecule. • Molecules are held together by chemical bonding.

Bonding – chemical matrimony • Chemical bonding: – formation of a compound by combining two or more elements – manner in which electrons are distributed among atoms • In bonded atoms, electrons may be lost, gained, or shared. • 4 types of bonding: ionic covalent metallic van der Waals

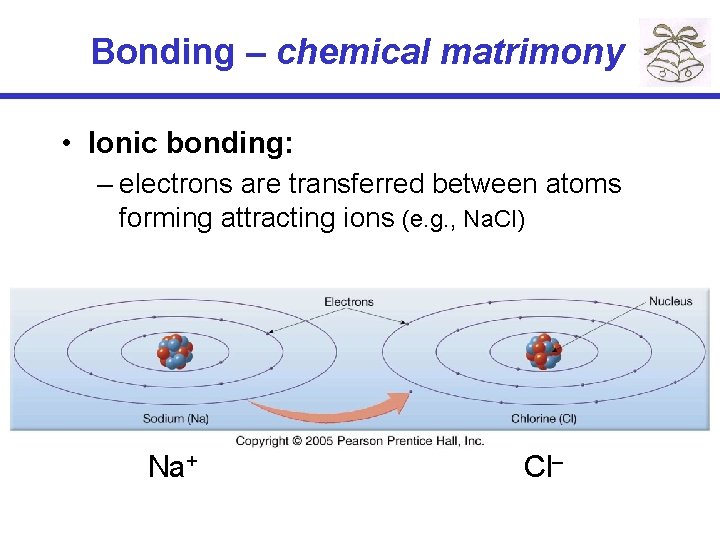
Bonding – chemical matrimony • Ionic bonding: – electrons are transferred between atoms forming attracting ions (e. g. , Na. Cl) Na+ Cl–

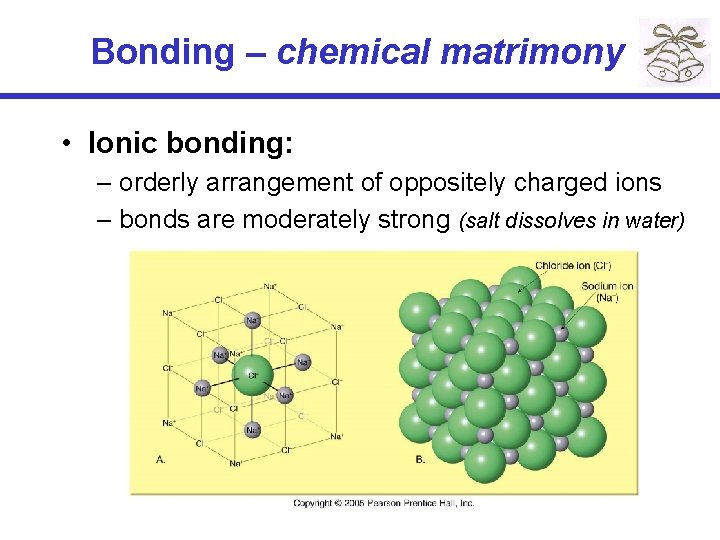
Bonding – chemical matrimony • Ionic bonding: – orderly arrangement of oppositely charged ions – bonds are moderately strong (salt dissolves in water)

Periodic Table of Elements

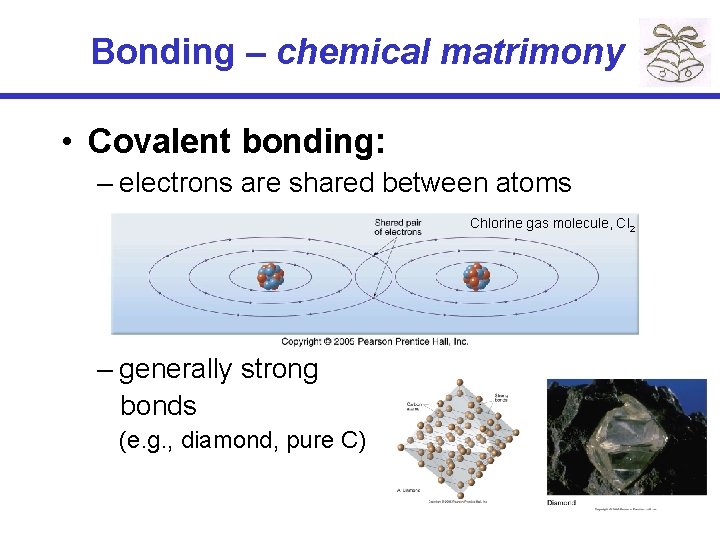
Bonding – chemical matrimony • Covalent bonding: – electrons are shared between atoms Chlorine gas molecule, Cl 2 – generally strong bonds (e. g. , diamond, pure C)

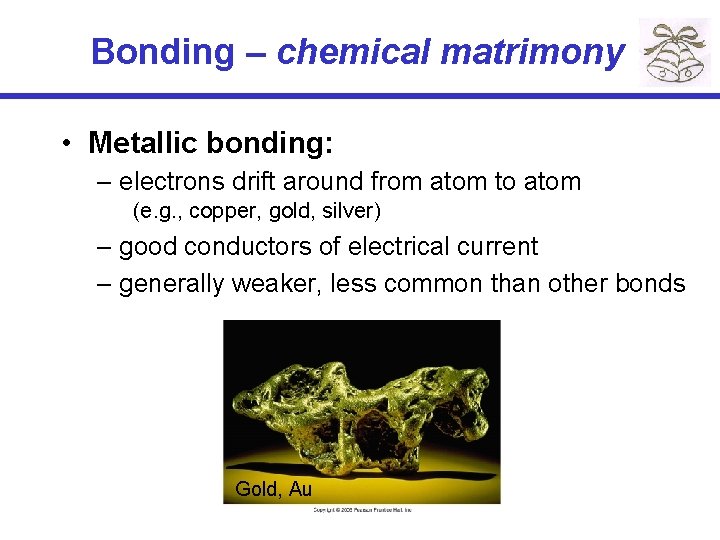
Bonding – chemical matrimony • Metallic bonding: – electrons drift around from atom to atom (e. g. , copper, gold, silver) – good conductors of electrical current – generally weaker, less common than other bonds Gold, Au

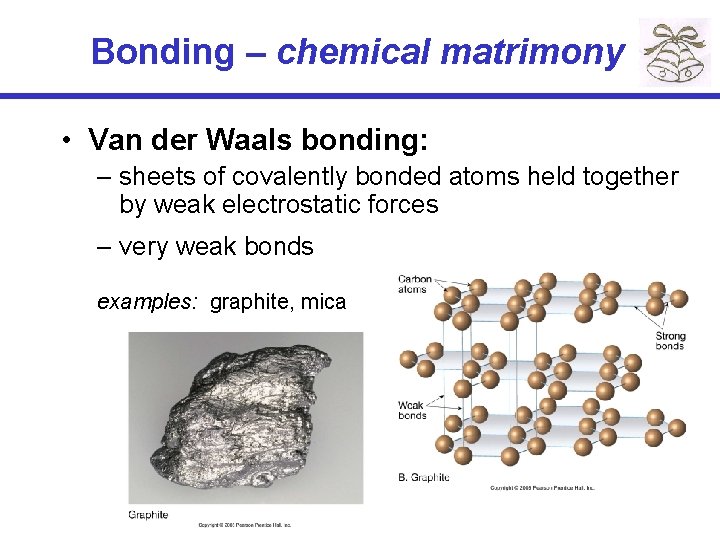
Bonding – chemical matrimony • Van der Waals bonding: – sheets of covalently bonded atoms held together by weak electrostatic forces – very weak bonds examples: graphite, mica

What kind of bonding do you prefer? In a covalent world… In an ionic world…

The stuff that makes up all matter • The make-up of solid matter on Earth: Atoms Elements Compounds Minerals Rocks (smallest) (largest)

Minerals: the building blocks of rocks • Definition of a Mineral: naturally occurring inorganic solid characteristic crystalline structure definite chemical composition • Definition of a Rock: • A solid aggregate (mixture) of minerals


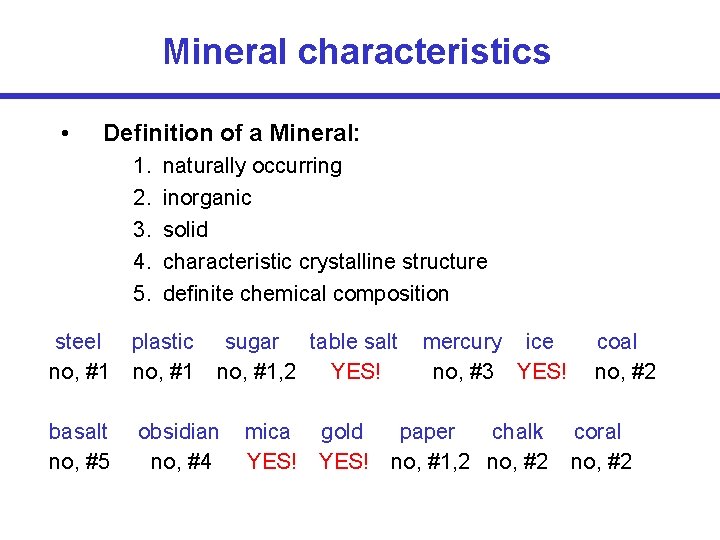
Mineral characteristics • Definition of a Mineral: 1. 2. 3. 4. 5. naturally occurring inorganic solid characteristic crystalline structure definite chemical composition steel plastic no, #1 basalt no, #5 sugar table salt no, #1, 2 YES! obsidian no, #4 mercury ice no, #3 YES! mica gold paper chalk YES! no, #1, 2 no, #2 coal no, #2 coral no, #2

Mineral characteristics • Naturally formed – No substance created artificially is a mineral. examples: plastic, steel, sugar, paper • Inorganic – Anything formed by a living organism and containing organic materials is not a mineral. examples: wood, plants, shells, coal • Solid – Liquids and gases are not minerals. examples: water, petroleum, lava, oxygen

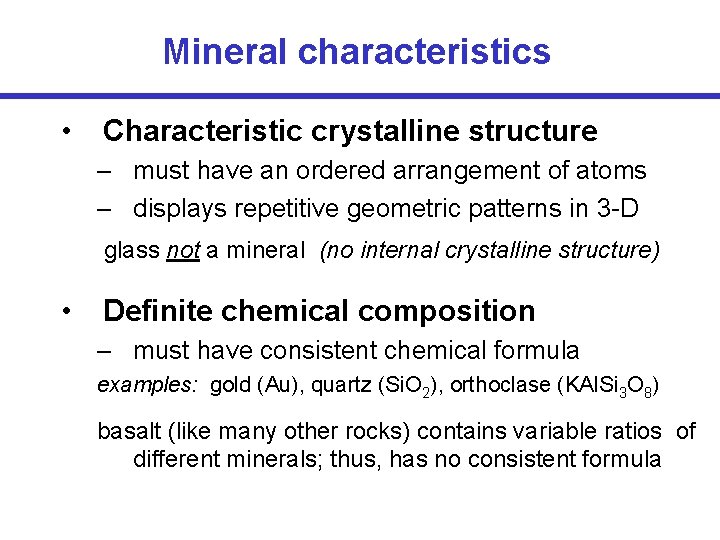
Mineral characteristics • Characteristic crystalline structure – must have an ordered arrangement of atoms – displays repetitive geometric patterns in 3 -D glass not a mineral (no internal crystalline structure) • Definite chemical composition – must have consistent chemical formula examples: gold (Au), quartz (Si. O 2), orthoclase (KAl. Si 3 O 8) basalt (like many other rocks) contains variable ratios of different minerals; thus, has no consistent formula

How many minerals are there? • Nearly 4, 000 types of minerals – Only ~30 occur commonly (whew!) – Why not more? • Some combinations are chemically impossible • Relative abundances of elements don’t allow more

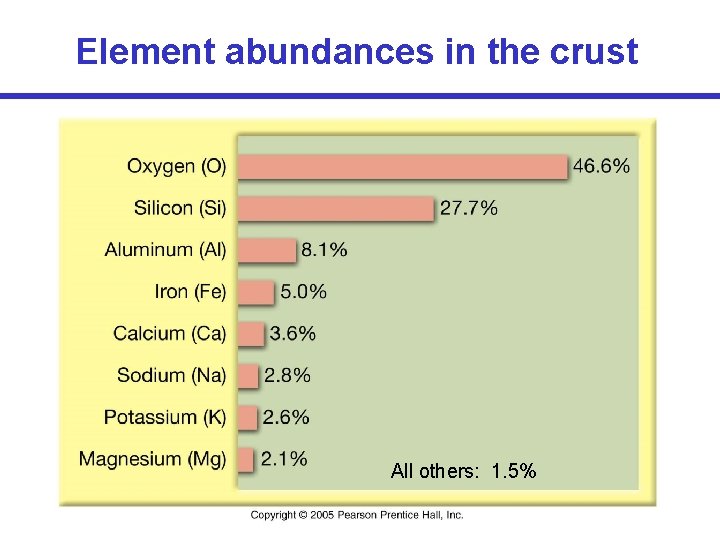
Element abundances in the crust All others: 1. 5%