CHAPTER 3 Introduction to radiant energy Objective At

CHAPTER 3. Introduction to radiant energy

Objective At the end of this chapter the student will be able to: • Definition of terms • Discuss radiant energy • Describe properties of EMR • Explain about interaction of EMR with matter • Discuss basic law of absorption: Beer-Lambert’s law
Outline of radiant energy lecture • • • Introduction to radiant energy Properties of EMR Interaction of EMR with matter Electromagnetic spectrum Absorption measurements: Beer-Lambert’s law, stray light
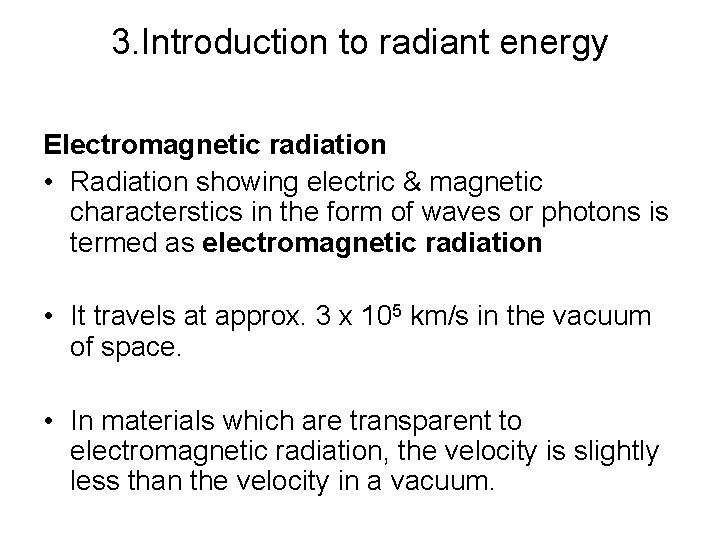
3. Introduction to radiant energy Electromagnetic radiation • Radiation showing electric & magnetic characterstics in the form of waves or photons is termed as electromagnetic radiation • It travels at approx. 3 x 105 km/s in the vacuum of space. • In materials which are transparent to electromagnetic radiation, the velocity is slightly less than the velocity in a vacuum.
1/16/2022 5
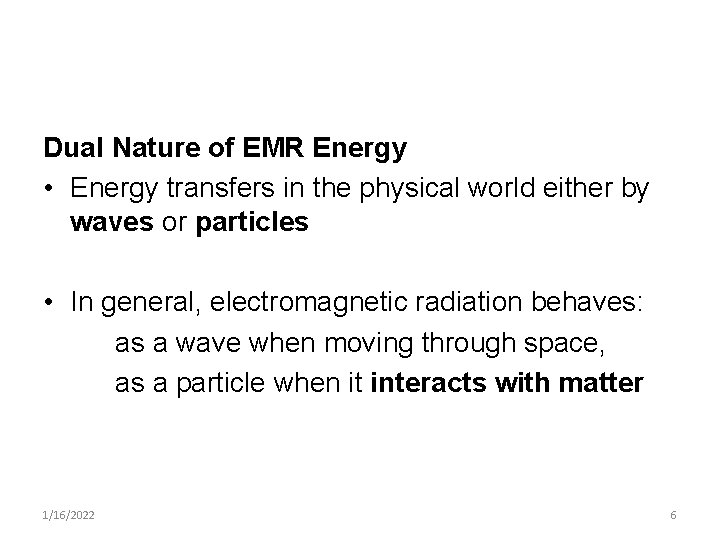
Dual Nature of EMR Energy • Energy transfers in the physical world either by waves or particles • In general, electromagnetic radiation behaves: as a wave when moving through space, as a particle when it interacts with matter 1/16/2022 6

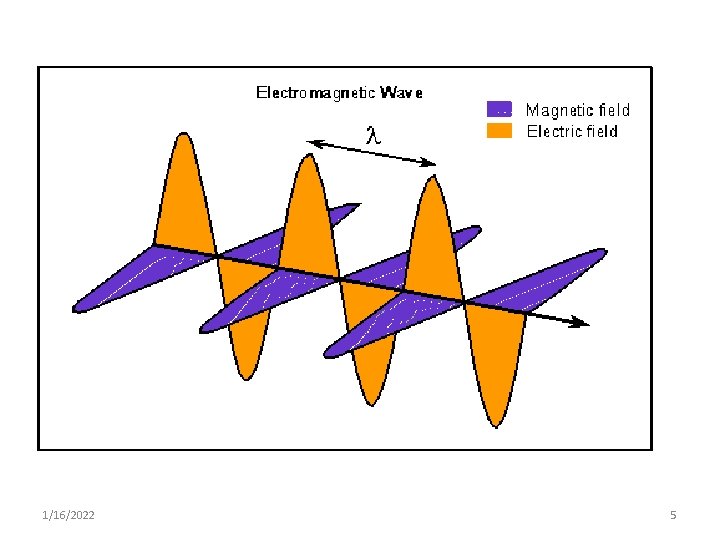
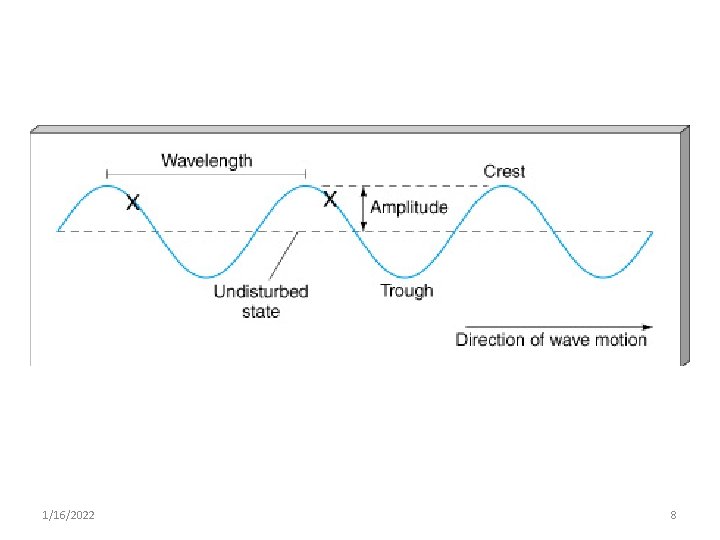
Wave Properties • Wave is the way of transferring an energy from one place to another • Consists of discrete packets of energy or quanta called photons • It Can be described by: 1. Velocity (c ) 2. Amplitude 3. Wave length (λ) 4. Frequency (ν) 1/16/2022 7
1/16/2022 8

1. Amplitude § The height the wave crest or troughs from the baseline § Governs brightness of light 2. Wavelength § distance between two wave crests or troughs § One full wave cycle (crest top to next crest top). 1/16/2022 9

3. Frequency qhow fast it oscillates (goes up & down) measured in cycles (remember crest to crest) per second. q. The number of wave crests per second, that is the number of wave crests that passes by a given point in one second. 1 cycle/second = 1 Hz (hertz) = 1 s-1. 1/16/2022 10

4. Speed of Light (velocity) § Speed of the wave § For example; – water - few meters per second – Sound wave – 340 m/sec – Light - 3 x 108 meters/second 1/16/2022 11
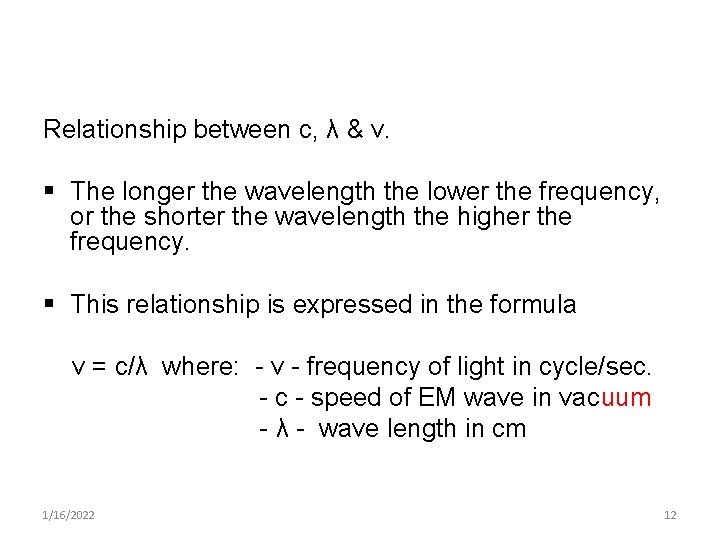
Relationship between c, λ & ν. § The longer the wavelength the lower the frequency, or the shorter the wavelength the higher the frequency. § This relationship is expressed in the formula ν = c/λ where: - ν - frequency of light in cycle/sec. - c - speed of EM wave in vacuum - λ - wave length in cm 1/16/2022 12
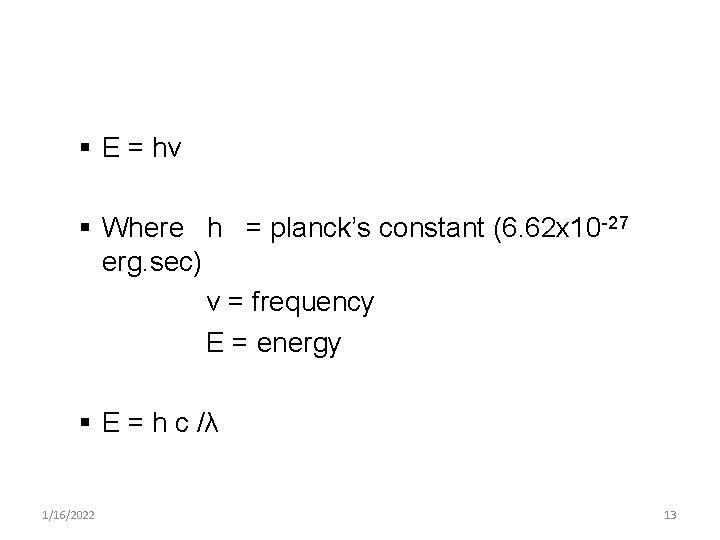
§ E = hv § Where h = planck’s constant (6. 62 x 10 -27 erg. sec) ν = frequency E = energy § E = h c /λ 1/16/2022 13
Interaction of EMR with matter • In order to use photometric instruments correctly & to be able to develop & modify spectroscopic techniques it is necessary to understand the principle of interaction of radiation with matter. • The only way to observe electromagnetic radiation is by its interaction with matter. 1/16/2022 14

• It involves: § Diffraction § Reflection § Refraction § Dispersion § Absorption & transmission 1/16/2022 15
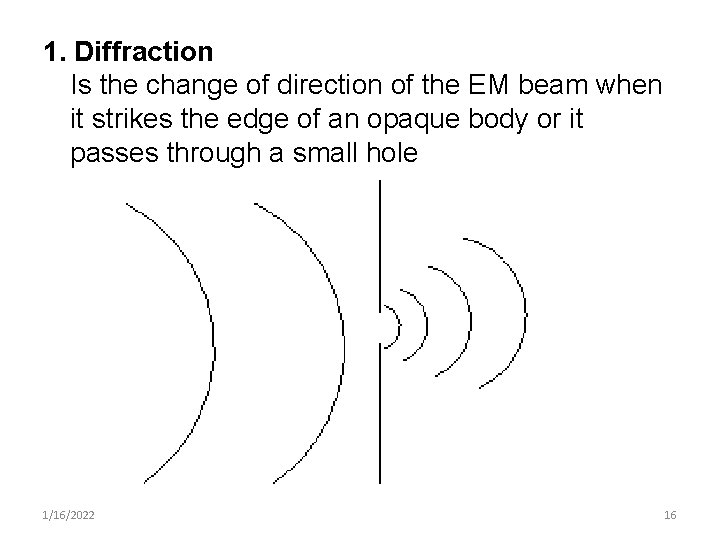
1. Diffraction Is the change of direction of the EM beam when it strikes the edge of an opaque body or it passes through a small hole 1/16/2022 16
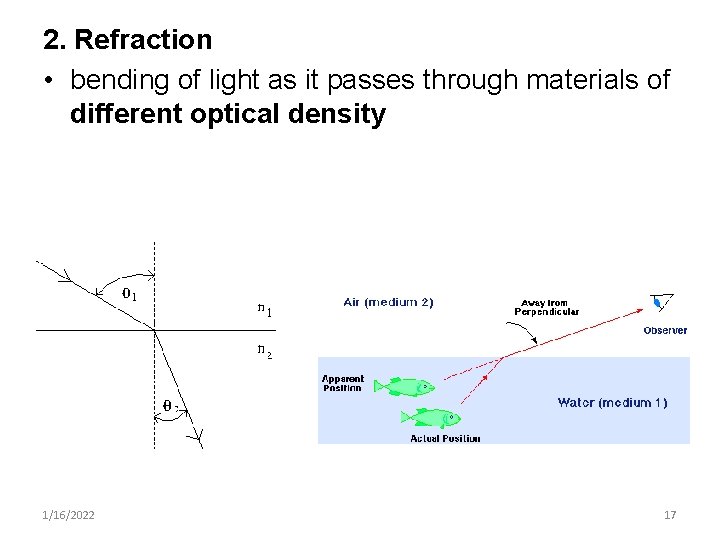
2. Refraction • bending of light as it passes through materials of different optical density 1/16/2022 17
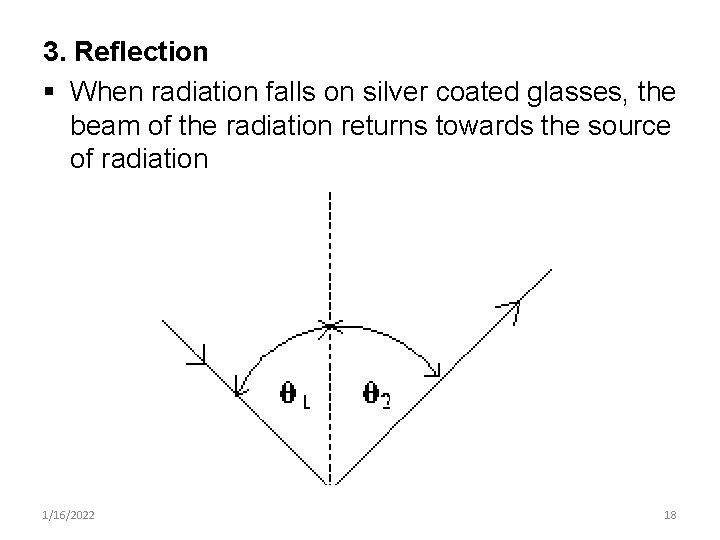
3. Reflection § When radiation falls on silver coated glasses, the beam of the radiation returns towards the source of radiation 1/16/2022 18

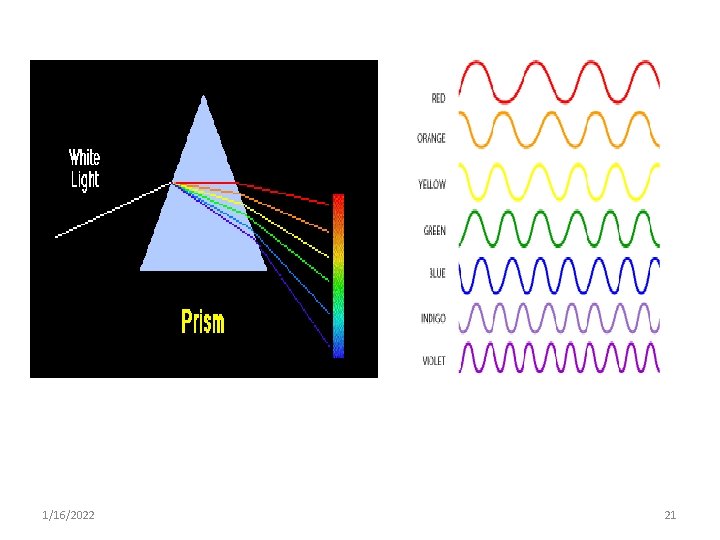
4. Dispersion • is the change in refractive index with a change in wavelength • The velocity of light in a materials & its refractive index depends on the wavelength of the light. • This causes the light to be refracted by different amounts according to the wavelength (or color). • This gives rise to the colors seen through a prism. 1/16/2022 19

• Rainbows are caused by dispersion of light inside the raindrop & total internal reflection of light from the back of raindrops. • The following is a chart giving the index of refraction for various wavelengths of light in glass 1/16/2022 20
1/16/2022 21
The electromagnetic Spectrum • Spectrum is an ordered arrangement of radiant energy according to the wavelength. A. Continuous spectrum • A spectrum which is composed of visible lights of all wavelengths are called Continuous spectrum • • It is a continuous spectrum because one color fades into another. E. g. sun light or light from ordinary incandescent bulb 1/16/2022 22

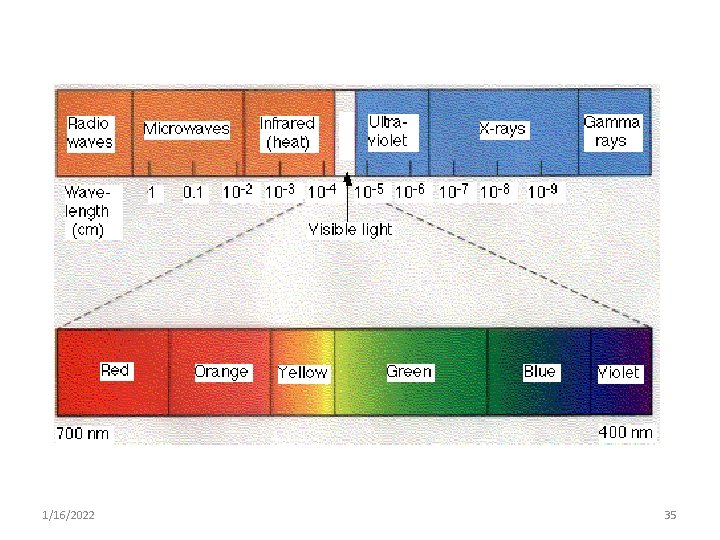
Component energies of the electromagnetic spectrum a. Radio waves § The longest- from a few meters to longer than the size of the earth. § They can travel long distance in the atmosphere b. Microwaves § The wavelength is from about 1 millimeter to 1 meter. § 1/16/2022 Used in communication, radar & cooking 23
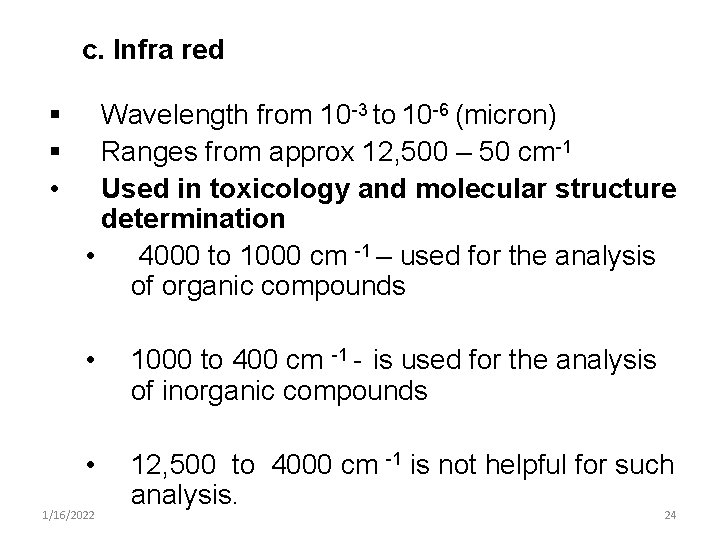
c. Infra red § § • Wavelength from 10 -3 to 10 -6 (micron) Ranges from approx 12, 500 – 50 cm-1 Used in toxicology and molecular structure determination • 4000 to 1000 cm -1 – used for the analysis of organic compounds • 1000 to 400 cm -1 - is used for the analysis of inorganic compounds • 12, 500 to 4000 cm -1 is not helpful for such analysis. 1/16/2022 24

d. Visible • It is a very small portion of the total EM spectrum visible to human eye • It ranges from 700 nm (at red light) to 400 nm (violet) • the visible color of a solution corresponds to the wavelength of the light that are transmitted, not absorbed, by the solution. 1/16/2022 25

• The different colors have different wavelengths & frequencies. • The rest of the EM spectrum is not visible to the human eye • Source of visible light: o tungsten lamp. 1/16/2022 26
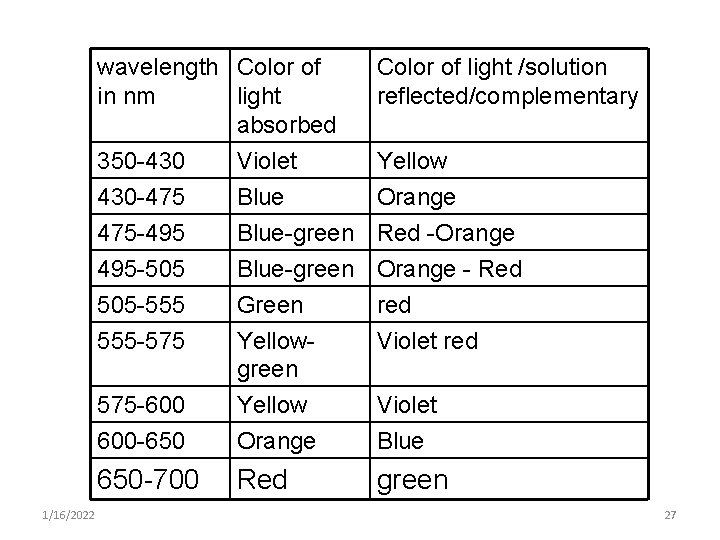
wavelength Color of in nm light absorbed 350 -430 Violet 1/16/2022 Color of light /solution reflected/complementary Yellow 430 -475 475 -495 495 -505 Blue Orange Blue-green Red -Orange Blue-green Orange - Red 505 -555 555 -575 Green Yellowgreen red Violet red 575 -600 600 -650 Yellow Orange Violet Blue 650 -700 Red green 27

Note: • A substance that absorbs green light at 500 nm reflects or transmits all other lights or wavelengths & appears as purple. • To measure the concentration of a blue solution, light at about 590 nm is passed through the solution • The amount of yellow light absorbed varies directly as the concentration of the absorbing substance in solution 28

§ The absorbed color is the complementary of the transmitted color. § Thus to make absorption measurement, one must use the wavelength at which a colored solution absorbs light. § For example, a red solution absorbs green light & transmitted red light. Therefore, a red solution should be measured at 490 to 550 nm 1/16/2022 29

• In photometer using filter used as a monochromator, the filter chosen is usually complementary to the color of the solution to be measured. § blue solution – yellow filter § Yellow solution –blue filter § Red solution – blue green filter § Blue green solution – red filter 1/16/2022 30

e. UV Light • It ranges from 400 to 100 nm • It is dangerous to tissue & cells (common sun burns) • It is obtained by energy transition in the valence electrons of the molecules. • Widely used in the quantitative & qualitative determination of clinical chemistry tests. 1/16/2022 31

• Source: – discharge tube containing hydrogen or deuterium at reduced pressure. – High pressure mercury & xenon arc lamps. • c + d + e = light 1/16/2022 32

f. X- ray • It ranges from 100 to 0. 1 nm • High frequency, high energy waves that can penetrate several centimeters into most solid matter. • Used in radiological diagnosis 1/16/2022 33

g. Gamma rays § it ranges from 0. 1 to less than 10 -16 § It is the highest energy ray in the EM spectrum § It is generally produced in nuclear reactions & not as common in nature, 1/16/2022 34
1/16/2022 35
B. Line/atomic emission spectrum • a spectrum with only certain colors. (NOT continuous like sunlight) • Samples of elements emit light when they are vaporized (heated) or electricity passes through them. • Every element has a unique line 1/16/2022 36

• The wavelength of the line are characteristics of a particular element • It can be used for qualitative identification & quantitative determination of elements in an unknown mixture • For example, flame photometer, atomic absorption spectrophotometer. 1/16/2022 37
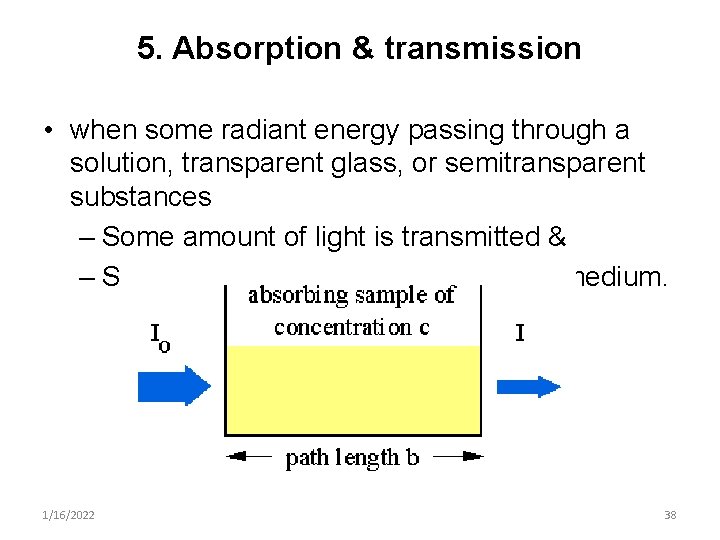
5. Absorption & transmission • when some radiant energy passing through a solution, transparent glass, or semitransparent substances – Some amount of light is transmitted & – Some is absorbed or trapped by the medium. 1/16/2022 38
Absorption measurement • Many determinations in clinical chemistry are based on the measurement of the radiant energy § Emitted (e. g Fluorometer) § Transmitted § Absorbed (absorption spectrophotometer) § Reflected (reflectance photometer) § Refracted (refractometer) 1/16/2022 39

Light transmittance • Is defined as the proportion of the incident light that is transmitted. 1/16/2022 40
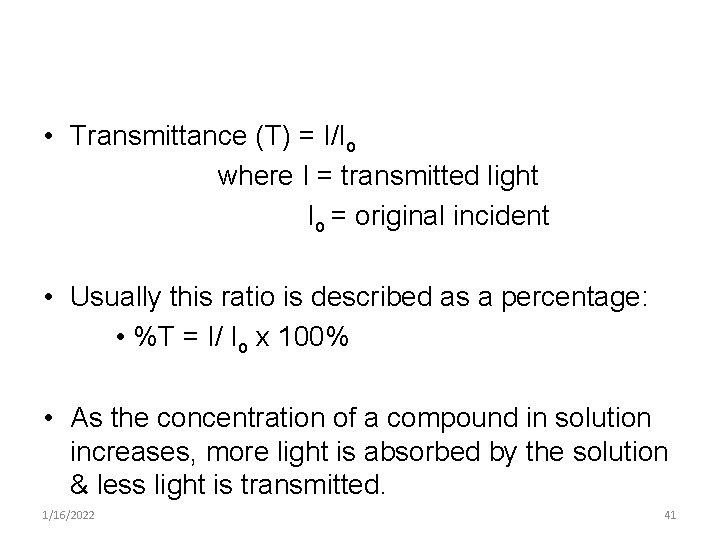
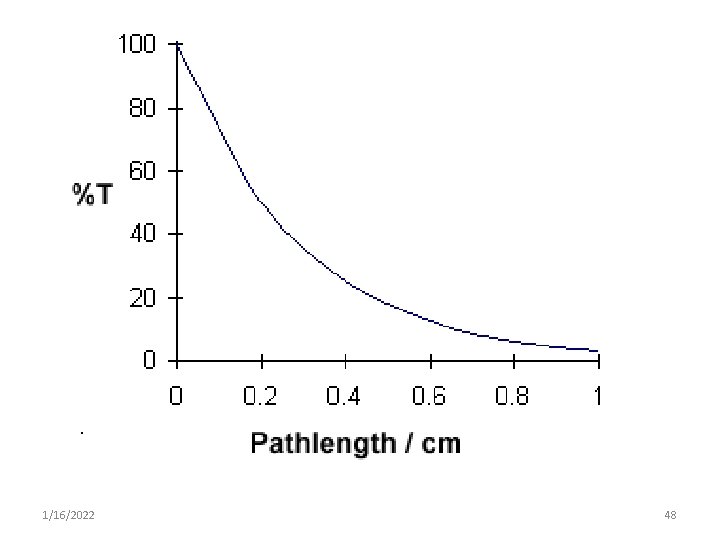
• Transmittance (T) = I/Io where I = transmitted light Io = original incident • Usually this ratio is described as a percentage: • %T = I/ Io x 100% • As the concentration of a compound in solution increases, more light is absorbed by the solution & less light is transmitted. 1/16/2022 41
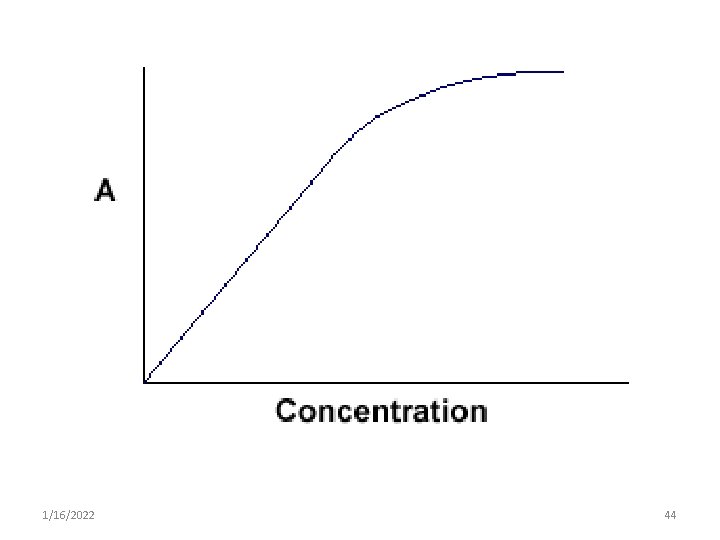
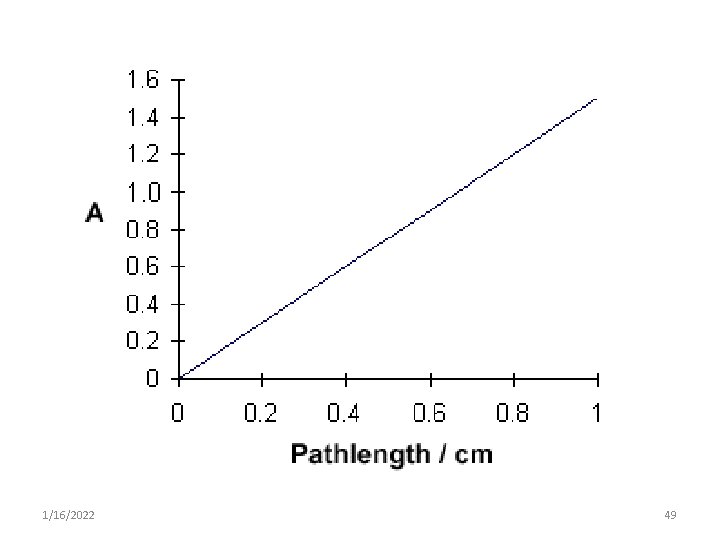
Light absorbance • The relationship between %T & concentration is not linear but varies inversely & logarithmically. • As a result it is more convenient to use the concept of absorbance to avoid the use of logarithmic units. • However the concept of transmittance is important because only transmitted light can be measured. 1/16/2022 42

• % T can be related light of absorbance of a solution by: • Absorbance, A = log 10 I 0 / I o A = log 10 1 / T o A = log 10 100 / %T o A = 2 - log 10 %T 1/16/2022 43
1/16/2022 44
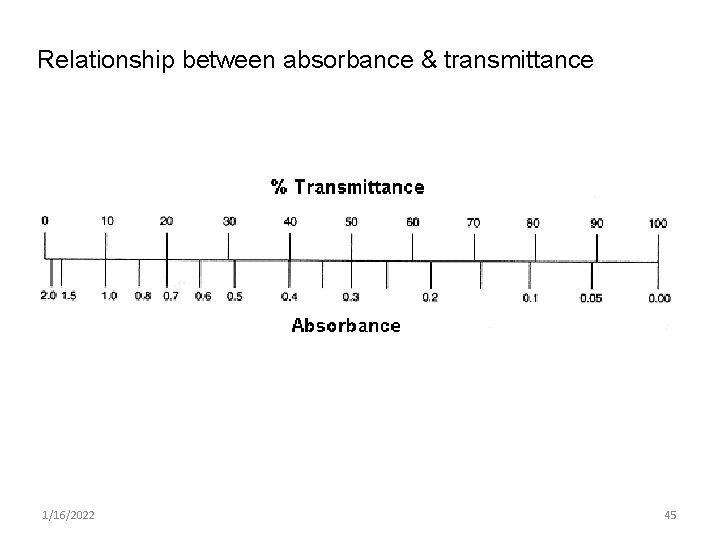
Relationship between absorbance & transmittance 1/16/2022 45

1. Absorption spectrophotometry Fundamental law of absorption a. Introduction • When a radiant energy, Io, passes through a solution to be analyzed, – some of the radiant energy will be absorbed – some of it will be transmitted 1/16/2022 46
• The transmitted light, I, is affected by factor such as: o Incident light o Optical path length o Concentration of solution 1/16/2022 47
1/16/2022 48
1/16/2022 49

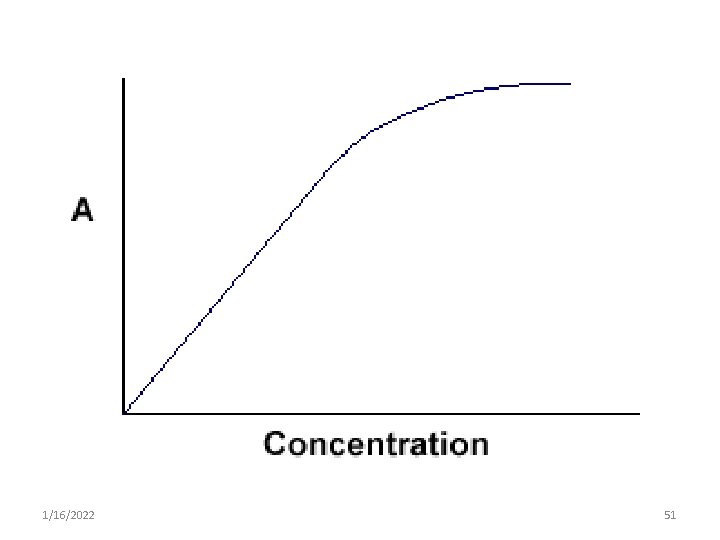
2. Beers- Lambert's law • It is commonly referred to as Beer’s Law 1. Beer’s law • It states that concentration of a substance is: o directly proportional to the amount of light absorbed by the solution & o inversely proportional to the logarithm of transmittance o A=C o A = a. c 1/16/2022 50
1/16/2022 51
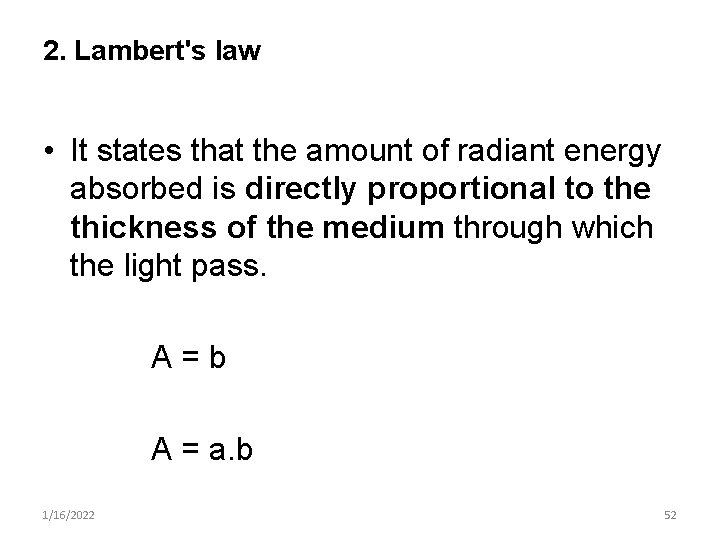
2. Lambert's law • It states that the amount of radiant energy absorbed is directly proportional to the thickness of the medium through which the light pass. A=b A = a. b 1/16/2022 52
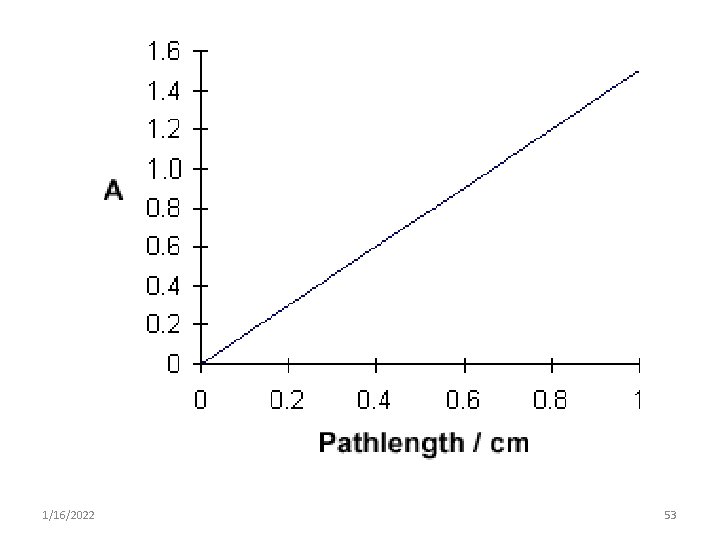
1/16/2022 53
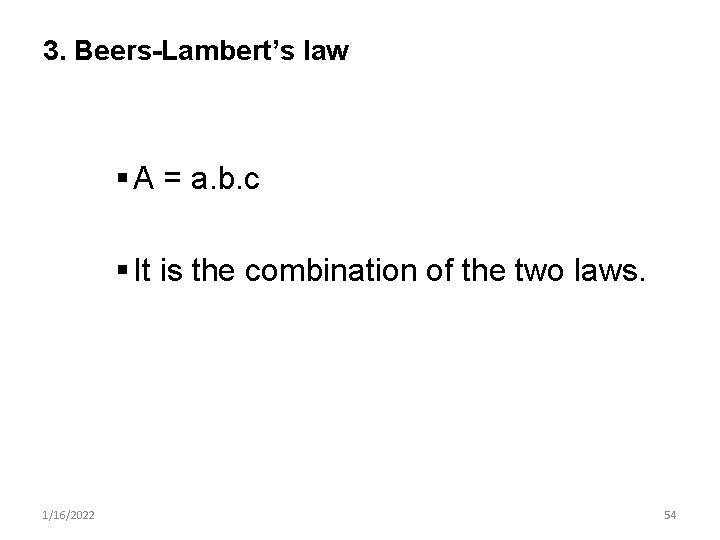
3. Beers-Lambert’s law § A = a. b. c § It is the combination of the two laws. 1/16/2022 54

• Beer-Lambert's law indicates a direct proportionality between A and c only if: – incident radiation is monochromatic – each molecule in solution acts as an independent absorbing species in solution – Absorption takes place in a solution of uniform cross-section (a well mixed solution) 1/16/2022 55

• Limitations of Beer’s law-cause for deviation from the law § non-monochromatic light § Elevated concentration § Solvent absorption § Transmitted light by other mechanisms § Non-parallel sides of cuvets 1/16/2022 56

Deviations from beer’s law a. Spectral interference • The beers-lambert’s law express the linear relationship b/n the concentration of the sample & the absorbance value recorded. • • However, the relationship is only an experimental one & not a fundamental law of nature. As a result, the linearity is only true under certain limiting conditions 1/16/2022 57
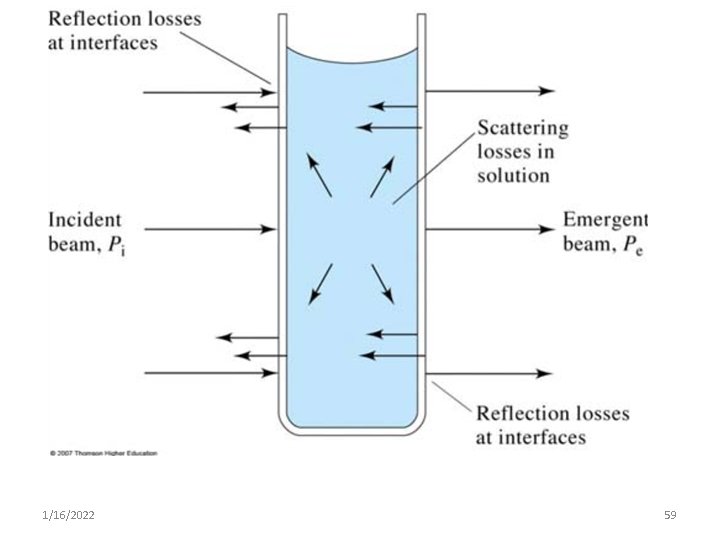
• Some amount of radiation will be – reflected from the surface of the sample holder, – absorbed by the material of which the cell is composed or – The solvent may also absorb or reflect radiation. • I 1/16/2022 o= absorbed + transmitted + others 58
1/16/2022 59

• To focus attention on the compound of interest, elimination of these factors is necessary. • This is done through the use of blank or reference solution • This blank should be identical to the test sample in all aspects except the presence of the test substance • Blank reading = Io - other loses 1/16/2022 60

• Hence: • Absorbed = blank – transmitted. • Types of blank solution 1. reagent blank 2. Sample blank 1/16/2022 3. Water saline or air blank 61

1. Reagent blank • reagent + solvent or • A solution of reagents with out sample • Used to correct high absorbance of the reagent 1/16/2022 62

2. Sample blank • Sample + diluent • A solution of sample & reagents missing a key reagents that initiate the rxn or cause formation of final rxn product. 1/16/2022 63
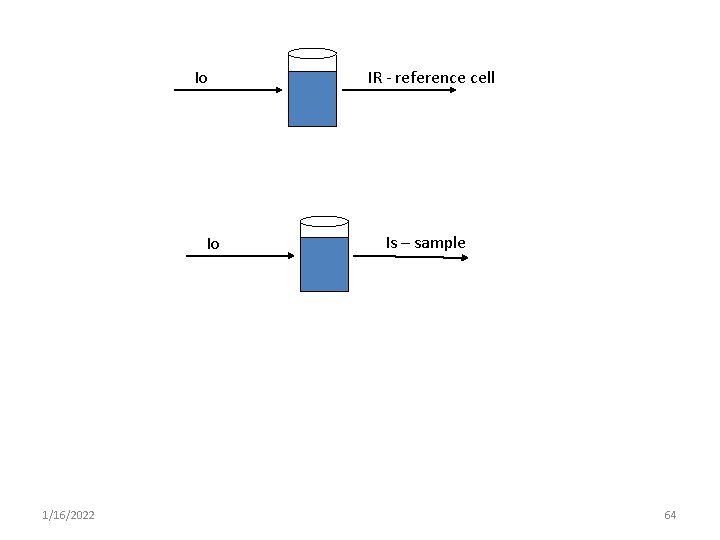
Io Io 1/16/2022 IR - reference cell Is – sample 64
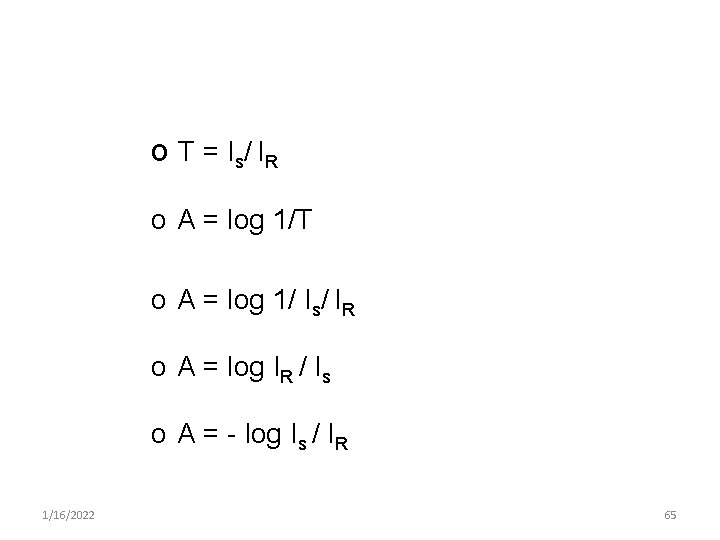
o T = I s/ I R o A = log 1/T o A = log 1/ Is/ IR o A = log IR / Is o A = - log Is / IR 1/16/2022 65

b. Stray light • Quantitative radiation rely on radiation that reach the detector passing through the sample • But it is impractical, because it is difficult to design instrument which are capable of effectively eliminating all extraneous radiation • Much of these unwanted radiation arises from the scattering of the incident radiation by irregularities in surfaces (by faults in manufacturers) or scratches 1/16/2022 66
• Such light, stray light, results in a deviation from Beer’s law & • The effect is that absorbance measurements are lower than they should be. • It is possible to asses the proportion of stray light by measuring the amount of radiation transmitted by samples which are optically opaque at the wavelength to be assessed but which transmit radiation of other wavelengths 1/16/2022 67
• The instrument is set to zero %T with blocking light path & 100% transmittance with a reagent blank in the normal way & opaque substance introduced into the sample compartment. • The amount of light transmitted by the sample, measured in percentage transmittance is quoted as the stray light at a specified wavelength. 1/16/2022
Summary • Radiant energy: radio waves (longest) to gamma rays (shorts) • Properties of Electromagnetic radiation as wave and particle. Visible light is 350 -700 nm. • Interaction of EMR with matter is one of six types: diffraction, reflection, dispersion, absorption or transmission. • Basic law of absorption: Beer-Lambert’s law which is A = a. b. c

Reference 1. Burtis, Carl A. , and Ashwood, Edward R. . Tietz: Fundamentals of Clinical Chemistry. Philadelphia, 2001. 2. Arneson, W and J Brickell: Clinical Chemistry: A Laboratory Perspective 1 st ed. 2007 FA Davis 3. Burtis, Carl A. , and Ashwood, Edward R. . Tietz: textbook of Clinical Chemistry. Philadelphia, 1999.

Next Chapter 4 • Analytical procedures and Instrumentation
- Slides: 71