Chapter 3 High Performance Liquid Chromatography HPLC Outline














![Equilibrium constant for ion-exchange reaction: [-NR 4+X-][Cl-] KX = [-NR +Cl-][X-] 4 Or [-SO Equilibrium constant for ion-exchange reaction: [-NR 4+X-][Cl-] KX = [-NR +Cl-][X-] 4 Or [-SO](https://slidetodoc.com/presentation_image_h2/4abf06a8d1a61394d8ae3bbaa95504fd/image-24.jpg)

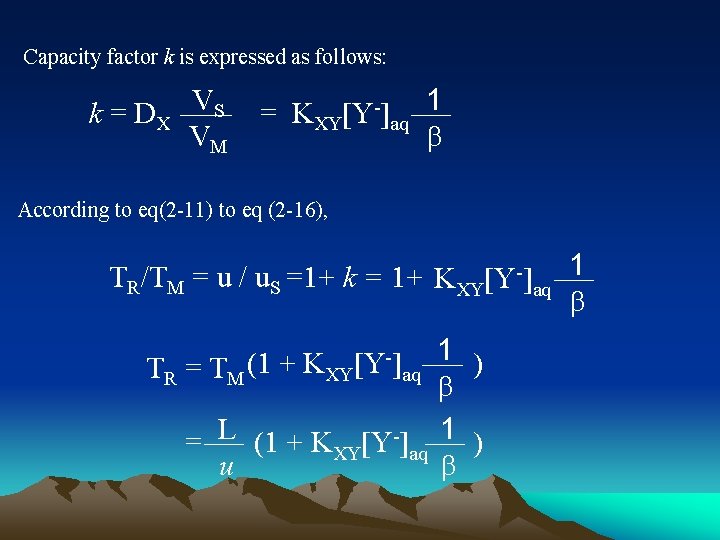
![It is obvious that rotention value increases with increasing KXY and [Y-]aq, KXY depends It is obvious that rotention value increases with increasing KXY and [Y-]aq, KXY depends](https://slidetodoc.com/presentation_image_h2/4abf06a8d1a61394d8ae3bbaa95504fd/image-30.jpg)
















- Slides: 68

Chapter 3 High Performance Liquid Chromatography (HPLC)

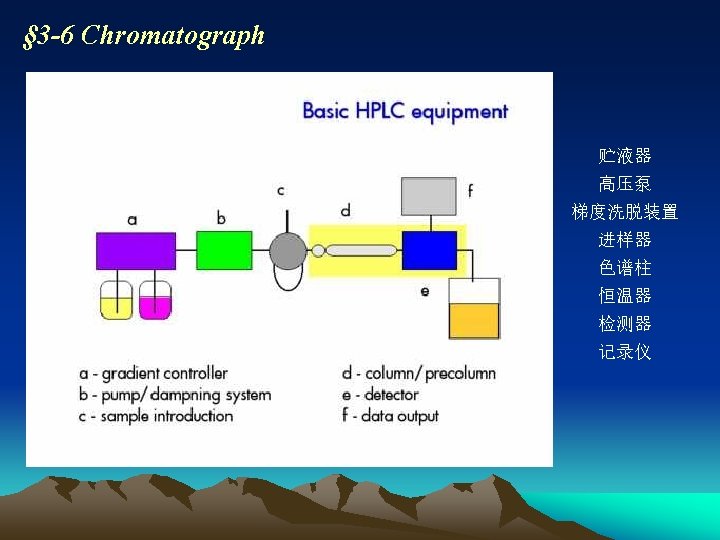
Outline of chapter 3 • Characteristics of HPLC(特点) • Structure of high performance liquid chromatographer (仪器构造) • Types and separation principles of HPLC methods(方法的类型及其 分离原理) • Factors influencing broadening of chromatographic peaks and separation(影响色谱峰扩展及色谱分离的因素) • Stationary phase of HPLC(液相色谱固定相) • Mobile phase of HPLC(液相色谱流动相) • Selection of chromatographic methods(色谱方法的选择) • Requirements: 要求掌握HPLC法的基本原理 • Emphases: 重点学会色谱分析方法的选择

§ 3 -1 Characteristics of HPLC ●High pressure Because carrier liquid moving through the column endures high resistance, high pressure must be provided Usually The pressures for supplying carrier liquid(供液压力)and for injection sample( 进样压力)reach 150 ~ 350× 105 Pa ●High velocity Flow rate of carrier liquid: 1 ~ 10 m. L. min-1, much faster than classical liquid chromatography. Analytical velocity is much faster than classical LC. It usually takes less than 1 hour

●High efficient Due to use of new-type of stationary phases, column efficiency of HPLC improved further (number of theoretical plates per meter is more than 3× 104/m ●High sensitivity Highly sensitive detectors’ application improves the analytical sensitivity. Minimum detectable quantity UV-detector: down to 10 -9 g Fluorescence detector: 10 -11 g Sample amount needed: Very little, micro-liter of test sample is enough for full analysis ●Wide application fields Available for organic compounds with high boiling point, poor thermal stability and high molecular weight(>400) and some of inorganic compounds

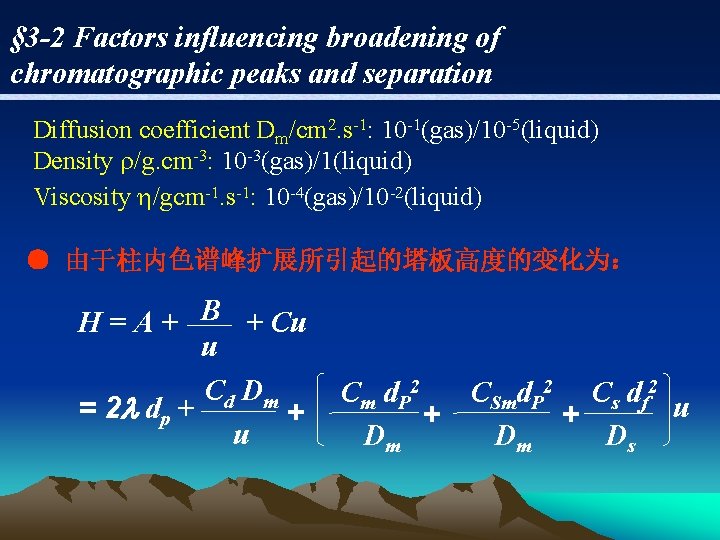
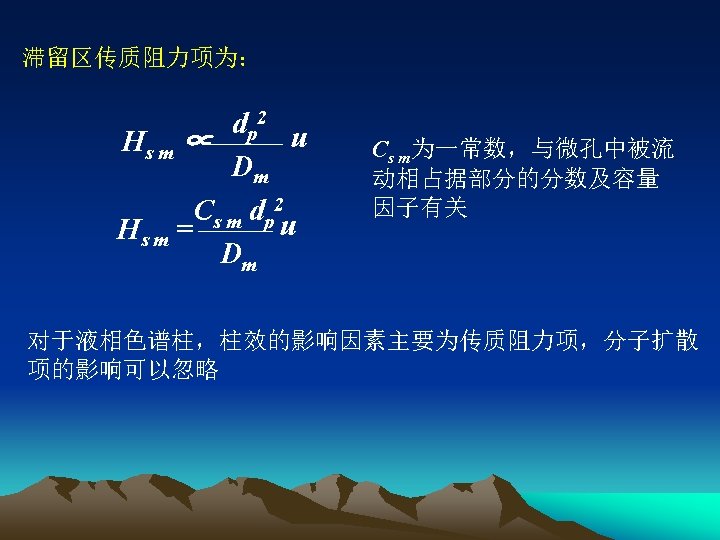
§ 3 -2 Factors influencing broadening of chromatographic peaks and separation Diffusion coefficient Dm/cm 2. s-1: 10 -1(gas)/10 -5(liquid) Density /g. cm-3: 10 -3(gas)/1(liquid) Viscosity /gcm-1. s-1: 10 -4(gas)/10 -2(liquid) ● 由于柱内色谱峰扩展所引起的塔板高度的变化为: B H=A+ + Cu u C d Dm = 2 l dp + + u Cm d. P 2 CSmd. P 2 Cs df 2 u + + Dm Dm Ds

1. 涡流扩散项He He = 2 ld. P 上式的含义及对峰扩展的影响同气相色谱 2. 纵向扩散项Hd Hd ∝ Dm u C d Dm Hd = u Due to very little diffusion coefficient of the molecules in liquid phase, when linear velocity is higher than 0. 5 cm. s-1, contribution of longitudinal diffusion term to peak broadening is neglectable.

3. 传质阻力项 ●Stationary phase mass transfer resistance term 固定相传质过程:试样分子从流动相进入固定液进行质量交换 Cs df 2 Hs = Ds u Cs is a coefficient depending on k How to eliminate or reduce peak broadening induced by mass transfer of stationary phase ?

▲ Improve mass transfer to quicken desorption of solute molecules on stationary phase ▲ Use thin layer of stationary phase for liquid-liquid partition chromatography ▲Use small grains with a little radii (小颗粒)as filling materials(填料)for adsorption, size-exclusion and ion-exchange chromatography ▲Use the stationary liquid with a big diffusion coefficient ▲Reduce rate of mobile phase However, too low mobile phase rate will deduce enhancement of molecular diffusion term and prolonging(延长)of analytical time.

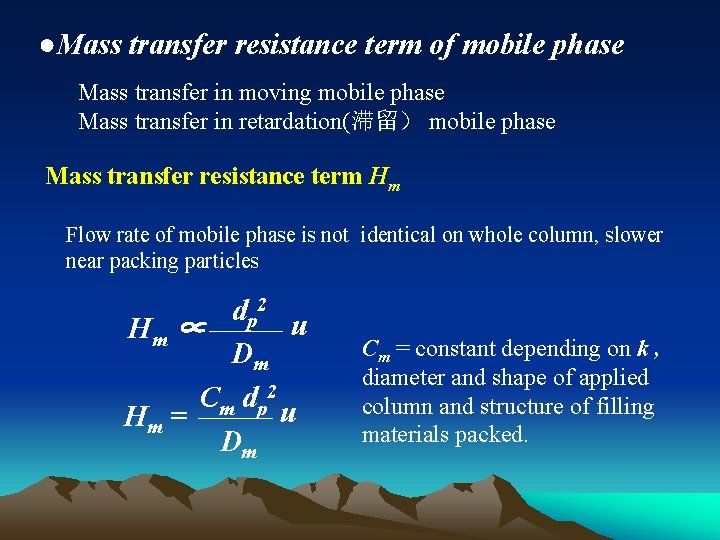
●Mass transfer resistance term of mobile phase Mass transfer in moving mobile phase Mass transfer in retardation(滞留) mobile phase Mass transfer resistance term Hm Flow rate of mobile phase is not identical on whole column, slower near packing particles dp 2 u Hm ∝ Dm Cm dp 2 u Hm = Dm Cm = constant depending on k , diameter and shape of applied column and structure of filling materials packed.

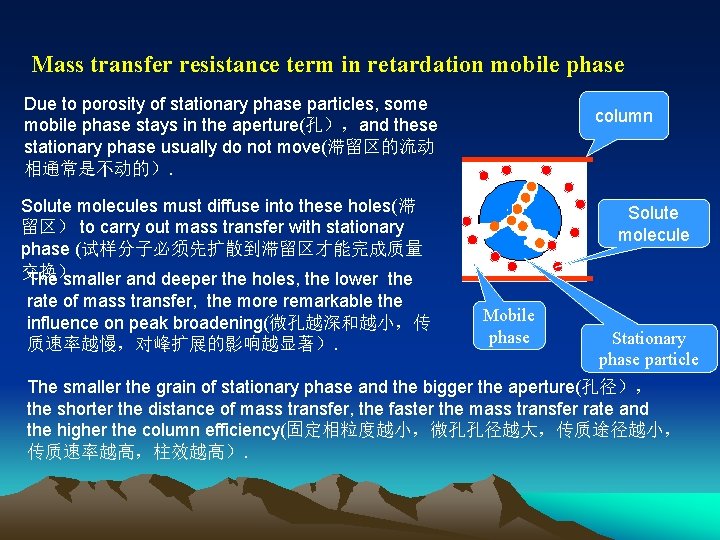
Mass transfer resistance term in retardation mobile phase Due to porosity of stationary phase particles, some mobile phase stays in the aperture(孔),and these stationary phase usually do not move(滞留区的流动 相通常是不动的). column Solute molecules must diffuse into these holes(滞 留区) to carry out mass transfer with stationary phase (试样分子必须先扩散到滞留区才能完成质量 交换) The smaller and deeper the holes, the lower the rate of mass transfer, the more remarkable the influence on peak broadening(微孔越深和越小,传 质速率越慢,对峰扩展的影响越显著). Solute molecule Mobile phase Stationary phase particle The smaller the grain of stationary phase and the bigger the aperture(孔径), the shorter the distance of mass transfer, the faster the mass transfer rate and the higher the column efficiency(固定相粒度越小,微孔孔径越大,传质途径越小, 传质速率越高,柱效越高).




§ 3 -3 Main types of HPLC methods and their separation principle • Liquid-liquid partition chromatography(液-液分配色 谱法) • Liquid-solid adsorption chromatography(液-固吸附 色谱法) • Ion-exchange chromatography(离子交换色谱法) • Ion-pair chromatography(离子对色谱法) • Ion chromatography(离子色谱法) • Steric exclusion chromatography(空间排阻色谱法)

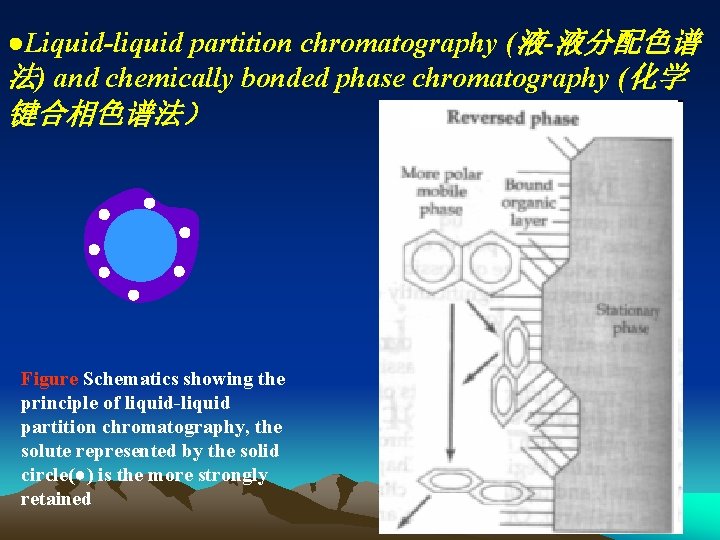
●Liquid-liquid partition chromatography (液-液分配色谱 法) and chemically bonded phase chromatography (化学 键合相色谱法) Figure Schematics showing the principle of liquid-liquid partition chromatography, the solute represented by the solid circle(●) is the more strongly retained

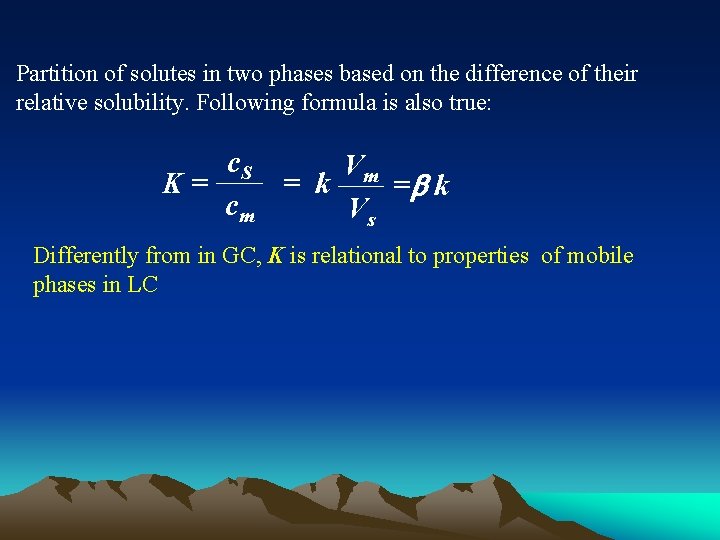
Partition of solutes in two phases based on the difference of their relative solubility. Following formula is also true: c. S Vm K= = k =b k cm Vs Differently from in GC, K is relational to properties of mobile phases in LC

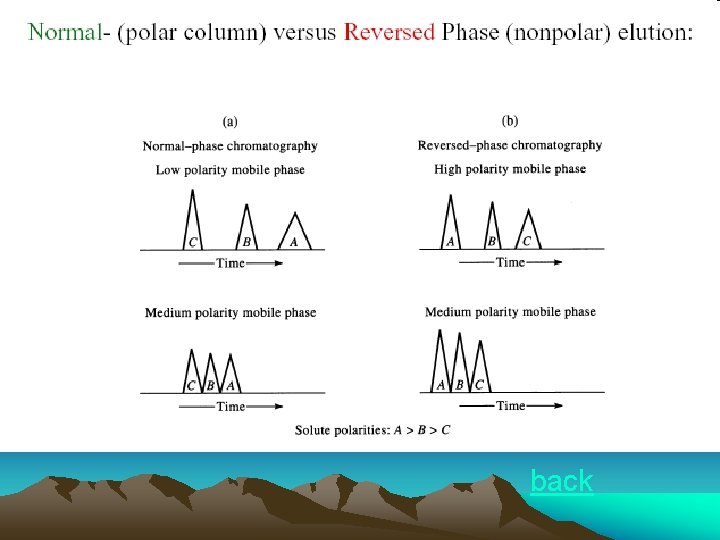
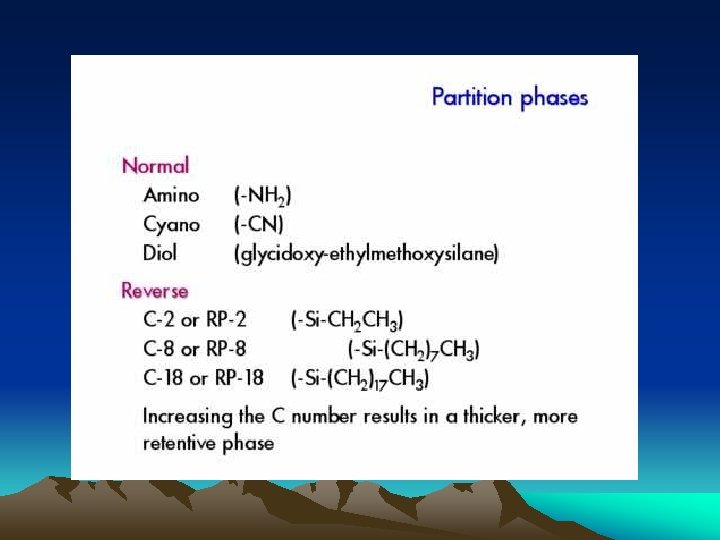
In order to reduce or eliminate loss of stationary liquid, following measures are usually employed: ●Normal phase liquid-liquid chromatography: Stationary liquid is more polar than mobile phase Stationary liquid: hydrophilic(亲水的) Mobile phase: hydrophobic(疏水的) ●Reverse phase liquid-liquid chromatography: Mobile phase is more polar than stationary liquid Stationary liquid: hydrophobic(疏水的) Mobile phase: hydrophilic(亲水的) Wider applications ●Chemically bonded stationary phase: 将各种不同有机基团(group)通过化学反应键合到硅胶(silica gel) 担体表面的游离羟基(dissociative hydroxyl)上,代替机械涂敷的液体固 定相。 应用广泛,可用于normal-, reverse-, ion-exchange-, ion pair色谱等 技术中。

back

●Liquid-Solid Adsorption Chromatography Figure Schematics showing the basis of separation in adsorption chromatography. The solute represented by the solid circle(●) is the more strongly retained. Mobile Phase: Liquid Stationary phase: Sorbent (吸附剂)

▲Mechanism of liquid-solid adsorption chromatography Separation based on difference of adsorptive actions of substances Competitive adsorption of solute molecule X and solvent molecule S on active surface of sorbent (溶质分子与溶剂分子在吸附剂活性表 面的竞争吸附): Xm + n. Sa Xa + n. Sm where subscripts m and a respectively stand for mobile phase and adsorption phase When adsorption reaches a equilibrium, following formula is true: [ Xa][ Sm]n K= [Xm][Sa]n Where K denotes adsorption equilibrium coefficient or partition coefficient

▲Applications: 1. Oil-soluble samples with a medium molecular weight(中等相对分 子质量) 2. Compounds linked with various functional groups and isomers( 异 构体) Applicable to the compounds which can be successfully separated by thin layer chromatography ▲Shortcoming: Tailing peak appears usually due to nonlinear isothermal(等温) adsorption back

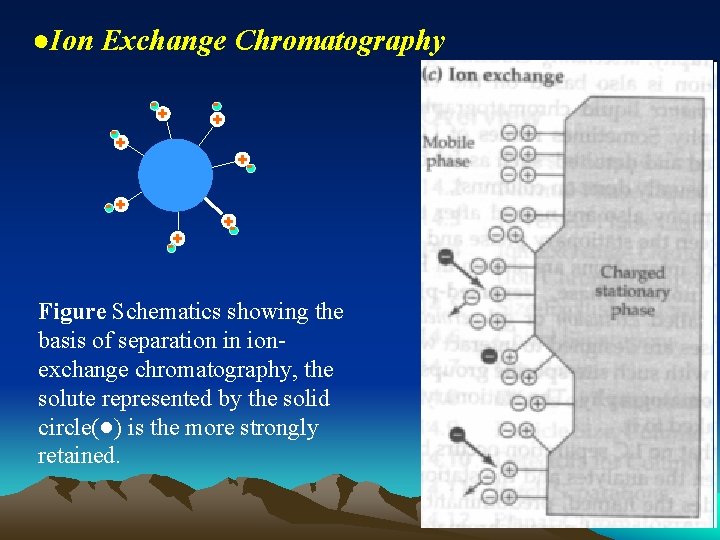
●Ion Exchange Chromatography + +- -+ -+ +- Figure Schematics showing the basis of separation in ionexchange chromatography, the solute represented by the solid circle(●) is the more strongly retained.

![Equilibrium constant for ionexchange reaction NR 4XCl KX NR ClX 4 Or SO Equilibrium constant for ion-exchange reaction: [-NR 4+X-][Cl-] KX = [-NR +Cl-][X-] 4 Or [-SO](https://slidetodoc.com/presentation_image_h2/4abf06a8d1a61394d8ae3bbaa95504fd/image-24.jpg)
Equilibrium constant for ion-exchange reaction: [-NR 4+X-][Cl-] KX = [-NR +Cl-][X-] 4 Or [-SO 3 -M+][Na+] KM = [-SO -Na+][M+] 3 Partition coefficient of anionic exchange DX [-NR 4+X-] DX = [X-] [-NR 4+Cl-] = KX [Cl-] Partition coefficient of cationic exchange DM [-SO 3 -M+] DM = [M+] [-SO 3 -Na+] = KM [Na+]

The higher the affinity of solute molecules with ion-exchange centre , the stronger the interaction of solute ions to ion-exchanger, the bigger partition coefficient and rotention value(溶质分子与离子交换中心的 亲合力越高,溶质的离子与离子交换剂的相互作用越强,分配系 数越大,保留值越大) ▲Applications: Used to separate various ions or dissociable(可离解的) compounds Inorganic species Organic species Biological molecules: for example, amino acids, nucleic acid, proteins ect.

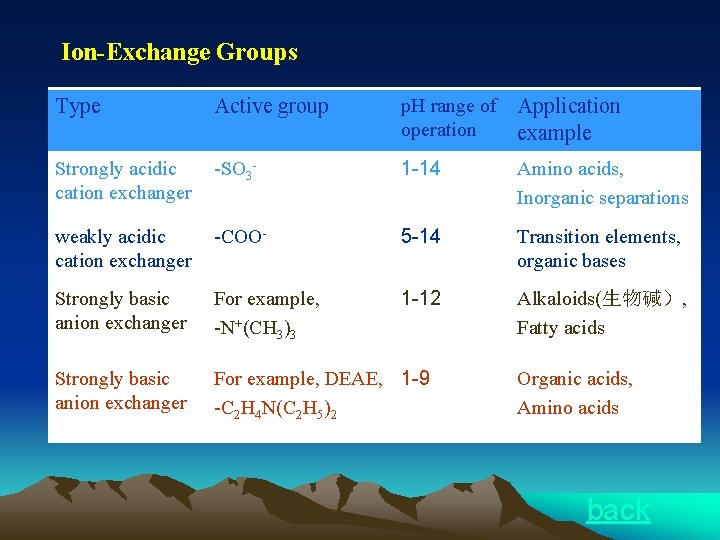
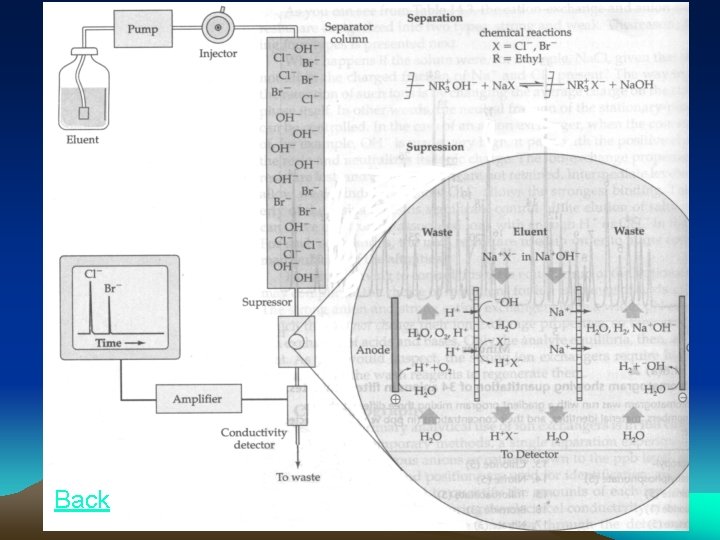
Ion-Exchange Groups Type Active group p. H range of operation Application example Strongly acidic cation exchanger -SO 3 - 1 -14 Amino acids, Inorganic separations weakly acidic cation exchanger -COO- 5 -14 Transition elements, organic bases Strongly basic anion exchanger For example, -N+(CH 3)3 1 -12 Alkaloids(生物碱), Fatty acids Strongly basic anion exchanger For example, DEAE, 1 -9 -C 2 H 4 N(C 2 H 5)2 Organic acids, Amino acids back

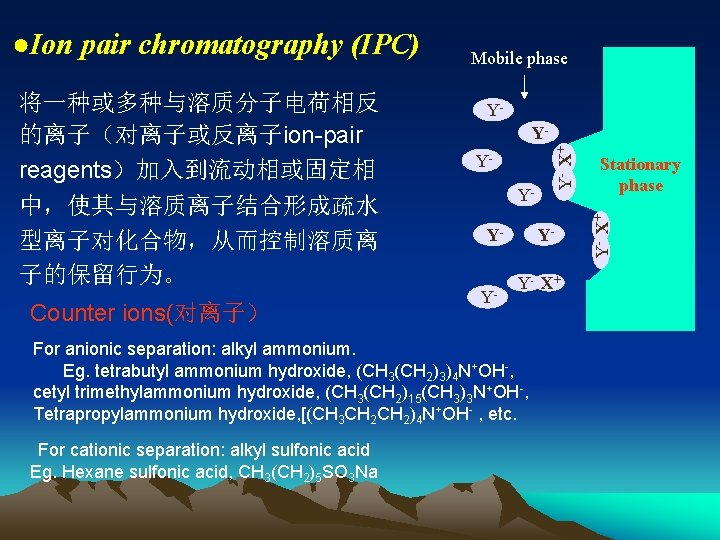
中,使其与溶质离子结合形成疏水 型离子对化合物,从而控制溶质离 子的保留行为。 Counter ions(对离子) YYYY- Y- X+ For anionic separation: alkyl ammonium. Eg. tetrabutyl ammonium hydroxide, (CH 3(CH 2)3)4 N+OH-, cetyl trimethylammonium hydroxide, (CH 3(CH 2)15(CH 3)3 N+OH-, Tetrapropylammonium hydroxide, [(CH 3 CH 2)4 N+OH- , etc. For cationic separation: alkyl sulfonic acid Eg. Hexane sulfonic acid, CH 3(CH 2)5 SO 3 Na Stationary phase Y- X+ 将一种或多种与溶质分子电荷相反 的离子(对离子或反离子ion-pair reagents)加入到流动相或固定相 Mobile phase Y- X+ ●Ion pair chromatography (IPC)

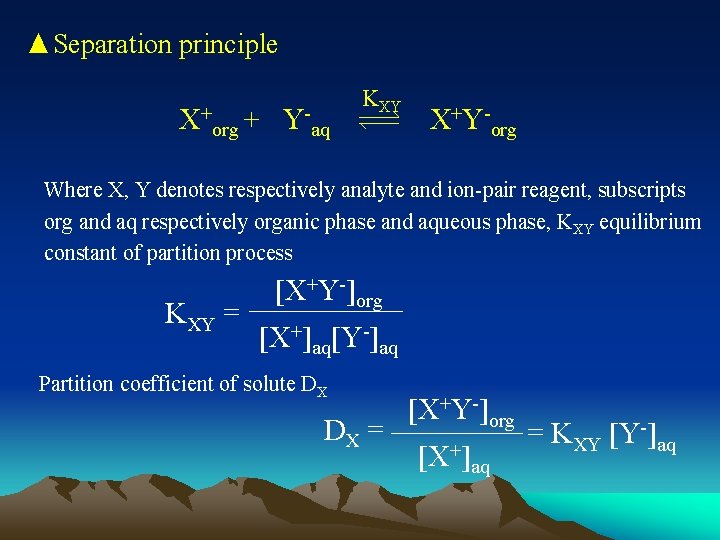
▲Separation principle X+ org + Y- KXY aq X+Y-org Where X, Y denotes respectively analyte and ion-pair reagent, subscripts org and aq respectively organic phase and aqueous phase, KXY equilibrium constant of partition process KXY = [X+Y-]org [X+]aq[Y-]aq Partition coefficient of solute DX DX = [X+Y-]org [X+]aq = KXY [Y-]aq
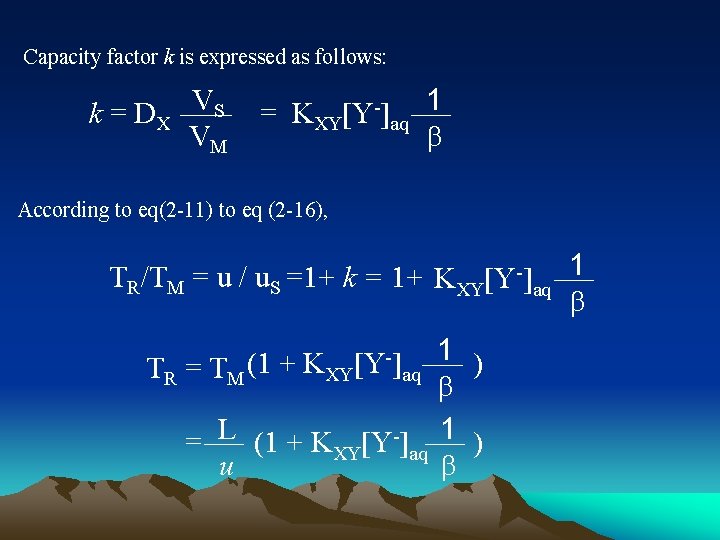
Capacity factor k is expressed as follows: k = D X VS VM = KXY[Y-]aq 1 b According to eq(2 -11) to eq (2 -16), TR/TM = u / u. S =1+ k = 1+ KXY[Y-]aq 1 b 1 ) TR = TM (1 + KXY aq b L 1 = (1 + KXY[Y ]aq ) u b [Y-]
![It is obvious that rotention value increases with increasing KXY and Yaq KXY depends It is obvious that rotention value increases with increasing KXY and [Y-]aq, KXY depends](https://slidetodoc.com/presentation_image_h2/4abf06a8d1a61394d8ae3bbaa95504fd/image-30.jpg)
It is obvious that rotention value increases with increasing KXY and [Y-]aq, KXY depends on properties of ion-pair reagent and organic phase 改变对离子浓度是控制反相离子对色谱溶质保留值的主要措施,可在 较大范围内改变分离的选择性。 ▲Applications ■Separate acids, bases, and some ionic, nonionic mixtures, especially, biochemical test samples. ■Easy to introduce some ultraviolet-adsorbed groups (chromaphore) or fluorescent groups to sample molecules to improve detection sensitivity ■ Reverse phase ion-pair chromatography is more common back


Back

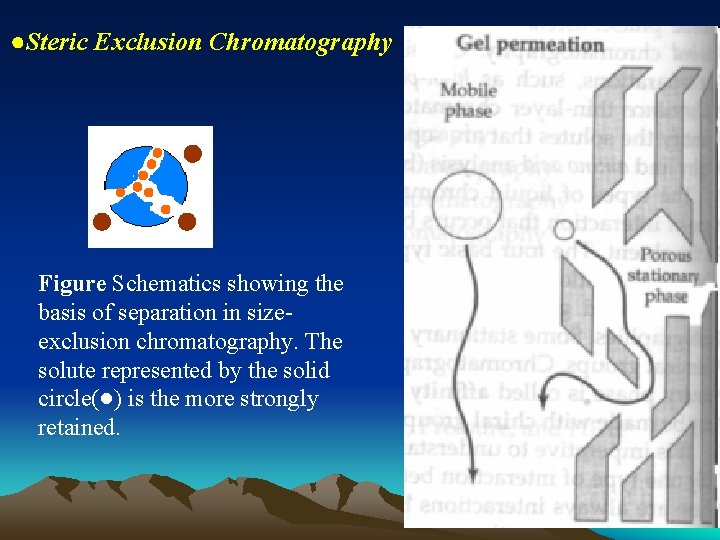
●Steric Exclusion Chromatography Figure Schematics showing the basis of separation in sizeexclusion chromatography. The solute represented by the solid circle(●) is the more strongly retained.



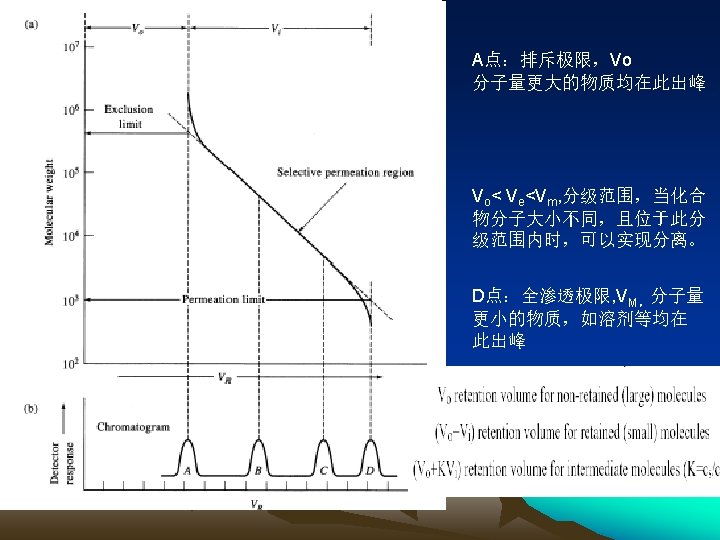
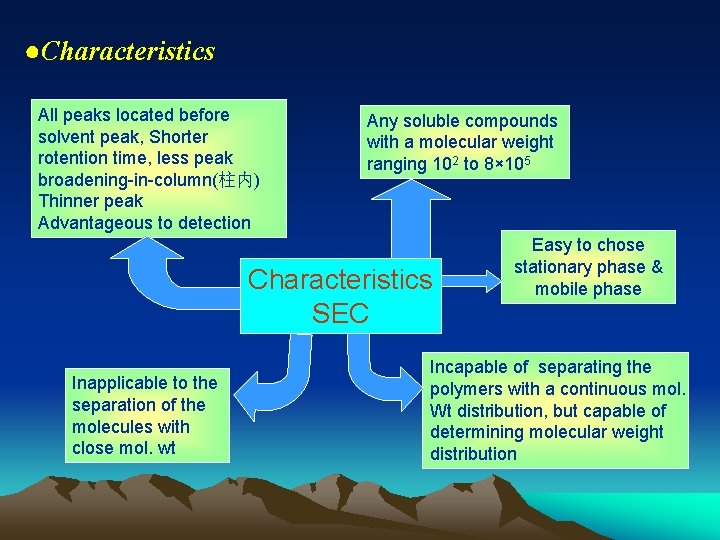
●Characteristics All peaks located before solvent peak, Shorter rotention time, less peak broadening-in-column(柱内) Thinner peak Advantageous to detection Any soluble compounds with a molecular weight ranging 102 to 8× 105 Characteristics SEC Inapplicable to the separation of the molecules with close mol. wt Easy to chose stationary phase & mobile phase Incapable of separating the polymers with a continuous mol. Wt distribution, but capable of determining molecular weight distribution

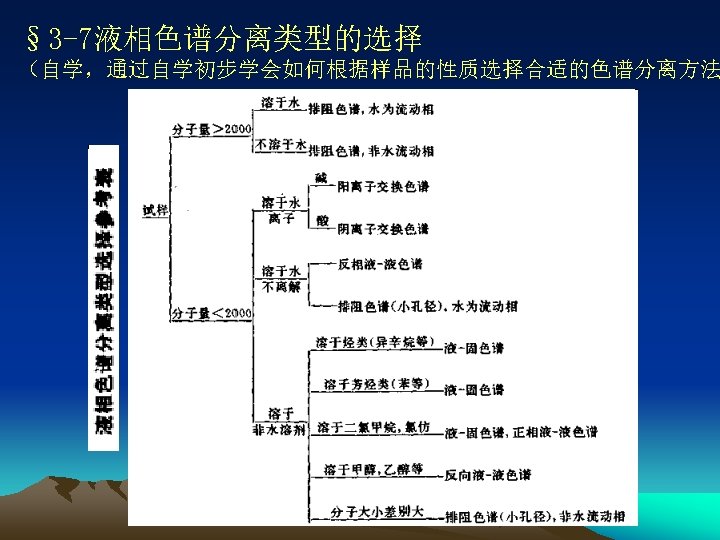
●Stationary and Mobile Phases of HPLC Self-study Problems What kinds of stationary phases do each HPLC method have? What characteristics do each stationary phase have? How to select mobile phase in HPLC? 详细内容


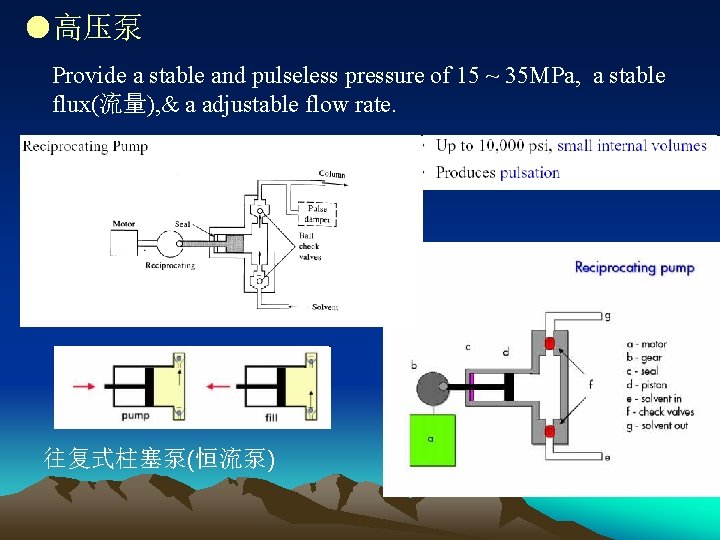
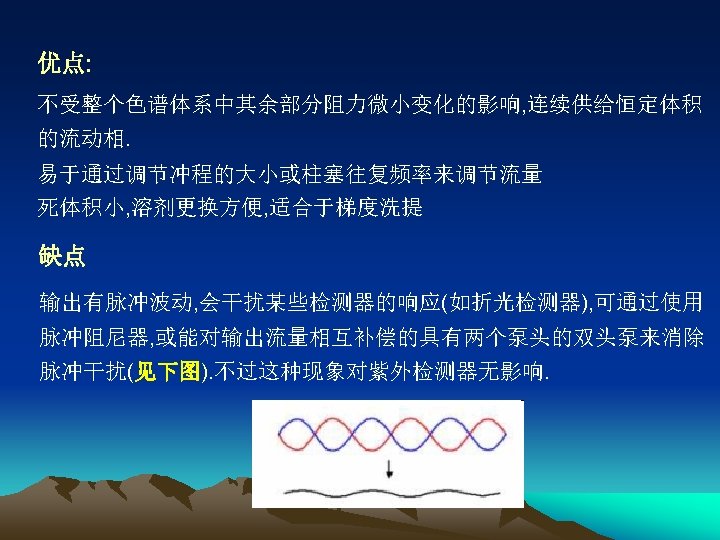
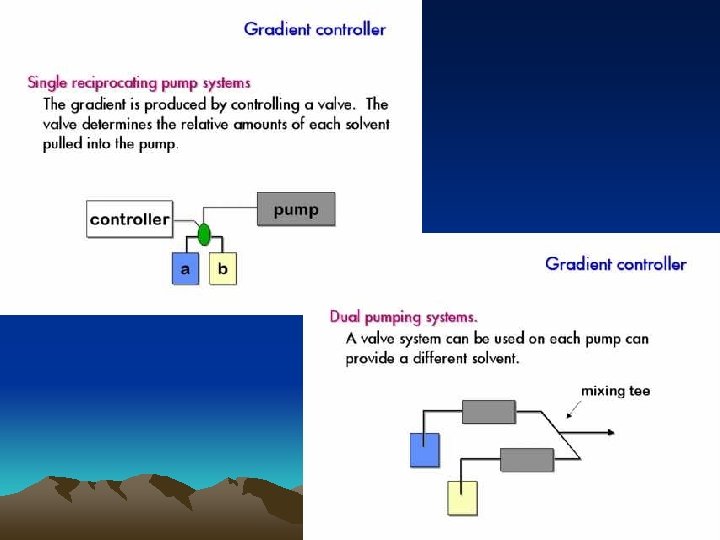
●高压泵 Provide a stable and pulseless pressure of 15 ~ 35 MPa, a stable flux(流量), & a adjustable flow rate. 往复式柱塞泵(恒流泵)


Air inlet Air outlet Solvent out 单向阀 Sealed underlay (密封垫) 柱塞 气动放大泵(恒压泵) Solvent let




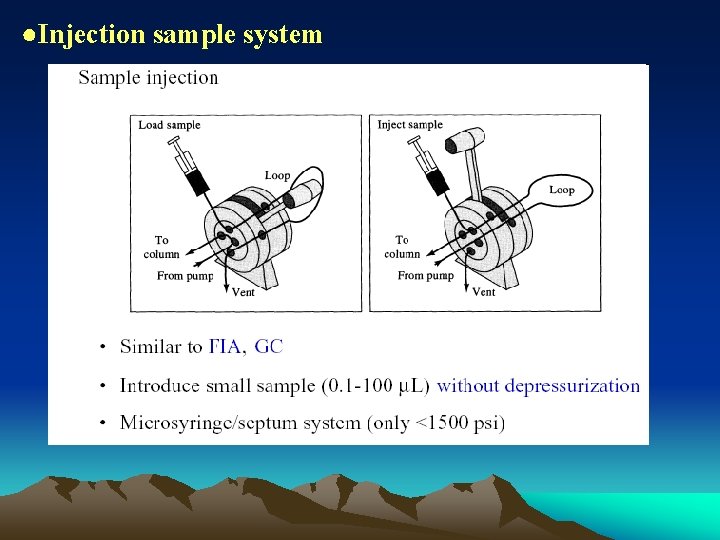
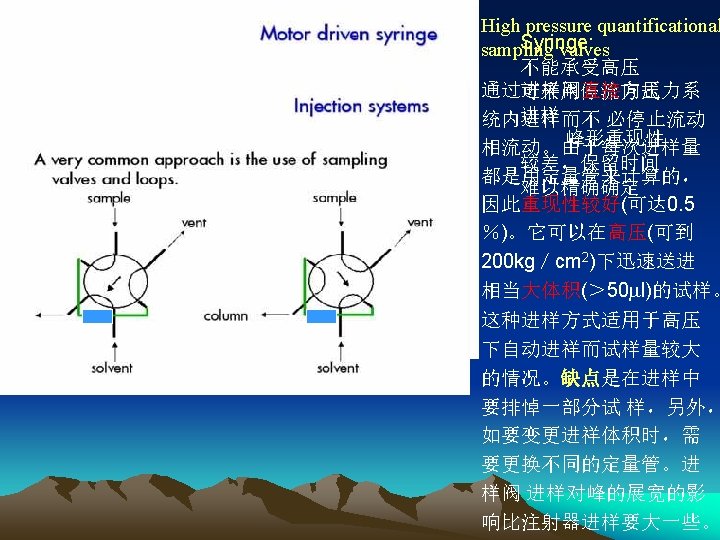
●Injection sample system





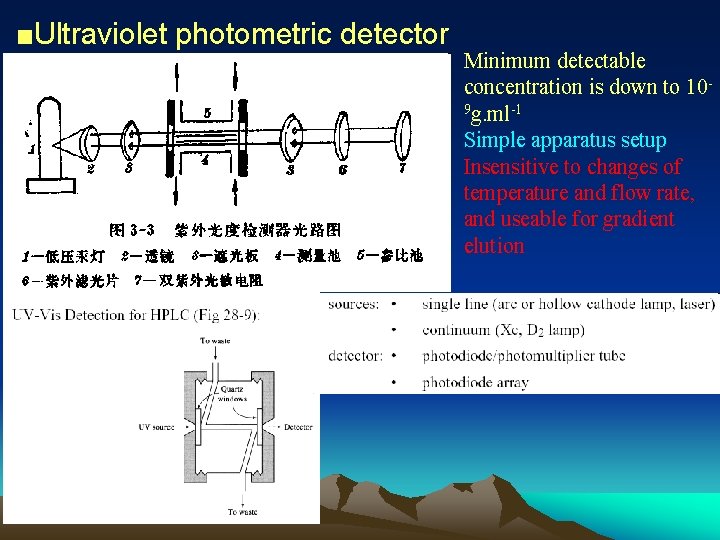
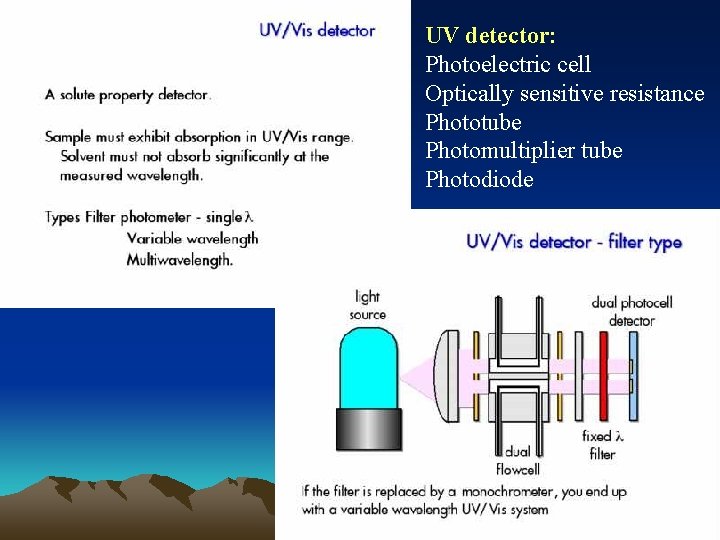
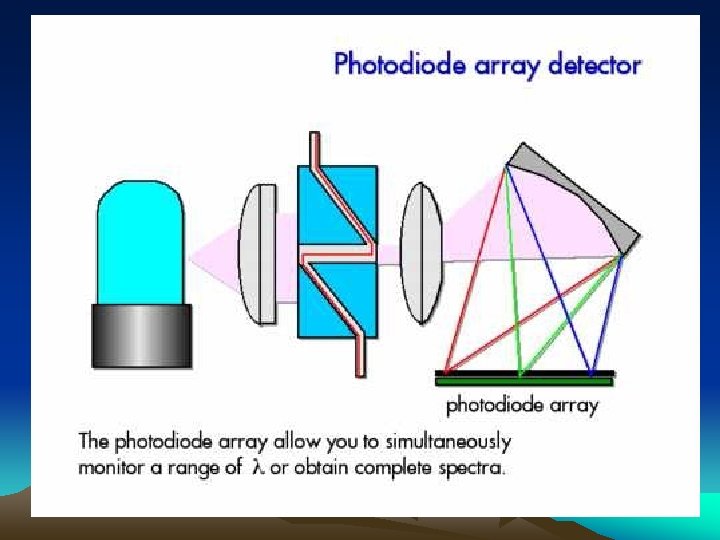
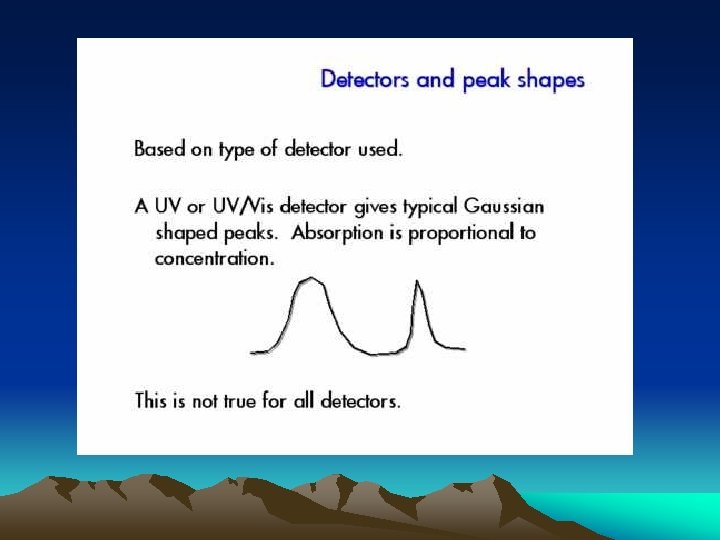
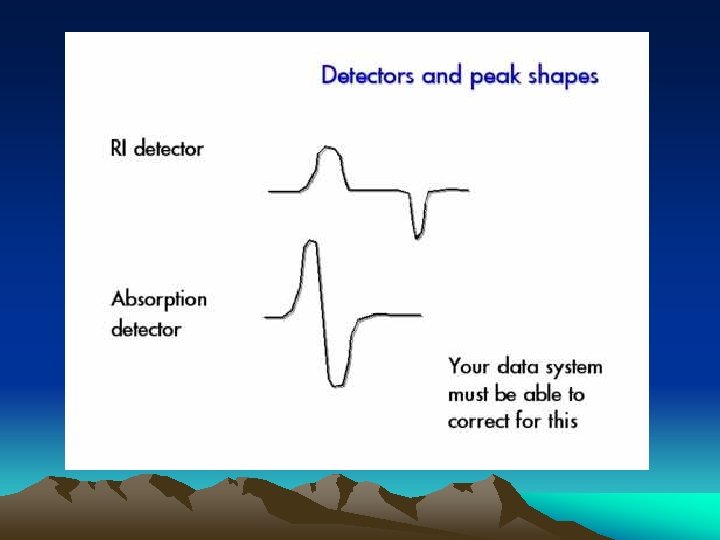
■Ultraviolet photometric detector Minimum detectable concentration is down to 109 g. ml-1 Simple apparatus setup Insensitive to changes of temperature and flow rate, and useable for gradient elution

UV detector: Photoelectric cell Optically sensitive resistance Phototube Photomultiplier tube Photodiode
















