Chapter 3 Free Radical Copolymerization 1 1 Introduction




![2. 1 Copolymer composition equation The overall rates of consumption of monomers [M 1], 2. 1 Copolymer composition equation The overall rates of consumption of monomers [M 1],](https://slidetodoc.com/presentation_image/1149f08937184ed51f0f0d9b10c3b30b/image-13.jpg)

![Line cross • Plot r 2 vs r 1 with different [M 1]/[M 2] Line cross • Plot r 2 vs r 1 with different [M 1]/[M 2]](https://slidetodoc.com/presentation_image/1149f08937184ed51f0f0d9b10c3b30b/image-46.jpg)



- Slides: 70

Chapter 3 Free Radical Copolymerization 1

1 Introduction 2

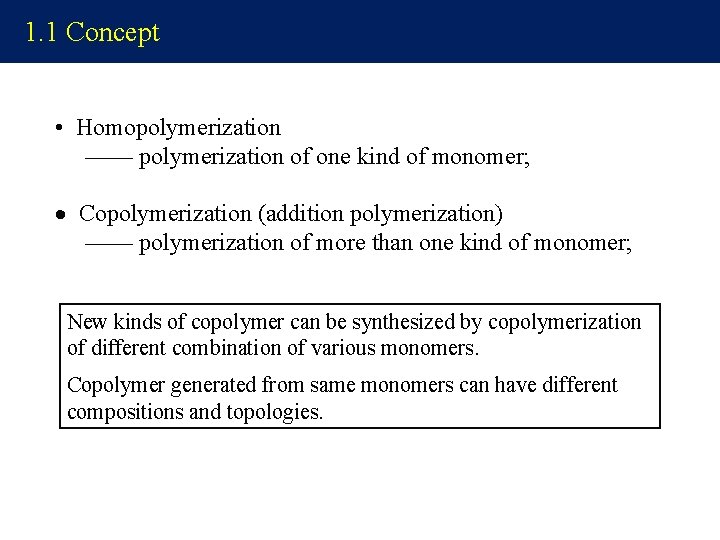
1. 1 Concept • Homopolymerization —— polymerization of one kind of monomer; Copolymerization (addition polymerization) —— polymerization of more than one kind of monomer; New kinds of copolymer can be synthesized by copolymerization of different combination of various monomers. Copolymer generated from same monomers can have different compositions and topologies.

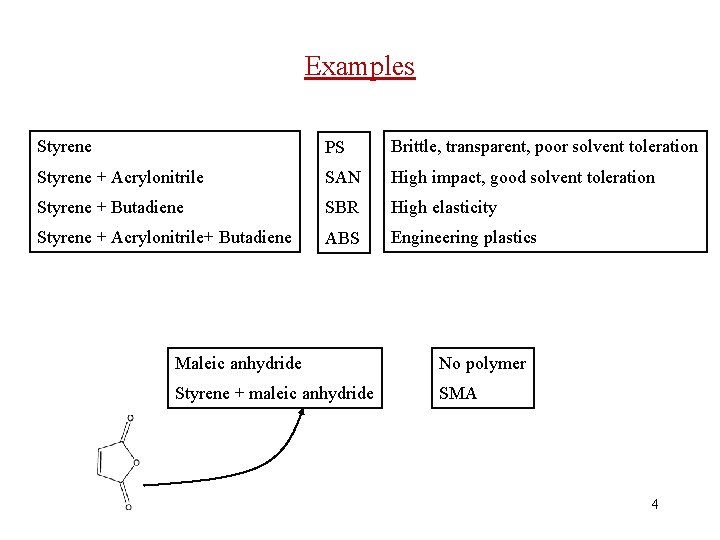
Examples Styrene PS Brittle, transparent, poor solvent toleration Styrene + Acrylonitrile SAN High impact, good solvent toleration Styrene + Butadiene SBR High elasticity Styrene + Acrylonitrile+ Butadiene ABS Engineering plastics Maleic anhydride No polymer Styrene + maleic anhydride SMA 4

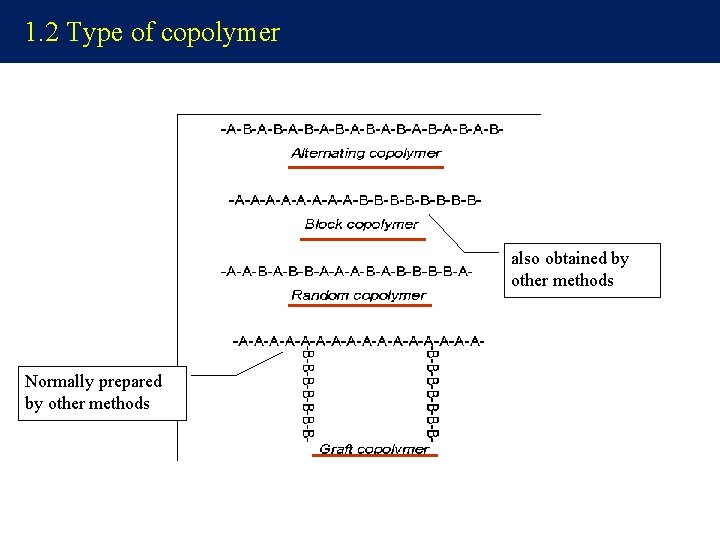
1. 2 Type of copolymer also obtained by other methods Normally prepared by other methods

1. 3 Nomenclature of copolymer poly(ethylene-co-propylene) 乙烯-丙烯共聚物 poly(ethylene-r-propylene) 乙烯-丙烯无规共聚物 poly(ethylene-b-propylene) 乙烯-丙烯嵌段共聚物 poly(ethylene-g-propylene) 乙烯-丙烯接枝共聚物 poly(ethylene-a-styrene) 乙烯-苯乙烯交替共聚物

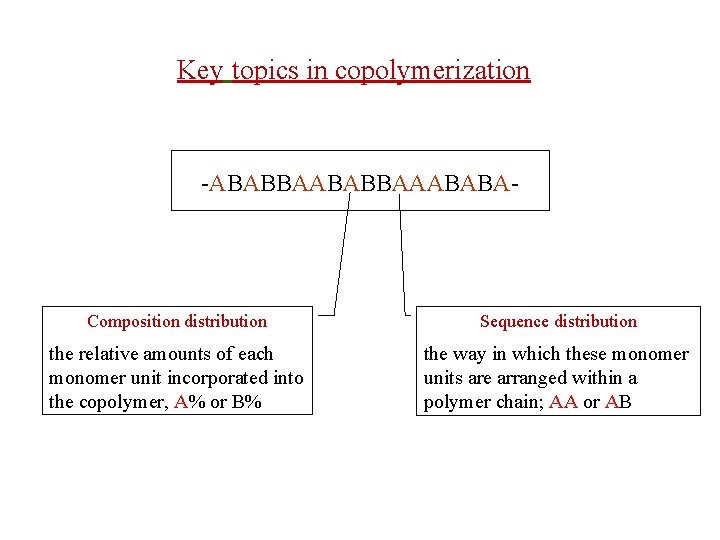
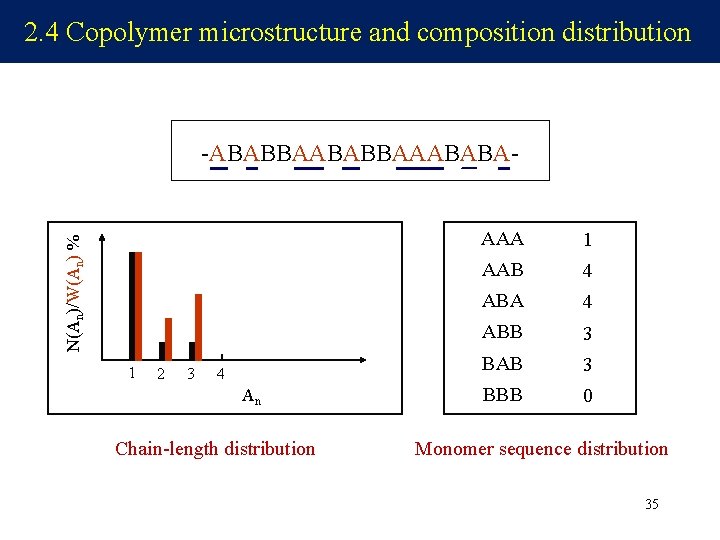
Key topics in copolymerization -ABABBAAABABA- Composition distribution Sequence distribution the relative amounts of each monomer unit incorporated into the copolymer, A% or B% the way in which these monomer units are arranged within a polymer chain; AA or AB

2. Copolymer composition 8

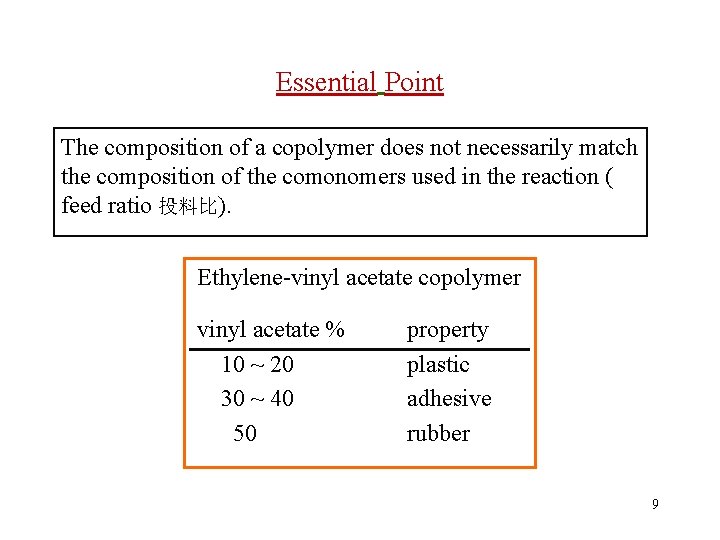
Essential Point The composition of a copolymer does not necessarily match the composition of the comonomers used in the reaction ( feed ratio 投料比). Ethylene-vinyl acetate copolymer vinyl acetate % 10 ~ 20 30 ~ 40 50 property plastic adhesive rubber 9

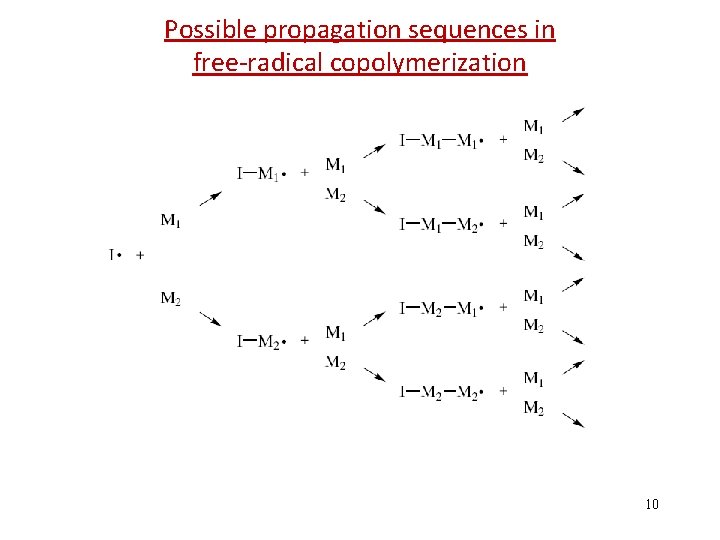
Possible propagation sequences in free-radical copolymerization 10

Assumptions in copolymerization • the rate of the propagation be chain-length-independent. • only the terminal unit of the polymer radical can affect its reactivity • no side reactions, such as depropagation, monomer partitioning, and various forms of complex formation 11

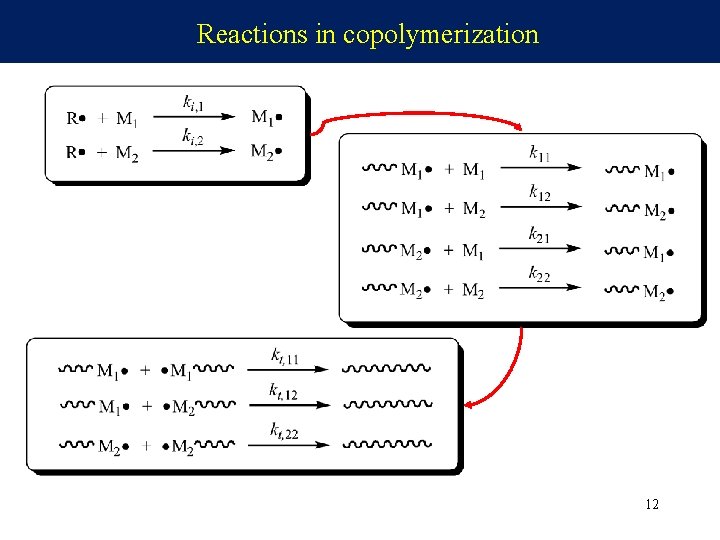
Reactions in copolymerization 12
![2 1 Copolymer composition equation The overall rates of consumption of monomers M 1 2. 1 Copolymer composition equation The overall rates of consumption of monomers [M 1],](https://slidetodoc.com/presentation_image/1149f08937184ed51f0f0d9b10c3b30b/image-13.jpg)
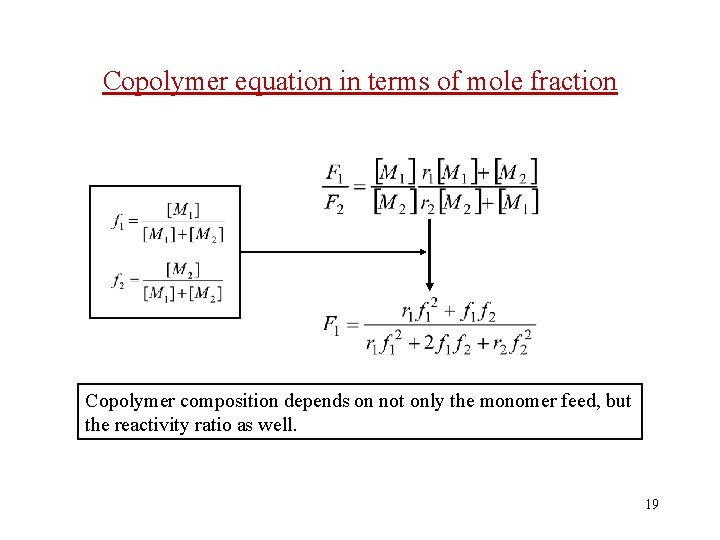
2. 1 Copolymer composition equation The overall rates of consumption of monomers [M 1], [M 2] then follow these expressions: ignore the initiation The expression for instantaneous copolymer composition (F 1/F 2) molar fraction of M 1 molar fraction of M 2 13

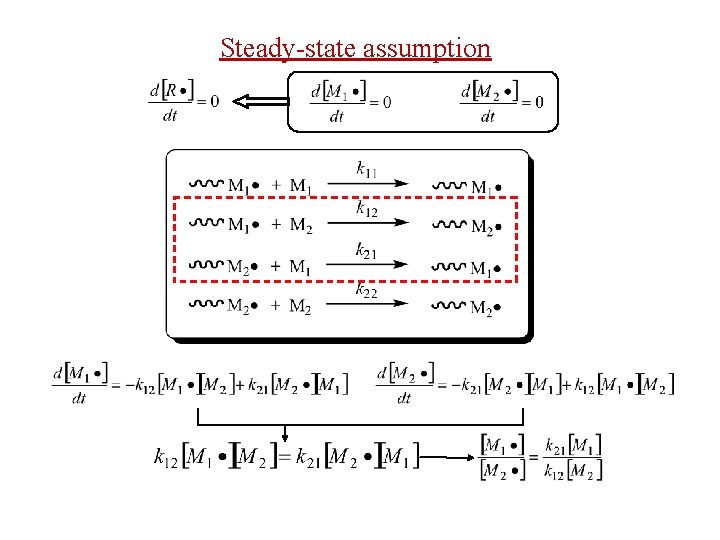
Steady-state assumption

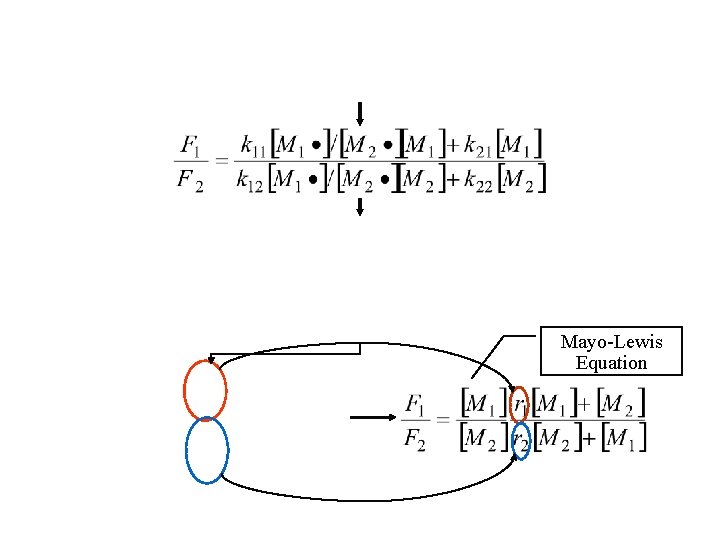
Mayo-Lewis Equation

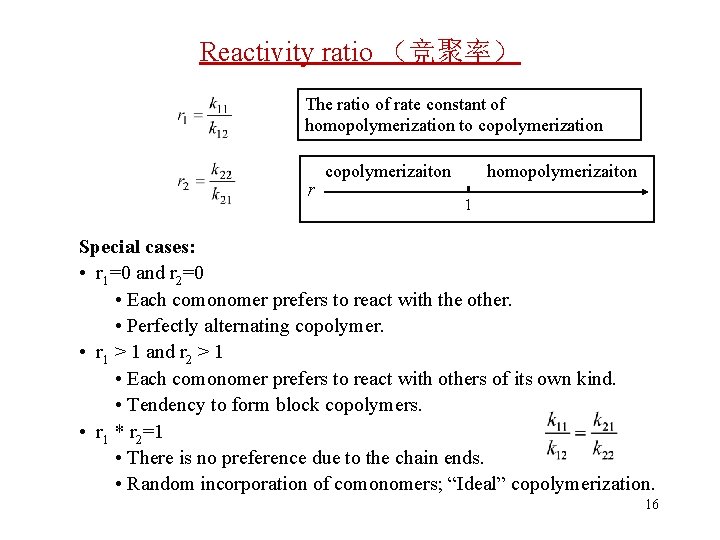
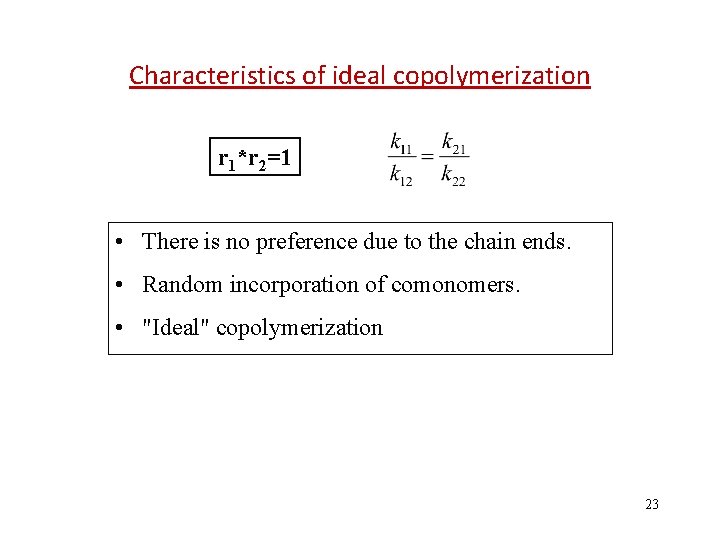
Reactivity ratio (竞聚率) The ratio of rate constant of homopolymerization to copolymerization r copolymerizaiton homopolymerizaiton 1 Special cases: • r 1=0 and r 2=0 • Each comonomer prefers to react with the other. • Perfectly alternating copolymer. • r 1 > 1 and r 2 > 1 • Each comonomer prefers to react with others of its own kind. • Tendency to form block copolymers. • r 1 * r 2=1 • There is no preference due to the chain ends. • Random incorporation of comonomers; “Ideal” copolymerization. 16

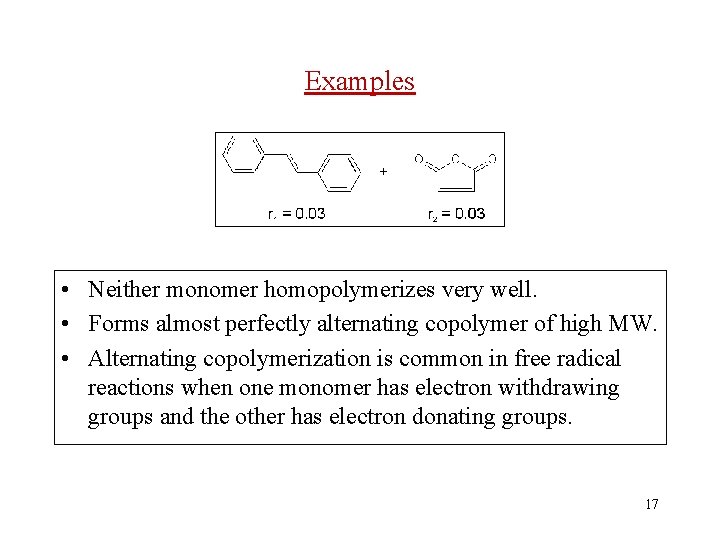
Examples • Neither monomer homopolymerizes very well. • Forms almost perfectly alternating copolymer of high MW. • Alternating copolymerization is common in free radical reactions when one monomer has electron withdrawing groups and the other has electron donating groups. 17

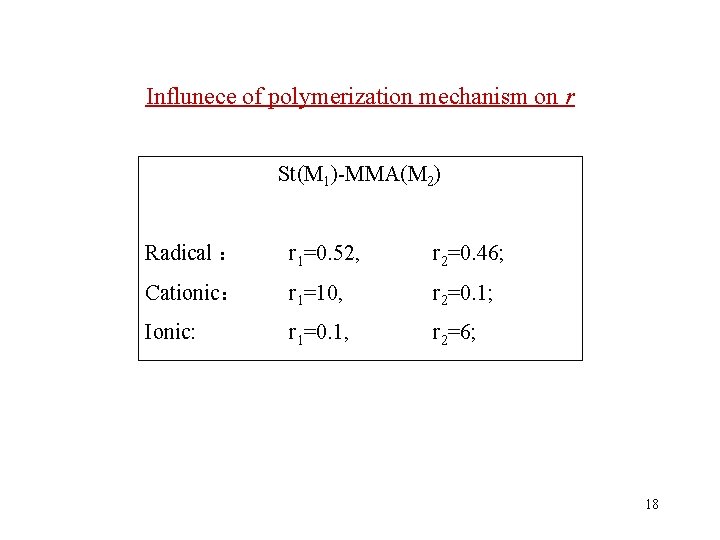
Influnece of polymerization mechanism on r St(M 1)-MMA(M 2) Radical : r 1=0. 52, r 2=0. 46; Cationic: r 1=10, r 2=0. 1; Ionic: r 1=0. 1, r 2=6; 18

Copolymer equation in terms of mole fraction Copolymer composition depends on not only the monomer feed, but the reactivity ratio as well. 19

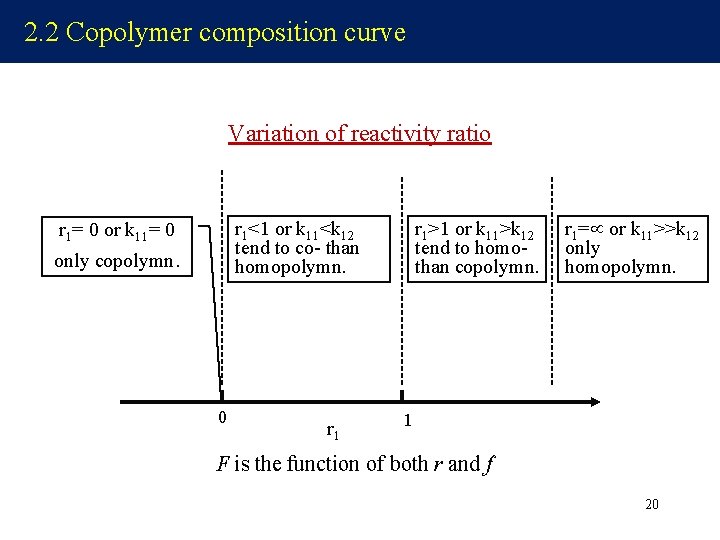
2. 2 Copolymer composition curve Variation of reactivity ratio r 1<1 or k 11<k 12 tend to co- than homopolymn. r 1= 0 or k 11= 0 only copolymn. 0 r 1>1 or k 11>k 12 tend to homothan copolymn. r 1= or k 11>>k 12 only homopolymn. 1 F is the function of both r and f 20

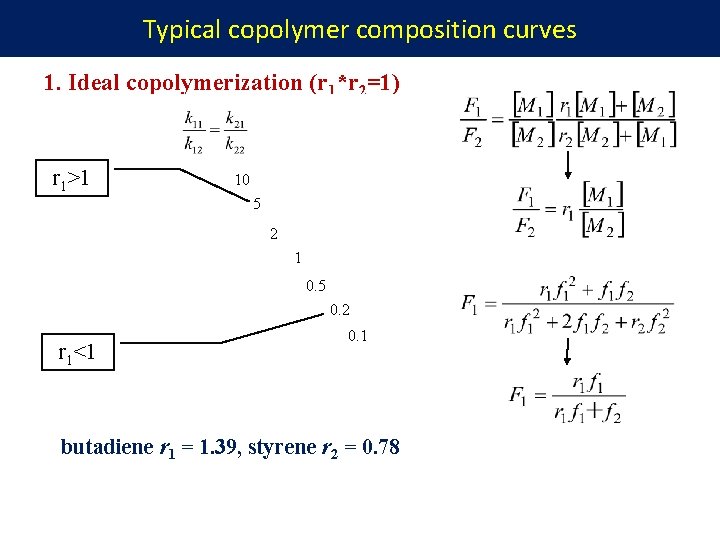
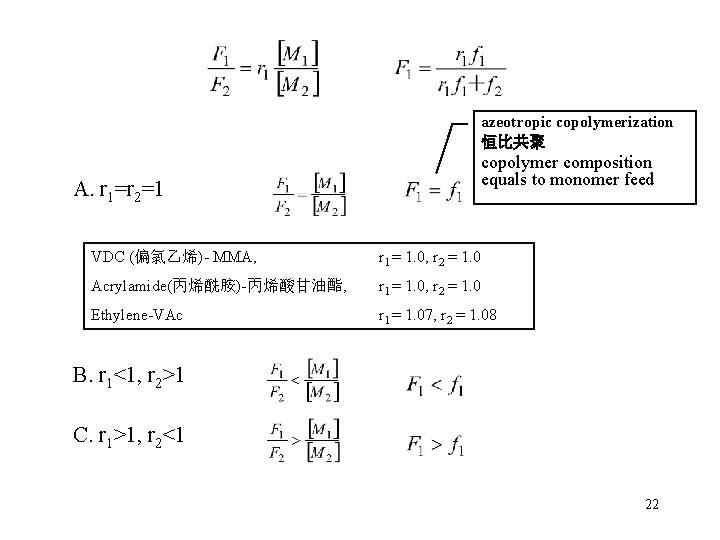
Typical copolymer composition curves 1. Ideal copolymerization (r 1*r 2=1) r 1>1 10 5 2 1 0. 5 0. 2 r 1<1 0. 1 butadiene r 1 = 1. 39, styrene r 2 = 0. 78

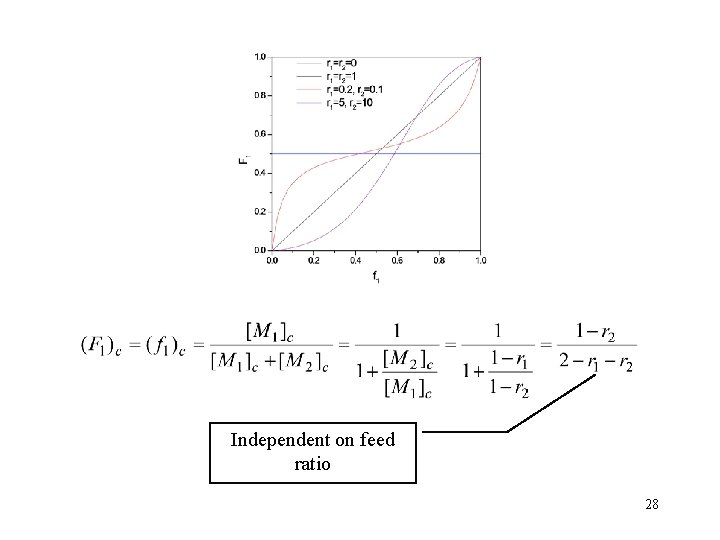
azeotropic copolymerization 恒比共聚 copolymer composition equals to monomer feed A. r 1=r 2=1 VDC (偏氯乙烯)- MMA, r 1 = 1. 0, r 2 = 1. 0 Acrylamide(丙烯酰胺)-丙烯酸甘油酯, r 1 = 1. 0, r 2 = 1. 0 Ethylene-VAc r 1 = 1. 07, r 2 = 1. 08 B. r 1<1, r 2>1 C. r 1>1, r 2<1 22

Characteristics of ideal copolymerization r 1*r 2=1 • There is no preference due to the chain ends. • Random incorporation of comonomers. • "Ideal" copolymerization 23

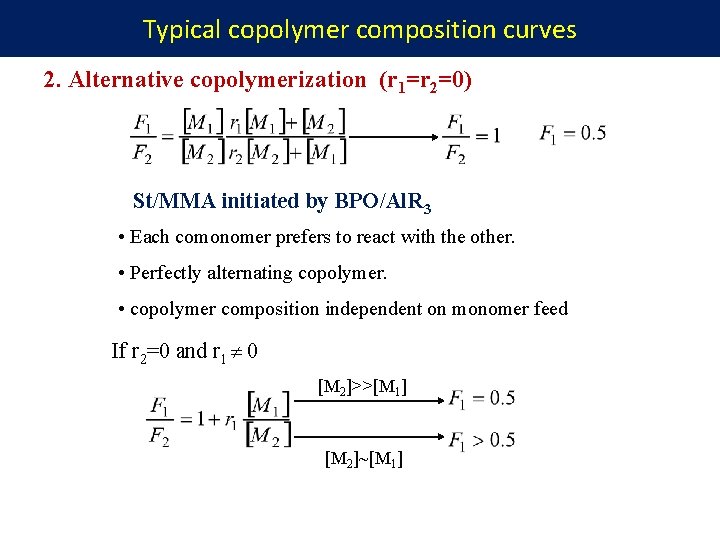
Typical copolymer composition curves 2. Alternative copolymerization (r 1=r 2=0) St/MMA initiated by BPO/Al. R 3 • Each comonomer prefers to react with the other. • Perfectly alternating copolymer. • copolymer composition independent on monomer feed If r 2=0 and r 1 0 [M 2]>>[M 1] [M 2]~[M 1]

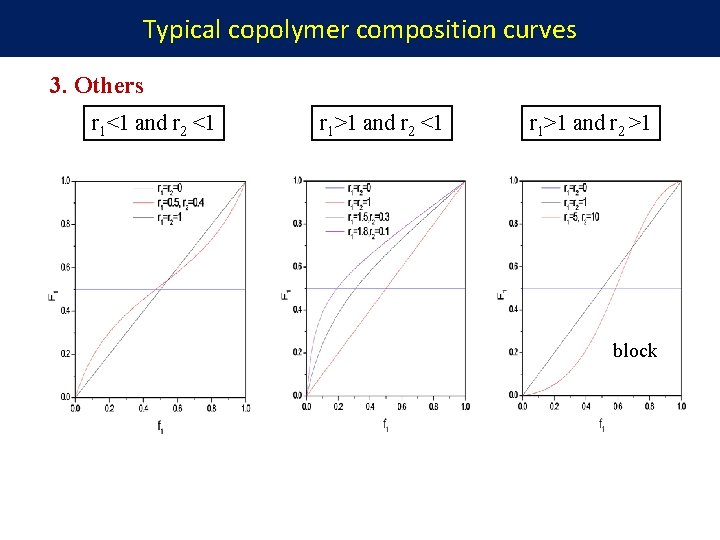
Typical copolymer composition curves 3. Others r 1<1 and r 2 <1 r 1>1 and r 2 >1 block

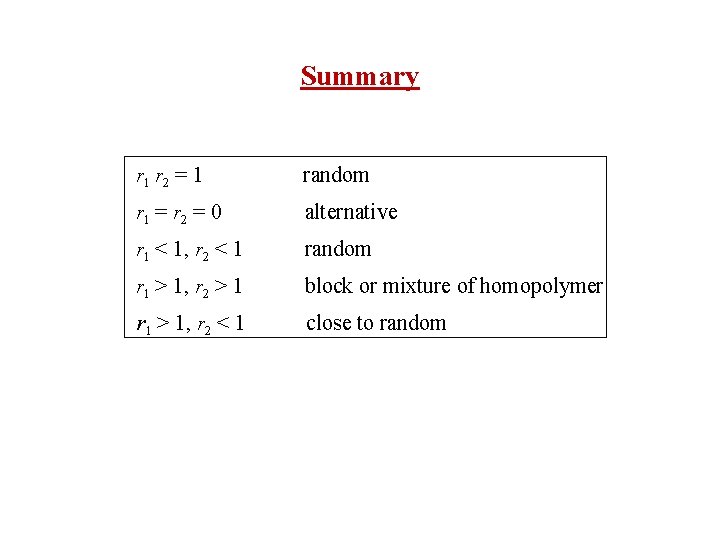
Summary r 1 r 2 = 1 random r 1 = r 2 = 0 alternative r 1 < 1, r 2 < 1 random r 1 > 1, r 2 > 1 block or mixture of homopolymer r 1 > 1, r 2 < 1 close to random

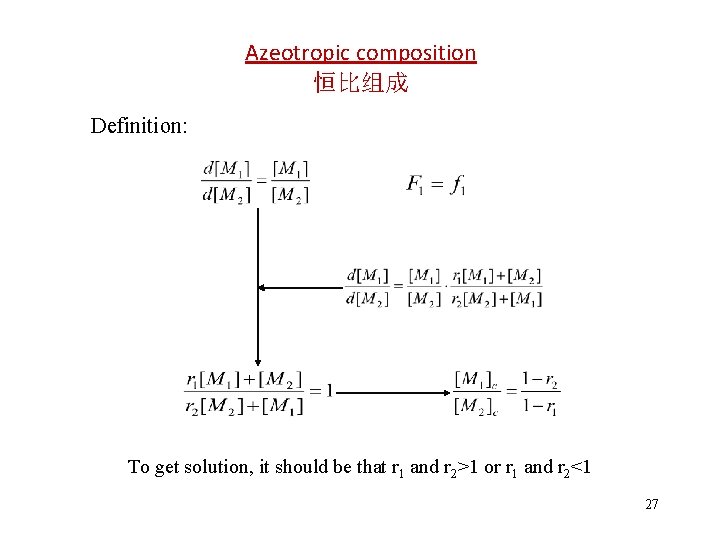
Azeotropic composition 恒比组成 Definition: To get solution, it should be that r 1 and r 2>1 or r 1 and r 2<1 27

Independent on feed ratio 28

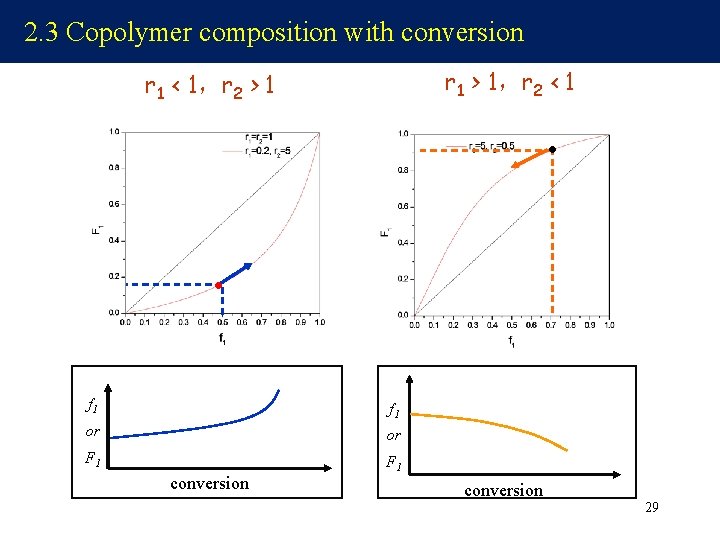
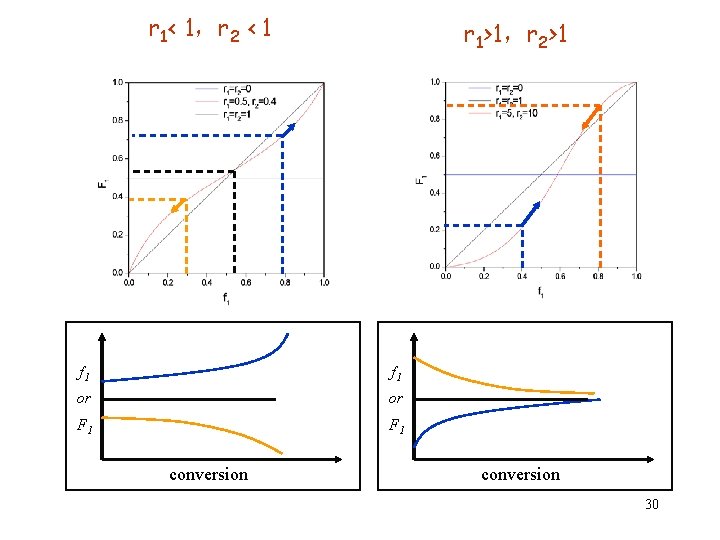
2. 3 Copolymer composition with conversion r 1 > 1,r 2 < 1 r 1 < 1,r 2 > 1 f 1 or or F 1 conversion 29

r 1< 1,r 2 < 1 r 1>1,r 2>1 f 1 or or F 1 conversion 30

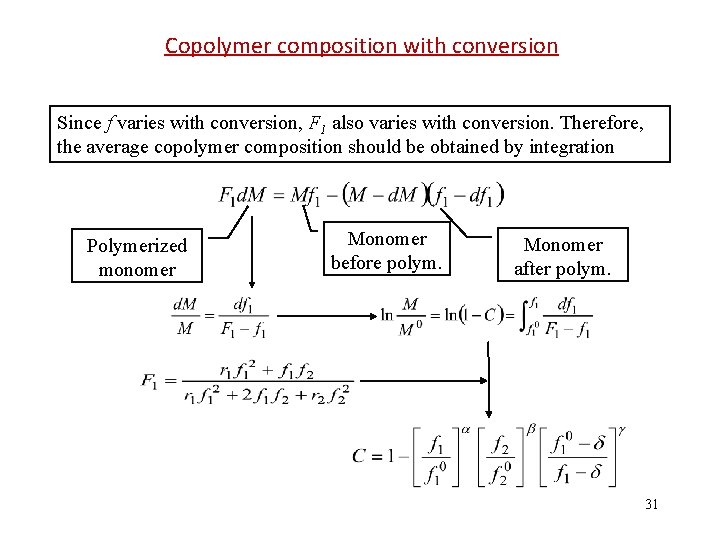
Copolymer composition with conversion Since f varies with conversion, F 1 also varies with conversion. Therefore, the average copolymer composition should be obtained by integration Polymerized monomer Monomer before polym. Monomer after polym. 31

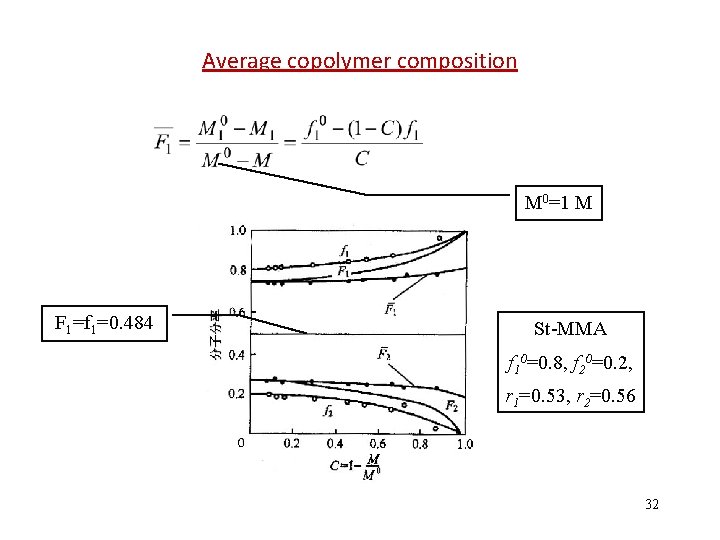
Average copolymer composition M 0=1 M F 1=f 1=0. 484 St-MMA f 10=0. 8, f 20=0. 2, r 1=0. 53, r 2=0. 56 32

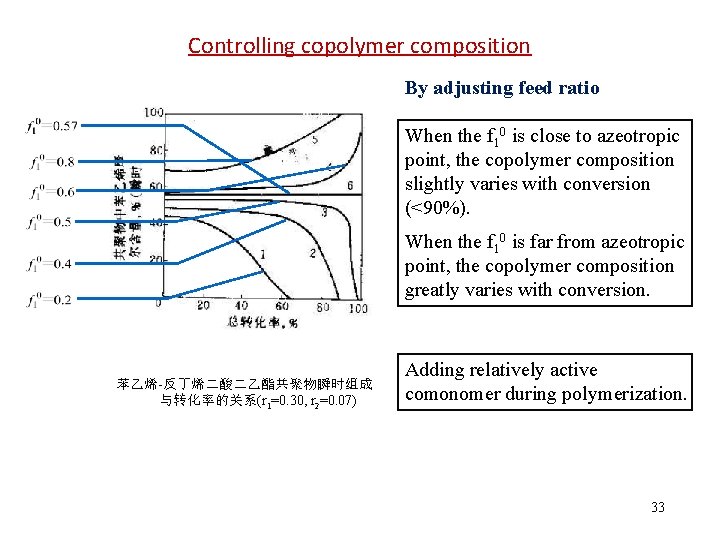
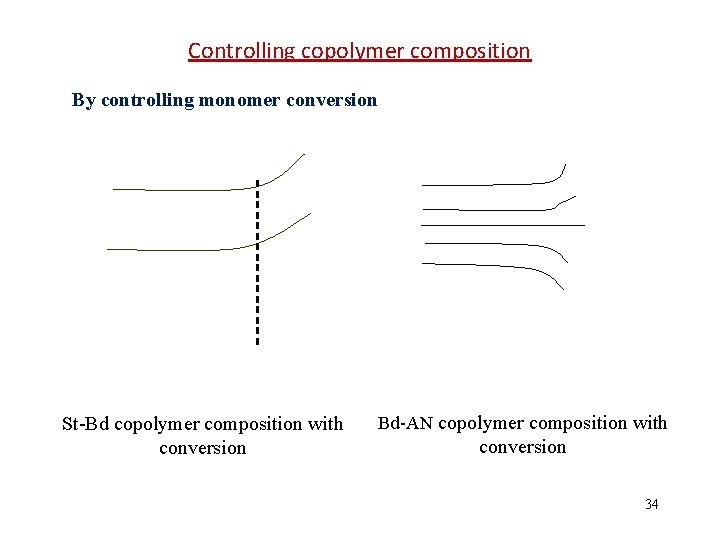
Controlling copolymer composition By adjusting feed ratio When the f 10 is close to azeotropic point, the copolymer composition slightly varies with conversion (<90%). When the f 10 is far from azeotropic point, the copolymer composition greatly varies with conversion. 苯乙烯-反丁烯二酸二乙酯共聚物瞬时组成 与转化率的关系(r 1=0. 30, r 2=0. 07) Adding relatively active comonomer during polymerization. 33

Controlling copolymer composition By controlling monomer conversion St-Bd copolymer composition with conversion Bd-AN copolymer composition with conversion 34

2. 4 Copolymer microstructure and composition distribution N(An)/W(An) % -ABABBAAABABA- 1 2 3 4 An Chain-length distribution AAA 1 AAB 4 ABA 4 ABB 3 BAB 3 BBB 0 Monomer sequence distribution 35

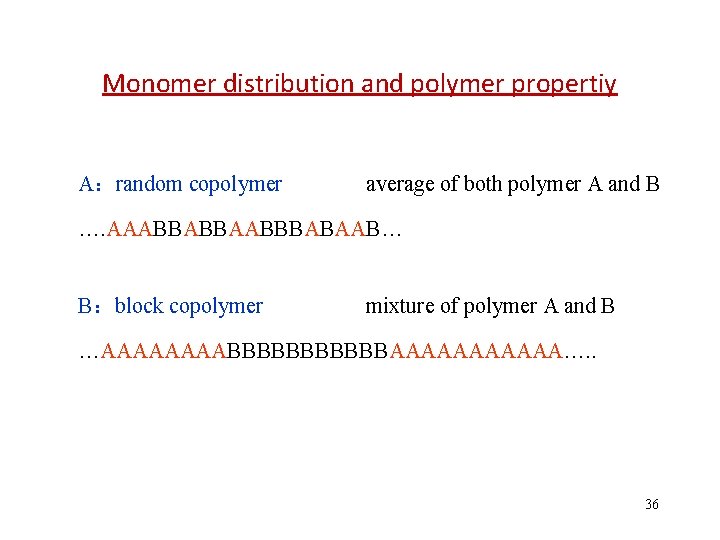
Monomer distribution and polymer propertiy A:random copolymer average of both polymer A and B …. AAABBABBAABBBABAAB… B:block copolymer mixture of polymer A and B …AAAABBBBBBAAAAAA…. . 36

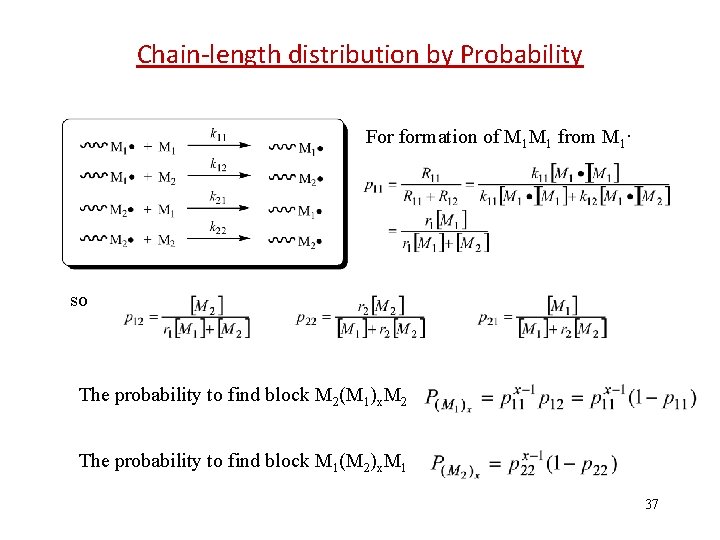
Chain-length distribution by Probability For formation of M 1 M 1 from M 1· so The probability to find block M 2(M 1)x. M 2 The probability to find block M 1(M 2)x. M 1 37

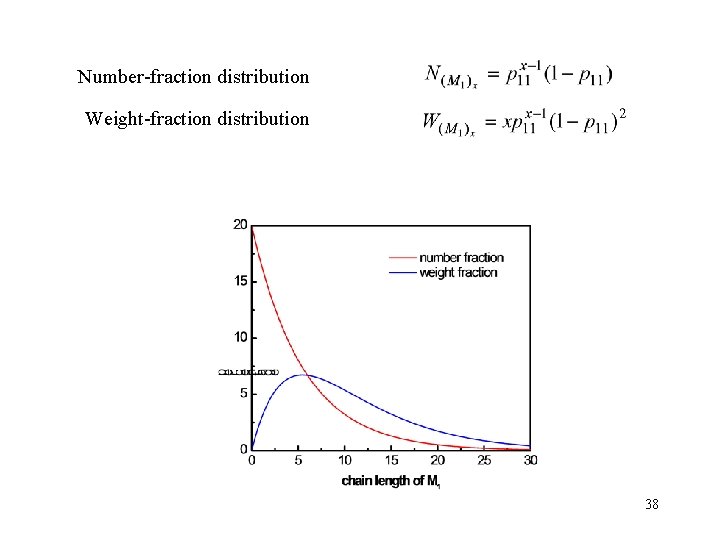
Number-fraction distribution Weight-fraction distribution 38

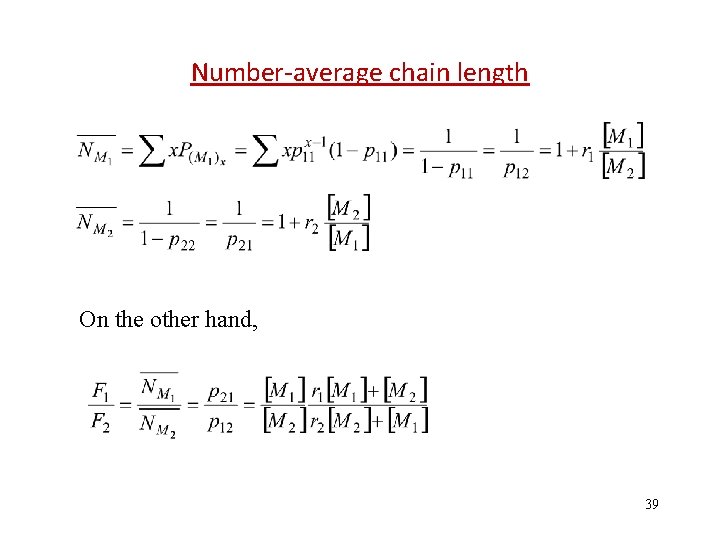
Number-average chain length On the other hand, 39

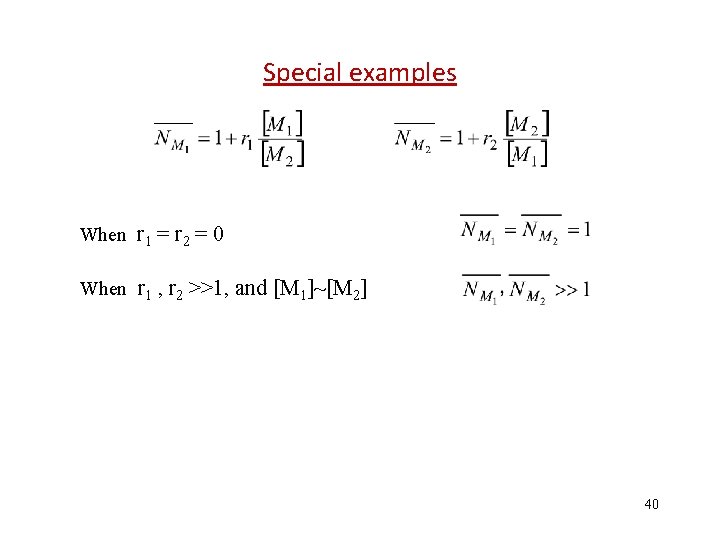
Special examples When r 1 = r 2 = 0 When r 1 , r 2 >>1, and [M 1]~[M 2] 40

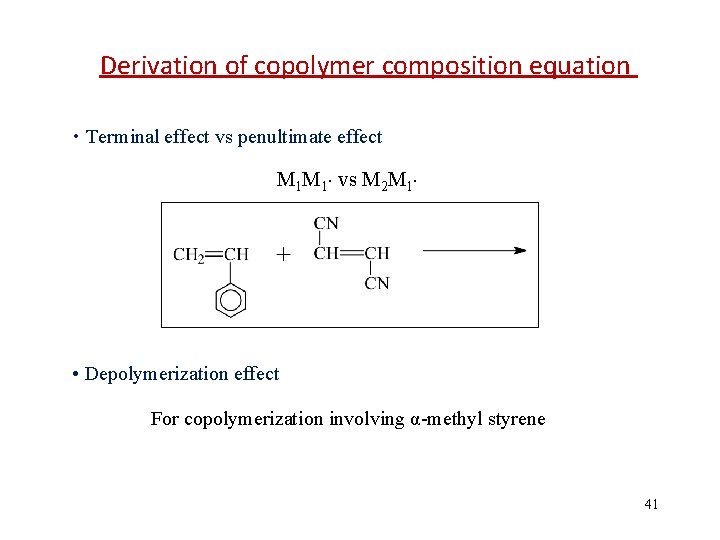
Derivation of copolymer composition equation • Terminal effect vs penultimate effect M 1 M 1 vs M 2 M 1 • Depolymerization effect For copolymerization involving α-methyl styrene 41

3. Multi-component copolymerization 42

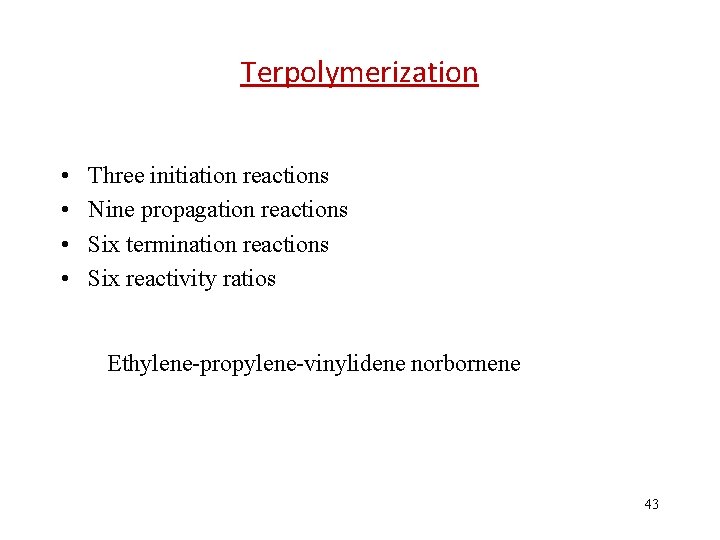
Terpolymerization • • Three initiation reactions Nine propagation reactions Six termination reactions Six reactivity ratios Ethylene-propylene-vinylidene norbornene 43

4. Experimental evaluation of reactivity ratios 44

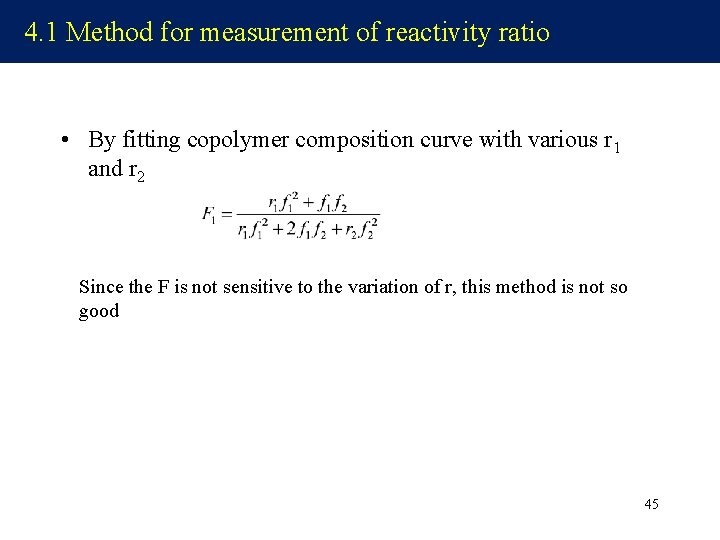
4. 1 Method for measurement of reactivity ratio • By fitting copolymer composition curve with various r 1 and r 2 Since the F is not sensitive to the variation of r, this method is not so good 45
![Line cross Plot r 2 vs r 1 with different M 1M 2 Line cross • Plot r 2 vs r 1 with different [M 1]/[M 2]](https://slidetodoc.com/presentation_image/1149f08937184ed51f0f0d9b10c3b30b/image-46.jpg)
Line cross • Plot r 2 vs r 1 with different [M 1]/[M 2] and F 1/F 2 46

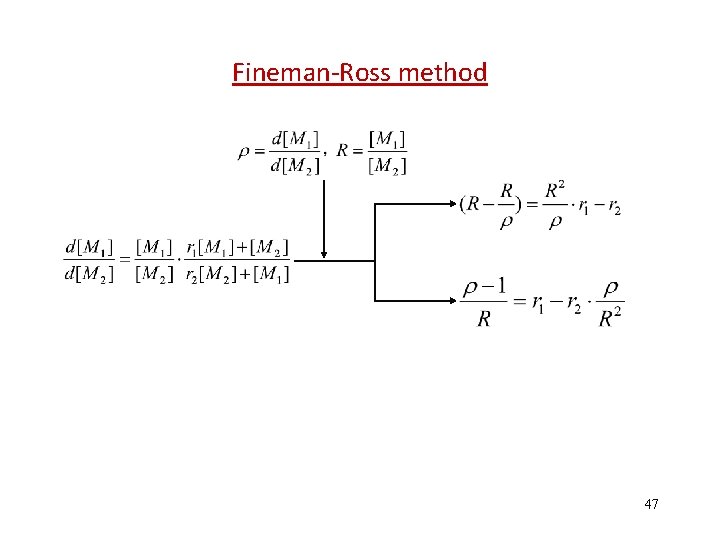
Fineman-Ross method 47

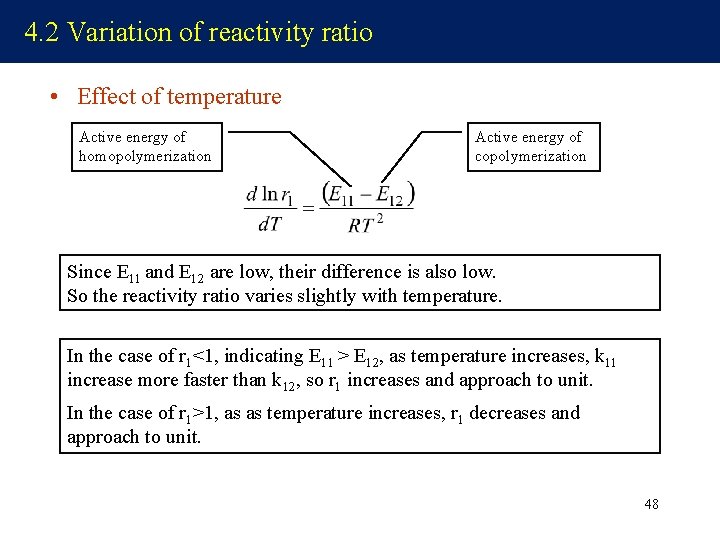
4. 2 Variation of reactivity ratio • Effect of temperature Active energy of homopolymerization Active energy of copolymerization Since E 11 and E 12 are low, their difference is also low. So the reactivity ratio varies slightly with temperature. In the case of r 1<1, indicating E 11 > E 12, as temperature increases, k 11 increase more faster than k 12, so r 1 increases and approach to unit. In the case of r 1>1, as as temperature increases, r 1 decreases and approach to unit. 48

• Effect of Pressure Same as temperature. • Effect of solvent • Effect of p. H 49

5. Monomer structure and the copolymerization reactivity 50

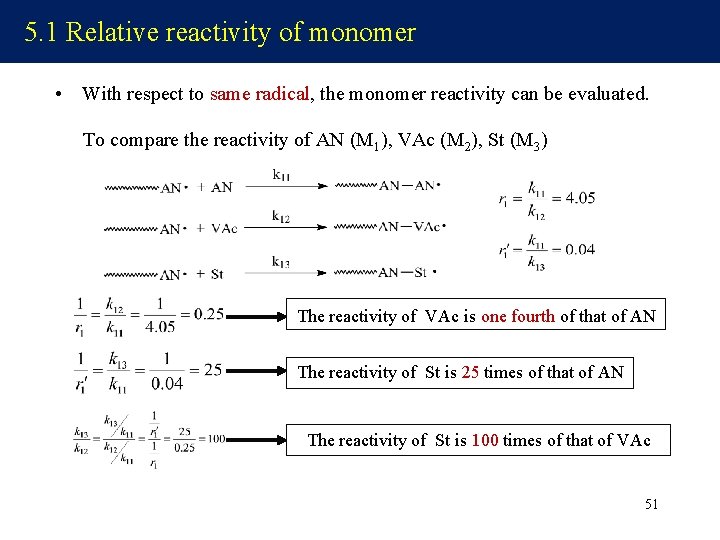
5. 1 Relative reactivity of monomer • With respect to same radical, the monomer reactivity can be evaluated. To compare the reactivity of AN (M 1), VAc (M 2), St (M 3) The reactivity of VAc is one fourth of that of AN The reactivity of St is 25 times of that of AN The reactivity of St is 100 times of that of VAc 51

The monomer reactivity decreases in the order of : St>AN>VAc (100: 4: 1) So the 1/r 1 can be applied to estimate the monomer reactivity. The larger the reciprocal of r 1, the higher the reactivity of M 1 compared to M 2. 52

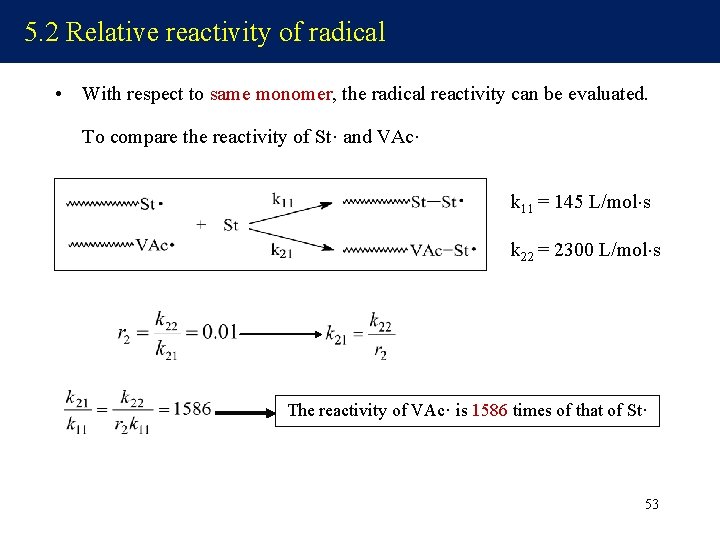
5. 2 Relative reactivity of radical • With respect to same monomer, the radical reactivity can be evaluated. To compare the reactivity of St· and VAc· k 11 = 145 L/mol s k 22 = 2300 L/mol s The reactivity of VAc· is 1586 times of that of St· 53

General rules • Monomer with low reactivity (VAc) generates corresponding radical with high reactivity(VAc ) • Monomer with high reactivity (St) generates corresponding radical with low reactivity(St ). • The rate of copolymerization is mainly determined by the reactivity of the radical 54

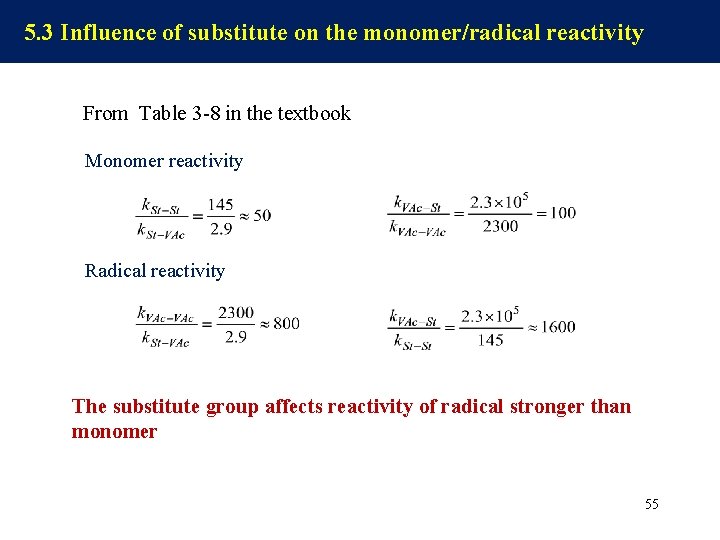
5. 3 Influence of substitute on the monomer/radical reactivity From Table 3 -8 in the textbook Monomer reactivity Radical reactivity The substitute group affects reactivity of radical stronger than monomer 55

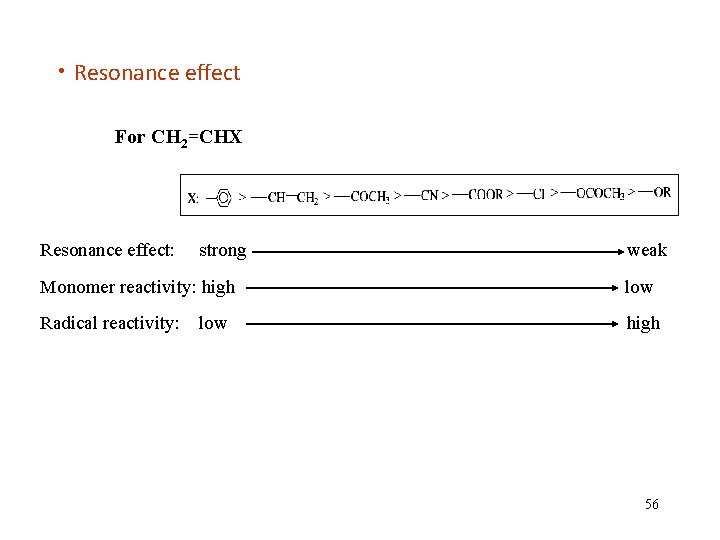
• Resonance effect For CH 2=CHX Resonance effect: strong weak Monomer reactivity: high low Radical reactivity: high low 56

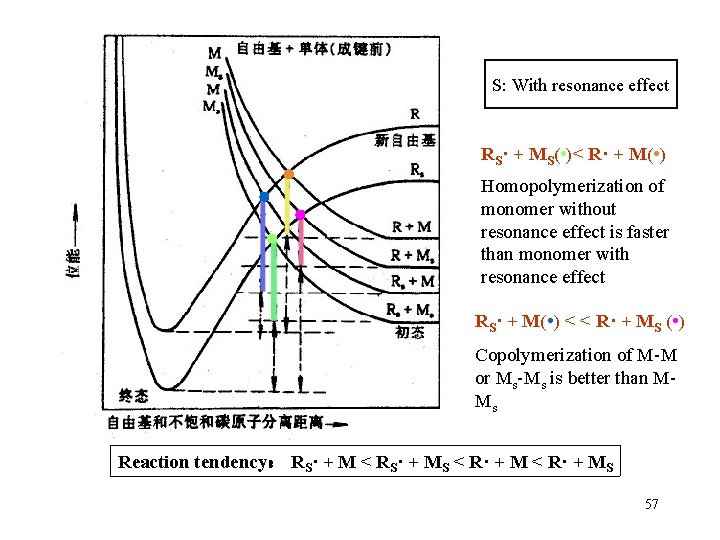
S: With resonance effect RS· + MS( • )< R· + M( • ) Homopolymerization of monomer without resonance effect is faster than monomer with resonance effect RS· + M( • ) < < R· + MS ( • ) Copolymerization of M-M or Ms-Ms is better than MMs Reaction tendency: RS· + M < RS· + MS < R· + MS 57

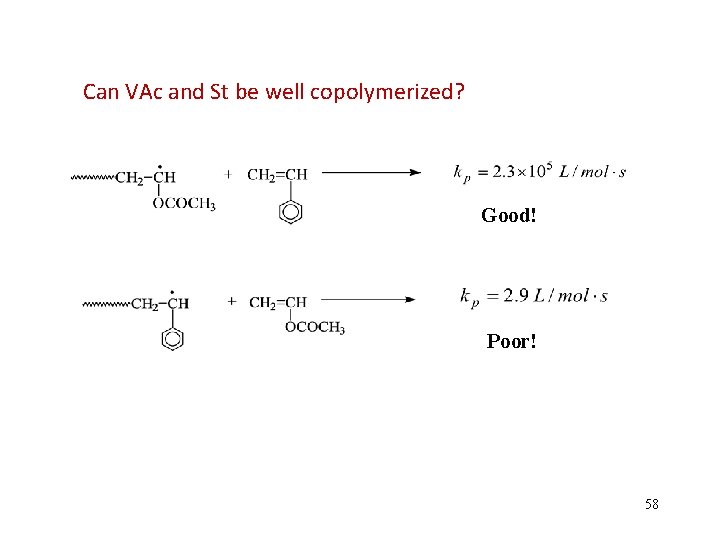
Can VAc and St be well copolymerized? Good! Poor! 58

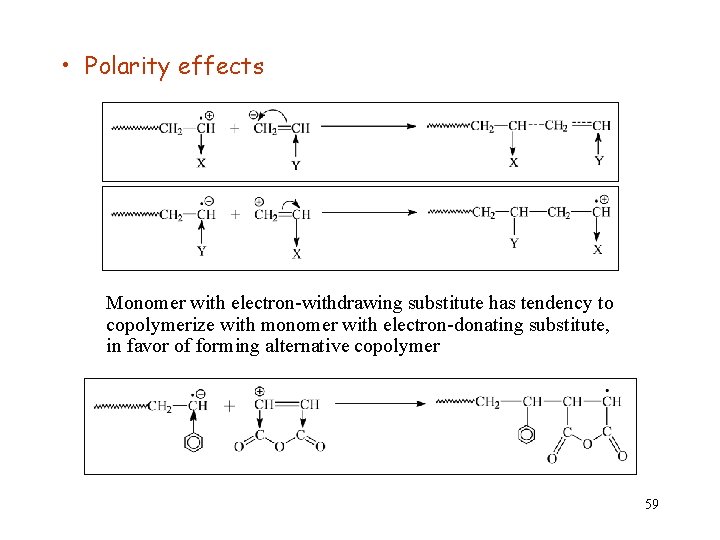
• Polarity effects Monomer with electron-withdrawing substitute has tendency to copolymerize with monomer with electron-donating substitute, in favor of forming alternative copolymer 59

Charge-transfer interaction Homopolymerization of the charge-transfer complex or donor-acceptor complex 60

• Steric effect • no influence on mono- or 1, 1 -disubstituted vinyl monomer • 1, 2 -disubstituted monomer can be copolymerized with monomer with less steric effect. Steric effect plays a more important role in homopolymerization than copolymerization. 61

6. Q-e concept 62

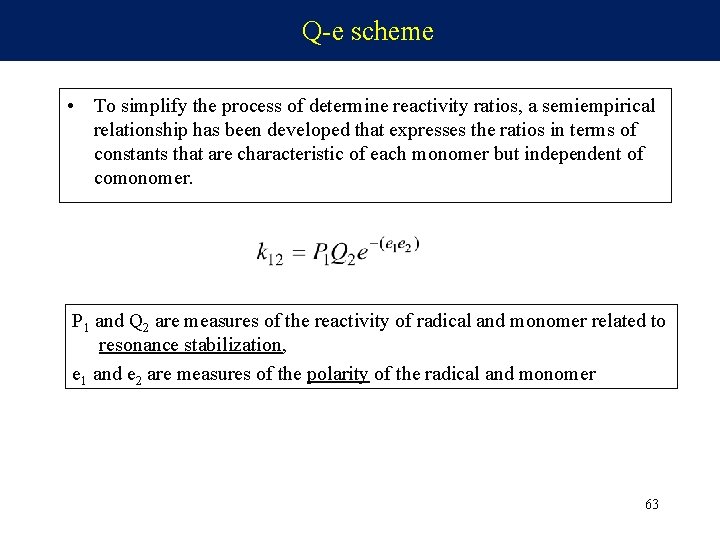
Q-e scheme • To simplify the process of determine reactivity ratios, a semiempirical relationship has been developed that expresses the ratios in terms of constants that are characteristic of each monomer but independent of comonomer. P 1 and Q 2 are measures of the reactivity of radical and monomer related to resonance stabilization, e 1 and e 2 are measures of the polarity of the radical and monomer 63

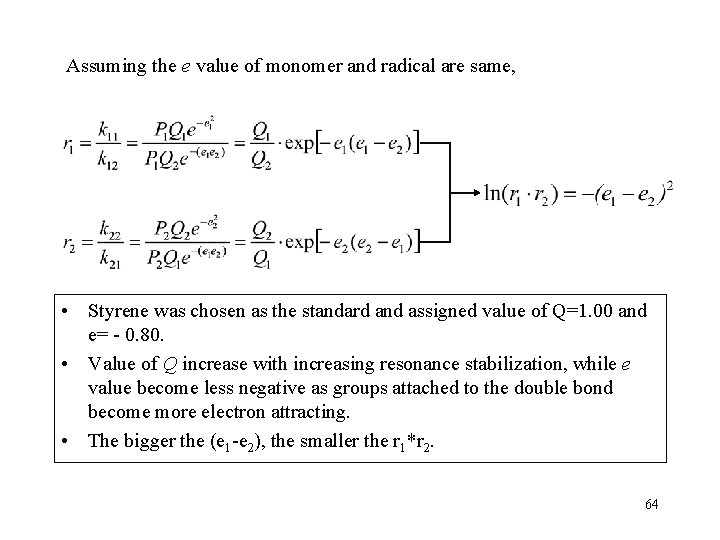
Assuming the e value of monomer and radical are same, • Styrene was chosen as the standard and assigned value of Q=1. 00 and e= - 0. 80. • Value of Q increase with increasing resonance stabilization, while e value become less negative as groups attached to the double bond become more electron attracting. • The bigger the (e 1 -e 2), the smaller the r 1*r 2. 64

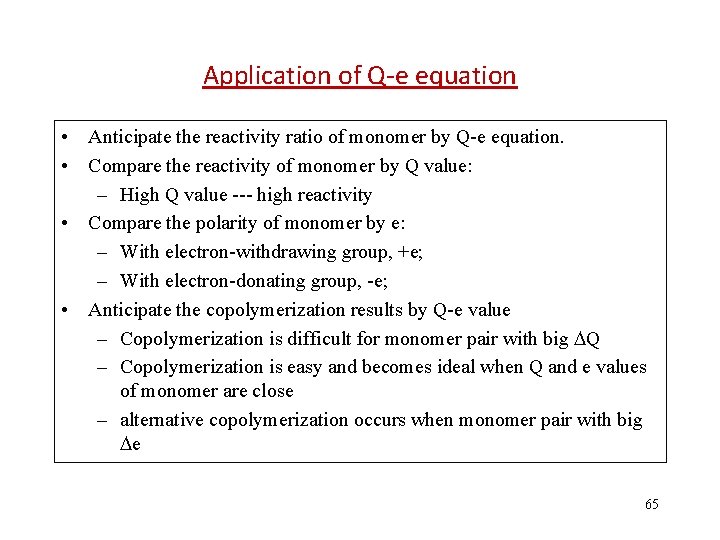
Application of Q-e equation • Anticipate the reactivity ratio of monomer by Q-e equation. • Compare the reactivity of monomer by Q value: – High Q value --- high reactivity • Compare the polarity of monomer by e: – With electron-withdrawing group, +e; – With electron-donating group, -e; • Anticipate the copolymerization results by Q-e value – Copolymerization is difficult for monomer pair with big Q – Copolymerization is easy and becomes ideal when Q and e values of monomer are close – alternative copolymerization occurs when monomer pair with big e 65

7. Copolymerization rate 66

• Chemically controlled termination • Diffusion controlled termination 67

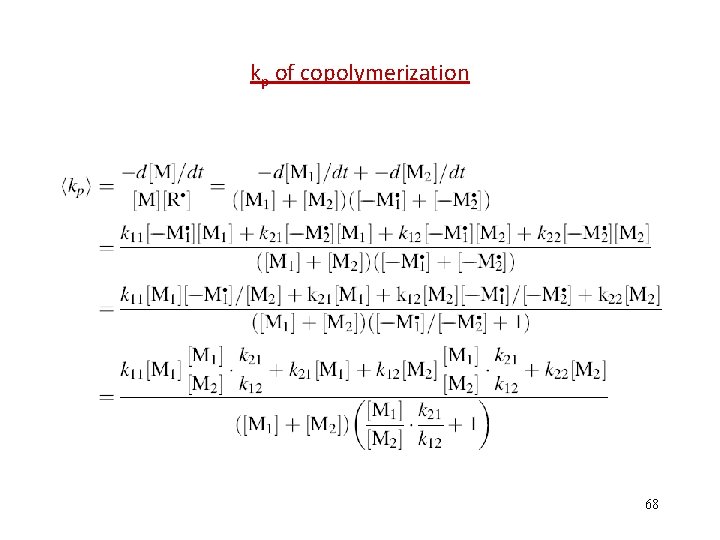
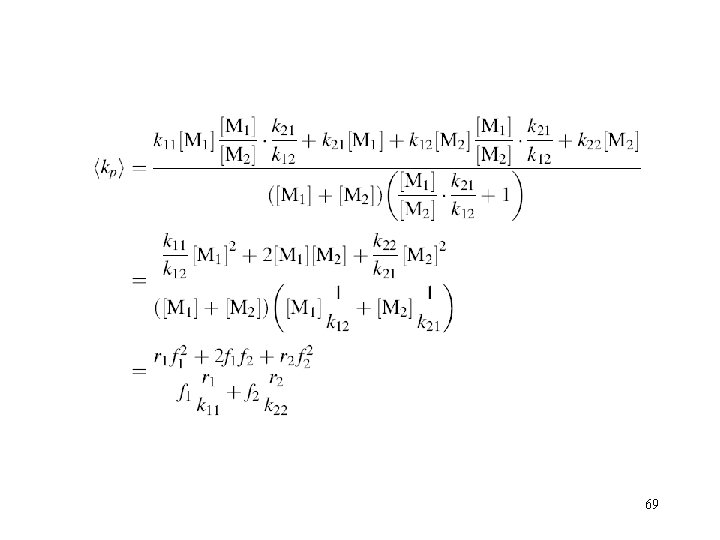
kp of copolymerization 68

69

Summary • • Nomenclature of copolymer Reactivity ratio and copolymer composition equation Copolymer composition curve Copolymer composition with conversion Control of copolymer composition Copolymer composition distribution The reactivity of monomer and radical Q-e equation 70