Chapter 3 Elements The Periodic Table VALENCE ELECTRONS

Chapter 3: Elements & The Periodic Table VALENCE ELECTRONS AND PERIODIC TABLE
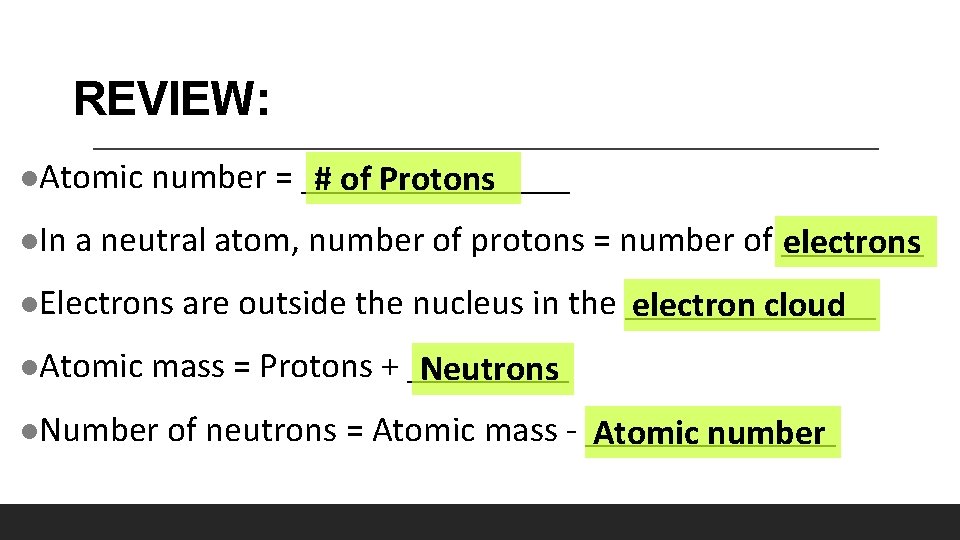
REVIEW: l. Atomic l. In number = ________ # of Protons a neutral atom, number of protons = number of ____ electrons l. Electrons l. Atomic are outside the nucleus in the _______ electron cloud mass = Protons + _____ Neutrons l. Number of neutrons = Atomic mass - _______ Atomic number
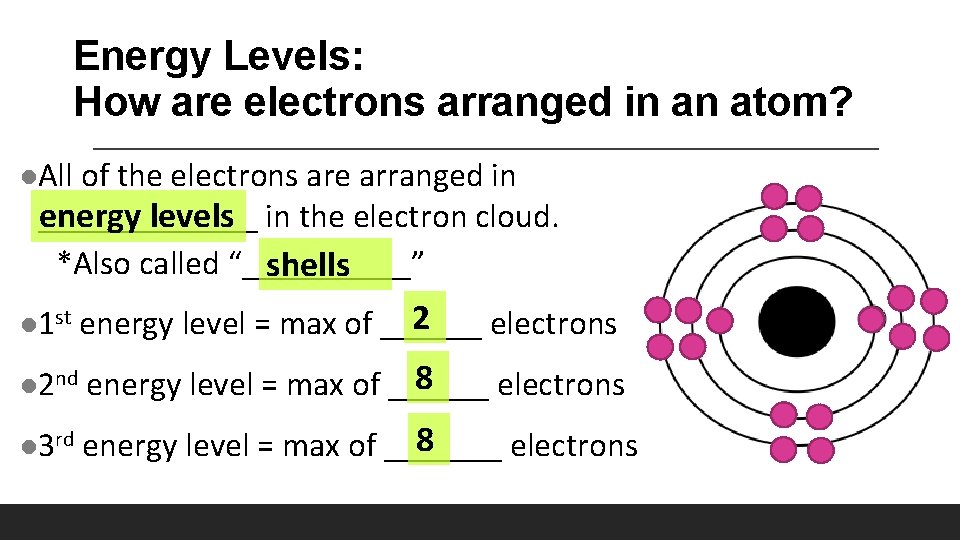
Energy Levels: How are electrons arranged in an atom? l. All of the electrons are arranged in _______ energy levels in the electron cloud. *Also called “_____” shells l 1 st 2 energy level = max of ______ electrons l 2 nd 8 energy level = max of ______ electrons l 3 rd 8 energy level = max of _______ electrons
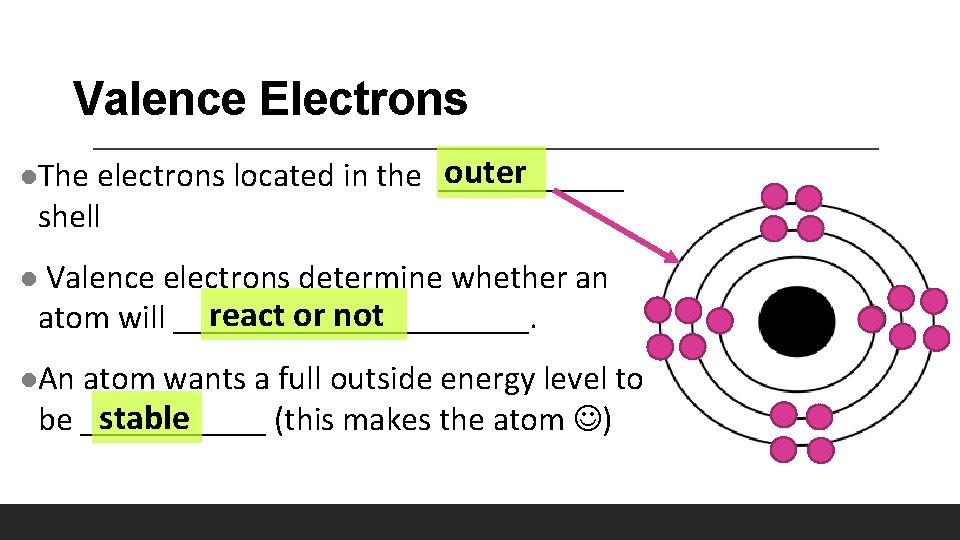
Valence Electrons outer electrons located in the ______ shell l. The Valence electrons determine whether an react or not atom will ___________. l l. An atom wants a full outside energy level to stable be ______ (this makes the atom )
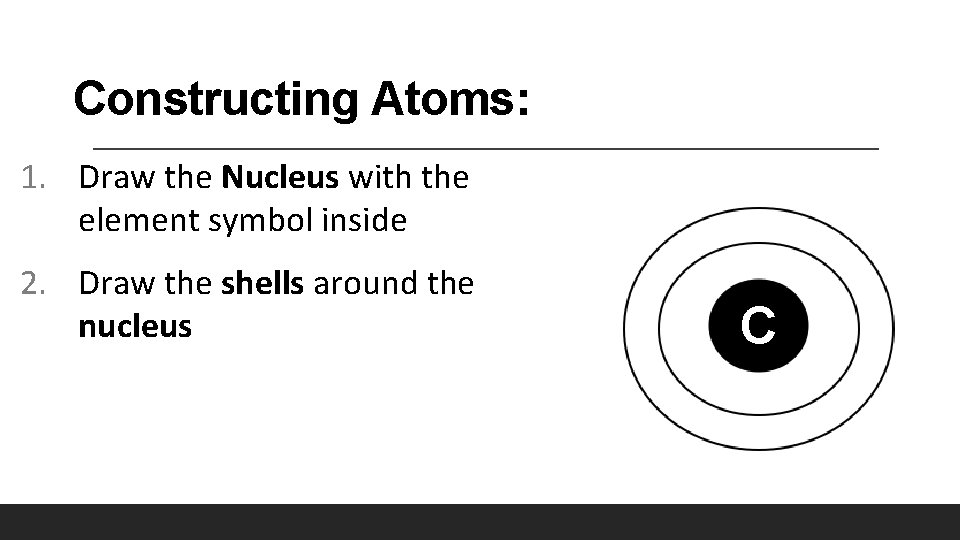
Constructing Atoms: 1. Draw the Nucleus with the element symbol inside 2. Draw the shells around the nucleus C
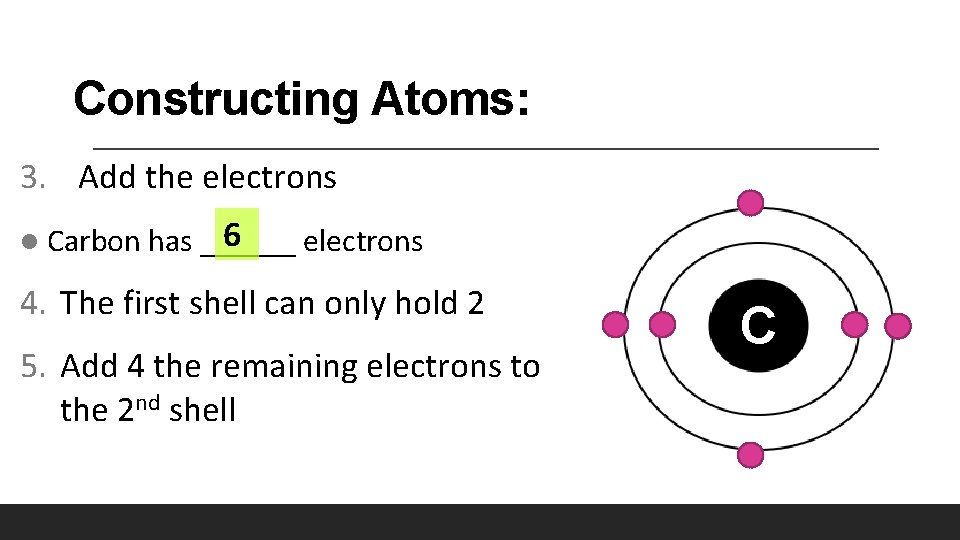
Constructing Atoms: 3. Add the electrons l 6 Carbon has ______ electrons 4. The first shell can only hold 2 5. Add 4 the remaining electrons to the 2 nd shell C
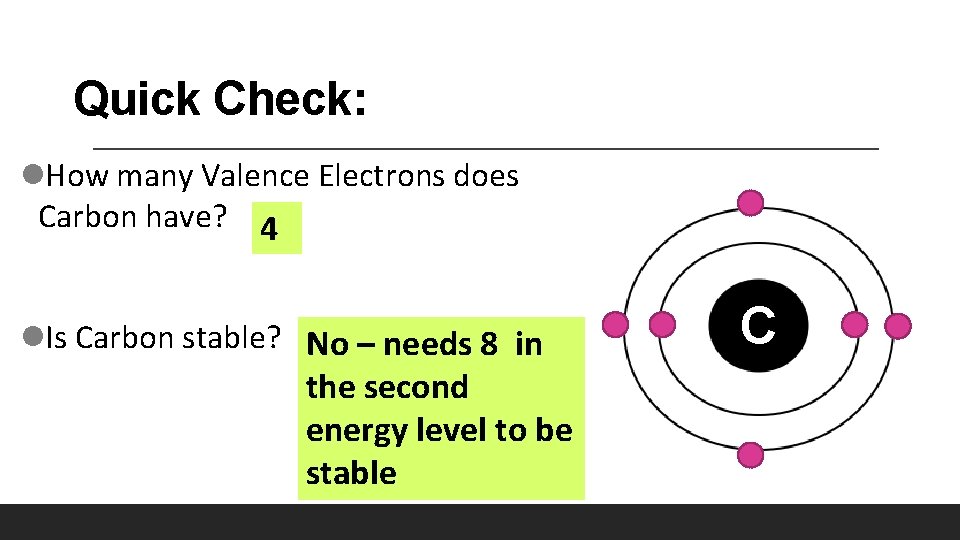
Quick Check: l. How many Valence Electrons does Carbon have? 4 l. Is Carbon stable? No – needs 8 in the second energy level to be stable C
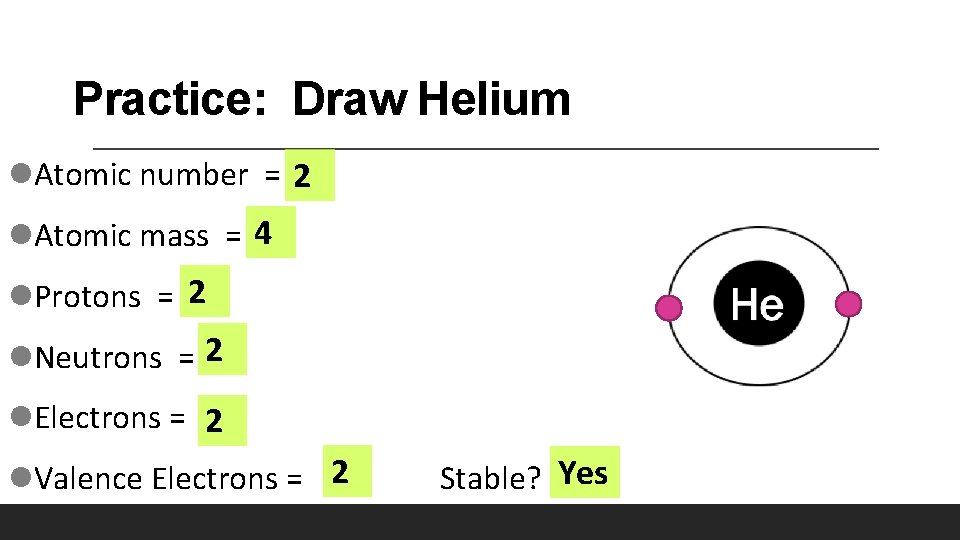
Practice: Draw Helium l. Atomic number = 2 l. Atomic mass = 4 l. Protons = 2 l. Neutrons = 2 l. Electrons = 2 l. Valence Electrons = 2 Stable? Yes
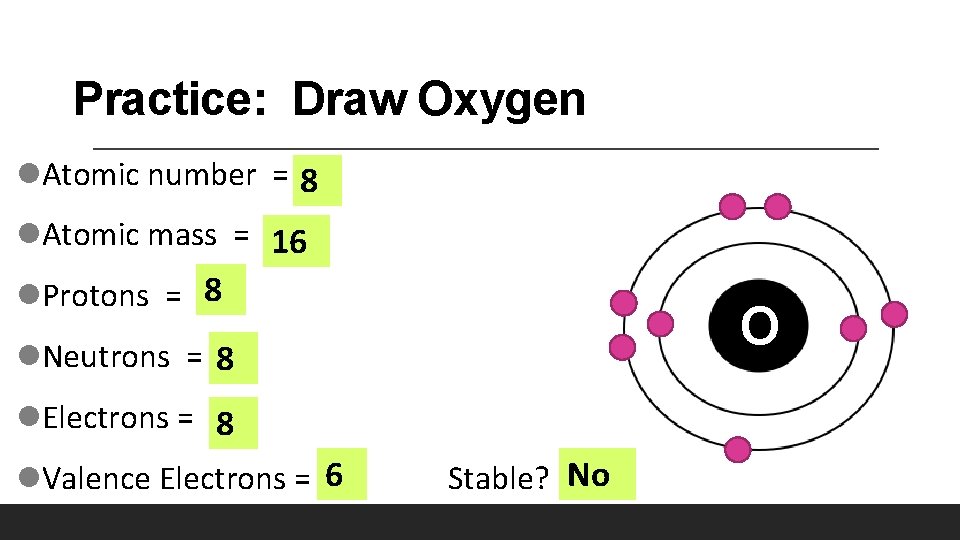
Practice: Draw Oxygen l. Atomic number = 8 l. Atomic mass = 16 l. Protons = 8 O l. Neutrons = 8 l. Electrons = 8 l. Valence Electrons = 6 Stable? No
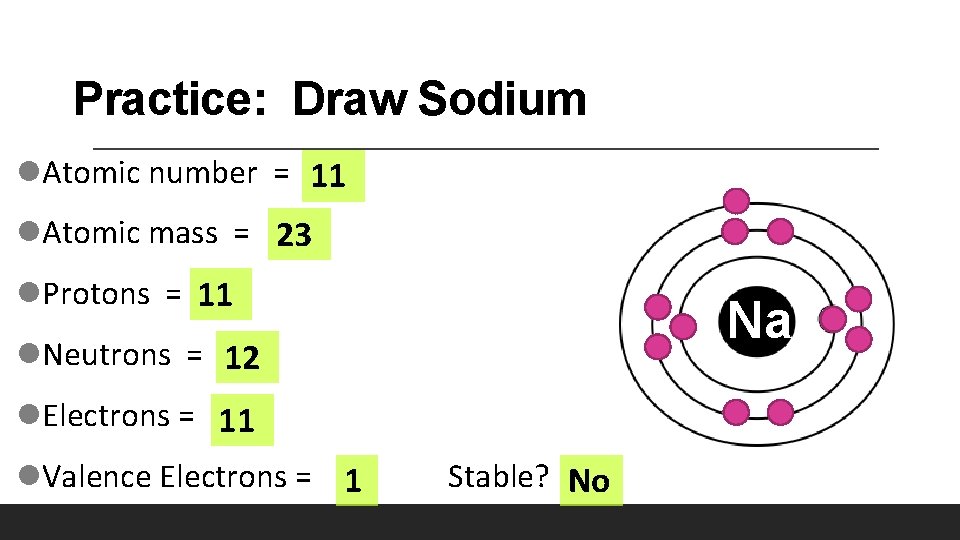
Practice: Draw Sodium l. Atomic number = 11 l. Atomic mass = 23 l. Protons = 11 Na l. Neutrons = 12 l. Electrons = 11 l. Valence Electrons = 1 Stable? No
- Slides: 10