Chapter 3 Electronic Structure and the Periodic Law



- Slides: 13

Chapter 3 Electronic Structure and the Periodic Law 3. 5 Another Look at the Periodic Table Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

Distinguishing Electrons The last and highest energy electron found in an element. l Nobel gas configurations can be used to write abbreviated electronic configurations. Instead of writing the configurations in their entirety, the symbols for the noble gases are used in brackets to represent the electrons found in their configuration. l 2

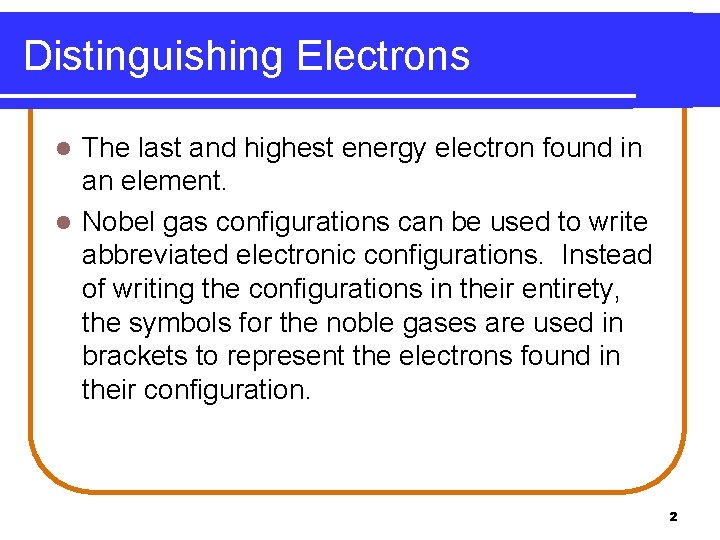
Abbreviated Electronic Configuration Form l For example, take Group 1 A(1), which contains Li, Na, K, Rb, Cs, and Fr with the electronic configuration for the first four elements shown below. Element Symbol Conventional Form Li Na K 1 s 2, 2 s 1 1 s 2, 2 s 2, 2 p 6, 3 s 1, 3 p 6, 4 s 1 Rb 1 s 2, 2 p 6, 3 s 1, 3 p 6, 4 s 2, 3 d 10, 4 p 6, 5 s 1 3

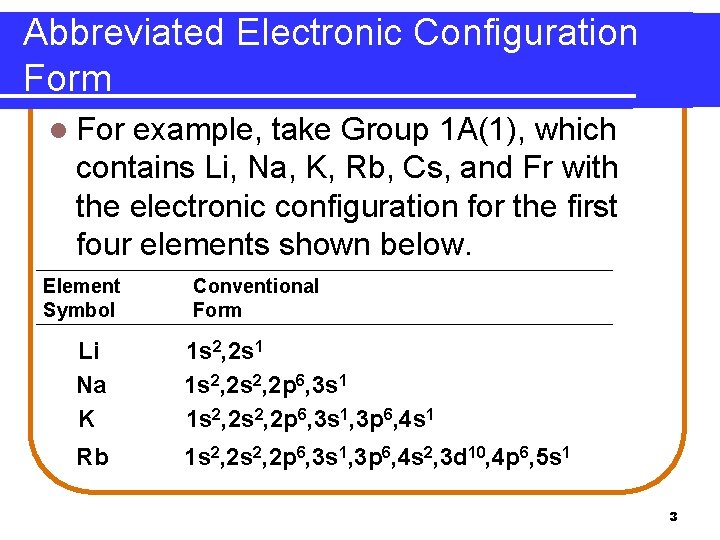
Abbreviated Electronic Configuration Form l The abbreviated form can be written using the noble gas configuration as a shorthand notation in brackets. Element Symbol Conventional Form Abbreviated Form Li 1 s 2, 2 s 1 [He]2 s 1 Na 1 s 2, 2 p 6, 3 s 1 [Ne]3 s 1 K 1 s 2, 2 p 6, 3 s 1, 3 p 6, 4 s 1 [Ar]4 s 1 Rb 1 s 2, 2 p 6, 3 s 1, 3 p 6, 4 s 2, 3 d 10, 4 p 6, 5 s 1 [Kr]5 s 1 4

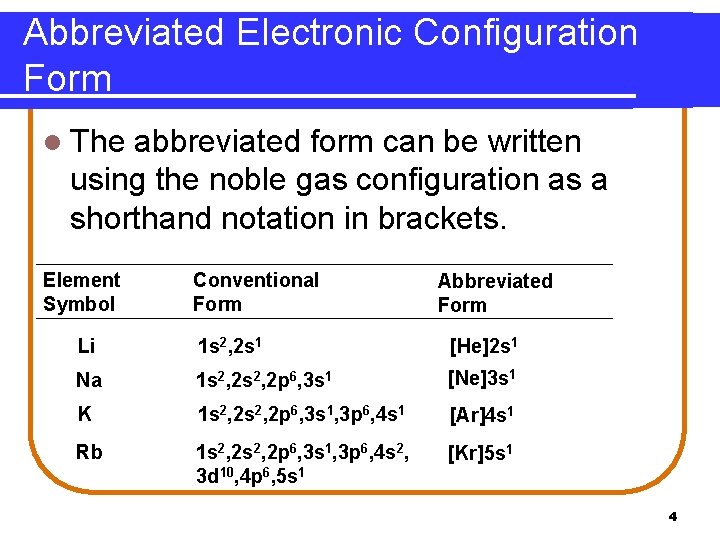
Abbreviated Electronic Configuration Form l Notice that each of these elements has a single electron in the valence shell, is located in the s subshell, and similar chemical properties of elements in the same group. Element Symbol Conventional Form Li Na K 1 s 2, 2 s 1 1 s 2, 2 s 2, 2 p 6, 3 s 1, 3 p 6, 4 s 1 Rb 1 s 2, 2 p 6, 3 s 1, 3 p 6, 4 s 2, 3 d 10, 4 p 6, 5 s 1 5

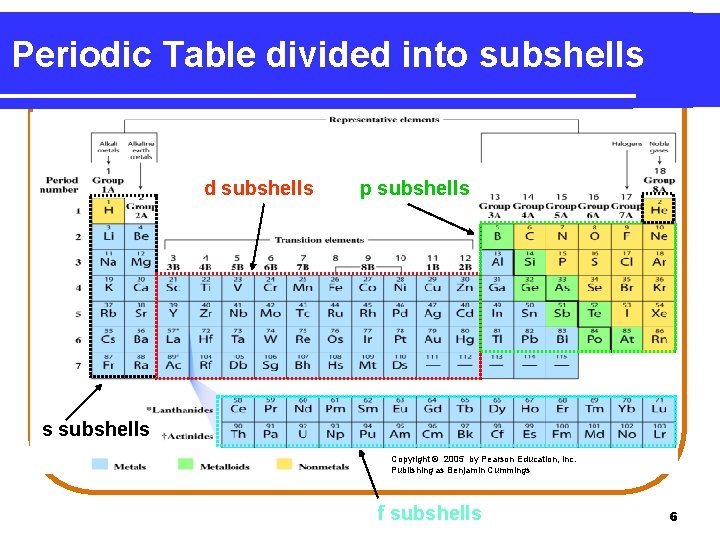
Periodic Table divided into subshells d subshells p subshells s subshells Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings f subshells 6

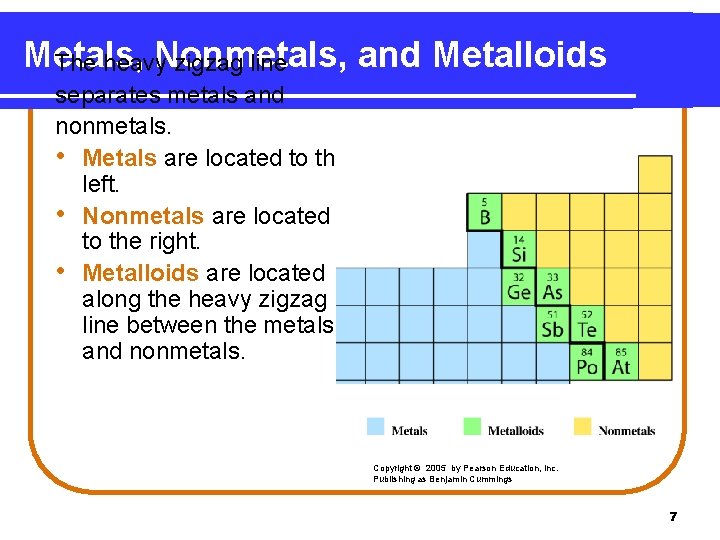
Metals, Nonmetals, and Metalloids The heavy zigzag line separates metals and nonmetals. • Metals are located to the left. • Nonmetals are located to the right. • Metalloids are located along the heavy zigzag line between the metals and nonmetals. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 7

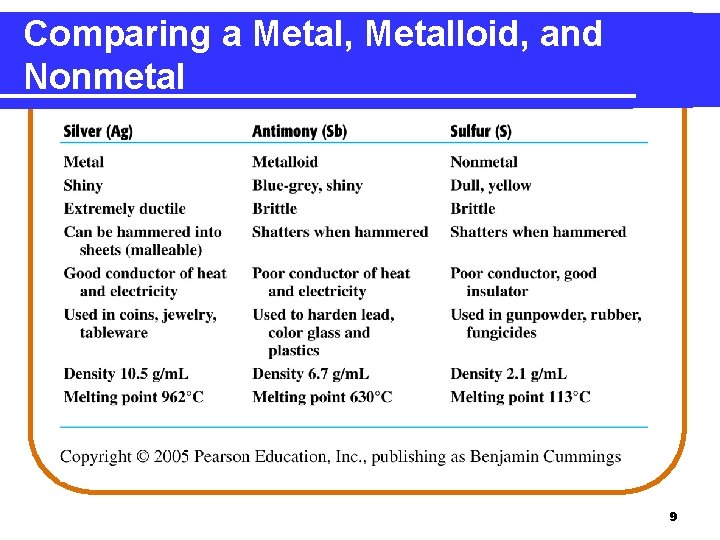
Properties of Metals, Nonmetals, and Metalloids Metals • are shiny and ductile. • are good conductors of heat and electricity. Nonmetals • are dull, brittle, and poor conductors. • are good insulators. Metalloids • are better conductors than nonmetals, but not as good as metals. • are used as semiconductors and insulators. 8

Comparing a Metal, Metalloid, and Nonmetal 9

Learning Check Identify each of the following elements as 1) metal 2) nonmetal 3) metalloid A. sodium B. chlorine C. silicon D. iron E. carbon ____ ____ 10

Solution Identify each of the following elements as 1) metal 2) nonmetal 3) metalloid A. sodium B. chlorine C. silicon D. iron E. carbon 1 metal 2 nonmetal 3 metalloid 1 metal 2 nonmetal 11

Learning Check Match the elements to the description. A. Metals in Group 4 A(14) 1) Sn, Pb 2) C, Si 3) C, Si, Ge, Sn B. Nonmetals in Group 5 A(15) 1) As, Sb, Bi 2) N, P 3) N, P, As, Sb C. Metalloids in Group 4 A(14) 1) C, Si, Ge, 2) Si, Ge 3) Si, Ge, Sn, Pb 12

Solution Match the elements to the description. A. Metals in Group 4 A (14) 1) Sn, Pb B. Nonmetals in Group 5 A(15) 2) N, P C. Metalloids in Group 4 A(14) 2) Si, Ge 13