Chapter 3 Ecosystems What Are They and How




















- Slides: 45

Chapter 3 Ecosystems: What Are They and How Do They Work?

LT 5

THE NATURE OF ECOLOGY Ø Ecology is a study of connections in nature. l How organisms interact with one another and with their nonliving environment. Figure 3 -2

Organisms and Species Ø Organisms, the different forms of life on earth, can be classified into different species based on certain characteristics. Figure 3 -3

Classification of Life Ø Domain Ø Kingdom Ø Phylum Ø Class Ø Order Ø Family Ø Genus Ø Species

Populations, Communities, and Ecosystems Ø Members of a species interact in groups called populations. Ø Populations of different species living and interacting in an area form a community. Ø A community interacting with its physical environment of matter and energy is an ecosystem.

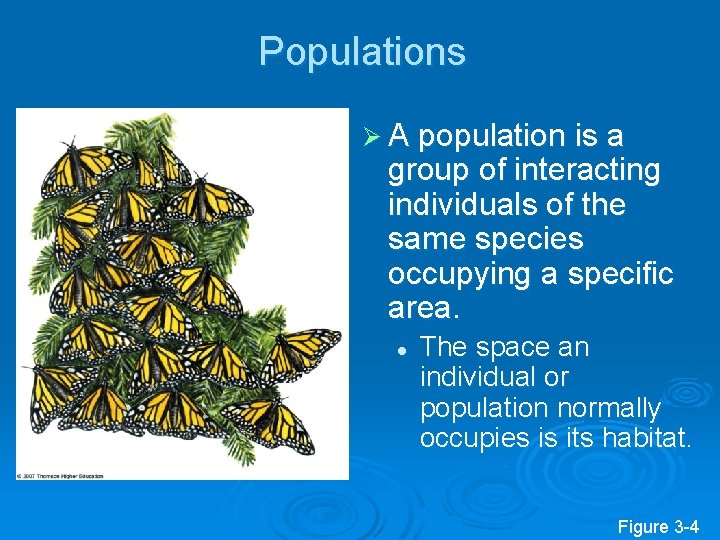
Populations Ø A population is a group of interacting individuals of the same species occupying a specific area. l The space an individual or population normally occupies is its habitat. Figure 3 -4

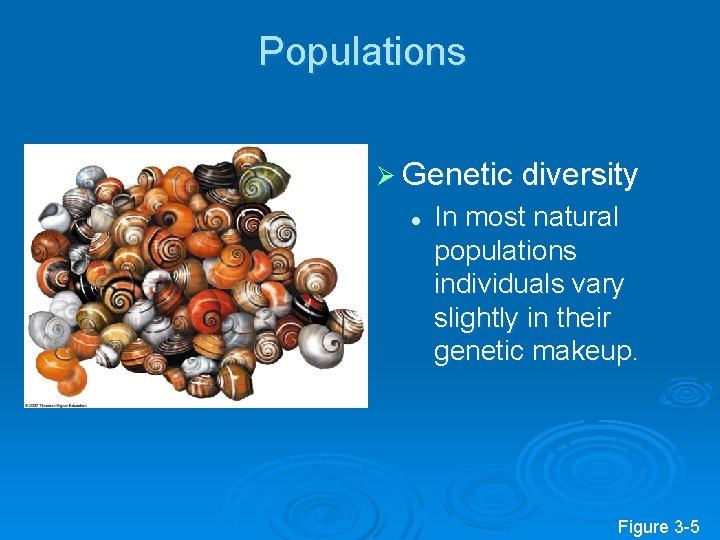
Populations Ø Genetic diversity l In most natural populations individuals vary slightly in their genetic makeup. Figure 3 -5

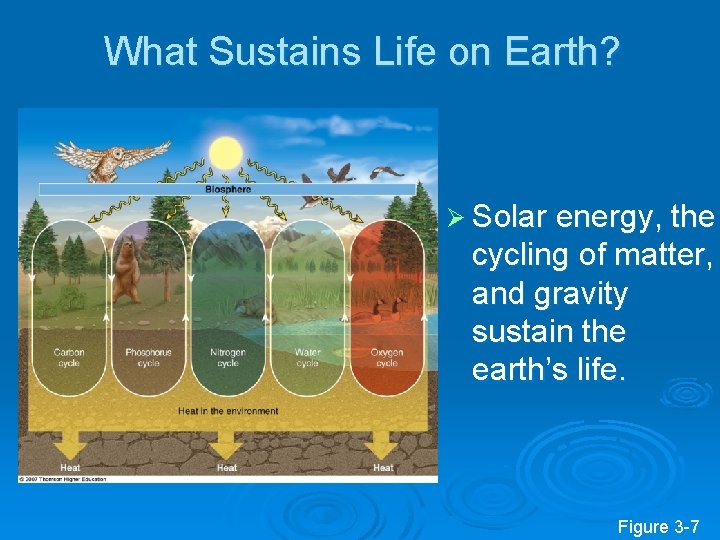
What Sustains Life on Earth? Ø Solar energy, the cycling of matter, and gravity sustain the earth’s life. Figure 3 -7

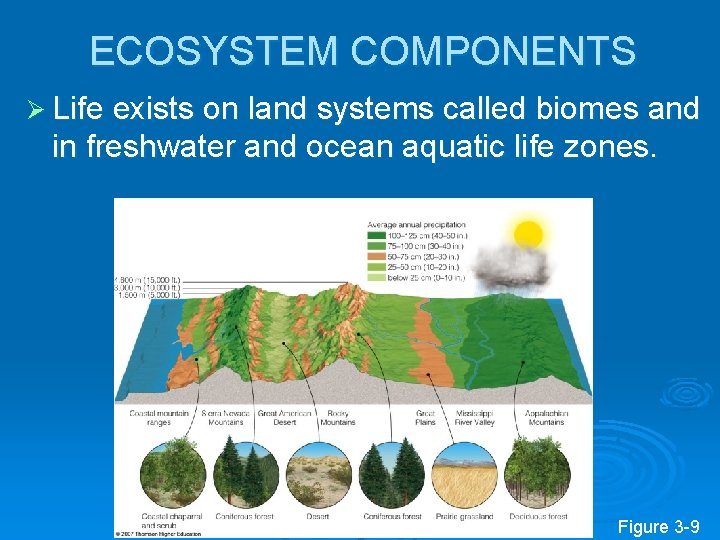
ECOSYSTEM COMPONENTS Ø Life exists on land systems called biomes and in freshwater and ocean aquatic life zones. Figure 3 -9

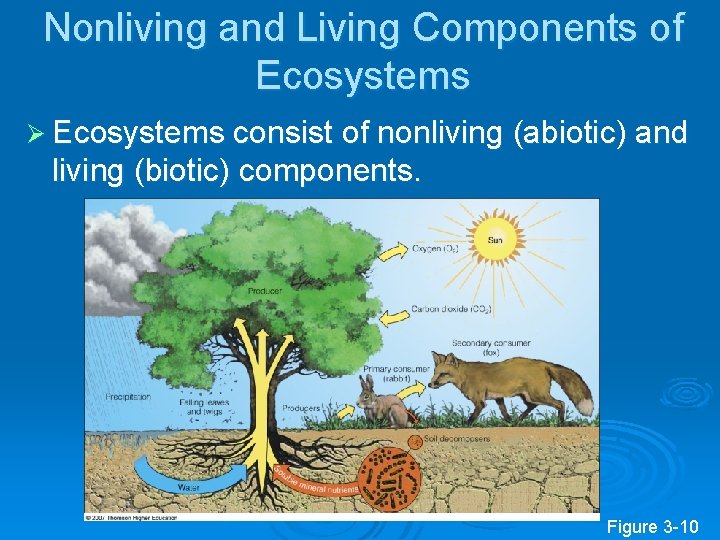
Nonliving and Living Components of Ecosystems Ø Ecosystems consist of nonliving (abiotic) and living (biotic) components. Figure 3 -10

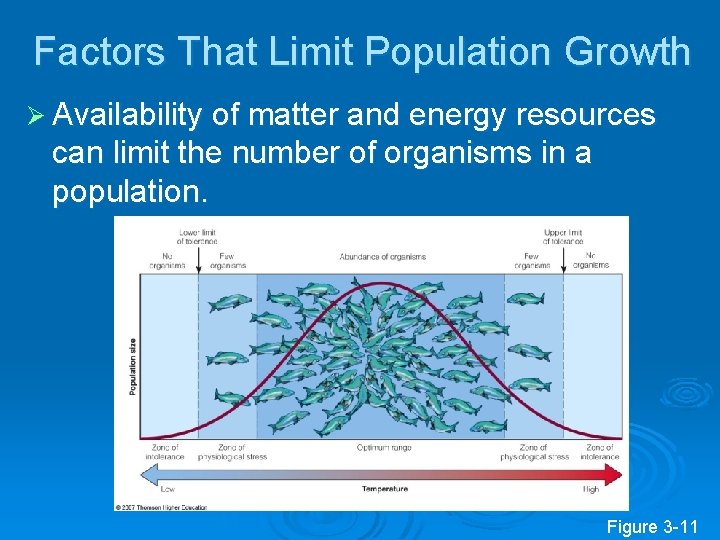
Factors That Limit Population Growth Ø Availability of matter and energy resources can limit the number of organisms in a population. Figure 3 -11

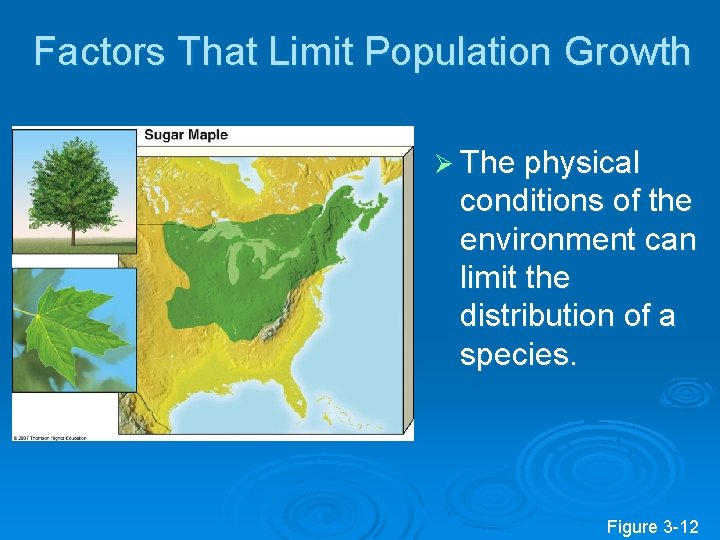
Factors That Limit Population Growth Ø The physical conditions of the environment can limit the distribution of a species. Figure 3 -12

LT 6

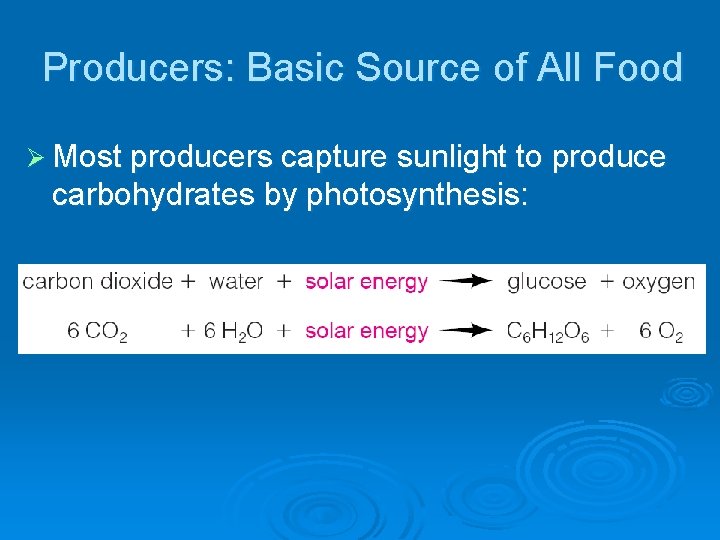
Producers: Basic Source of All Food Ø Most producers capture sunlight to produce carbohydrates by photosynthesis:

Producers: Basic Source of All Food Ø Chemosynthesis: l Some organisms such as deep ocean bacteria draw energy from hydrothermal vents and produce carbohydrates from hydrogen sulfide (H 2 S) gas.

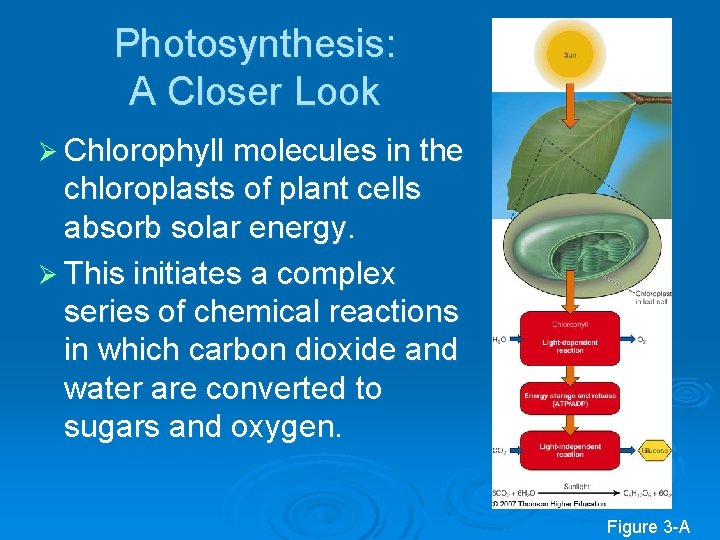
Photosynthesis: A Closer Look Ø Chlorophyll molecules in the chloroplasts of plant cells absorb solar energy. Ø This initiates a complex series of chemical reactions in which carbon dioxide and water are converted to sugars and oxygen. Figure 3 -A

Consumers: Eating and Recycling to Survive Ø Consumers (heterotrophs) get their food by eating or breaking down all or parts of other organisms or their remains. l Herbivores • Primary consumers that eat producers l Carnivores • Primary consumers eat primary consumers • Third and higher level consumers: carnivores that eat carnivores. l Omnivores • Feed on both plant and animals.

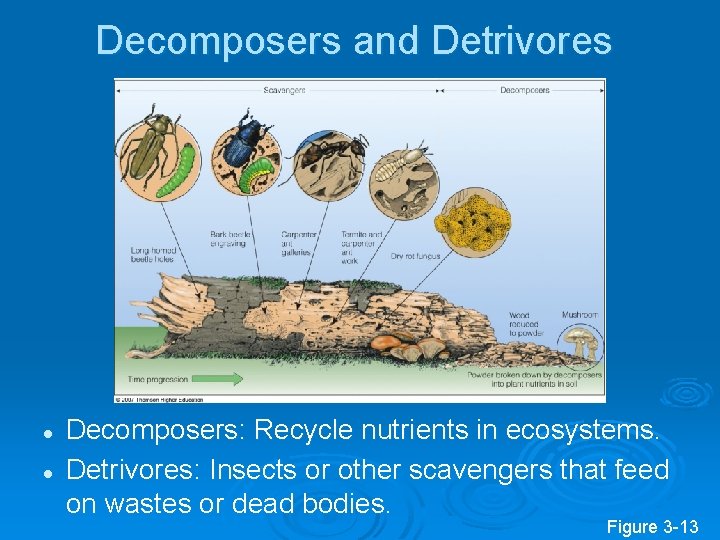
Decomposers and Detrivores l l Decomposers: Recycle nutrients in ecosystems. Detrivores: Insects or other scavengers that feed on wastes or dead bodies. Figure 3 -13

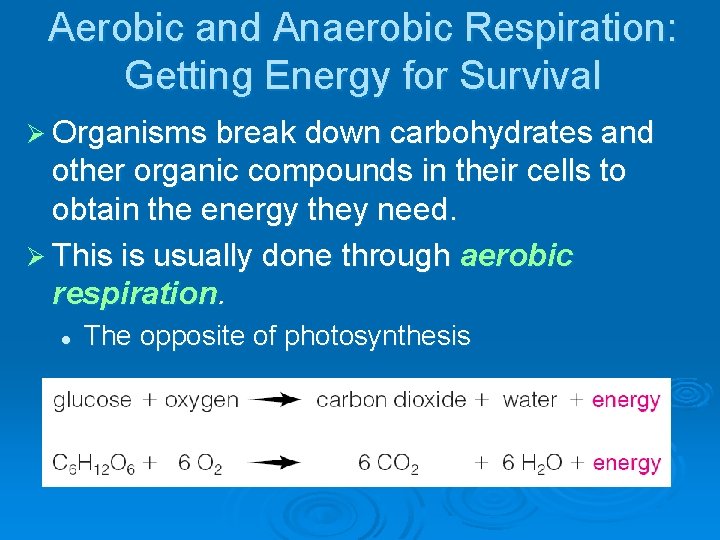
Aerobic and Anaerobic Respiration: Getting Energy for Survival Ø Organisms break down carbohydrates and other organic compounds in their cells to obtain the energy they need. Ø This is usually done through aerobic respiration. l The opposite of photosynthesis

Aerobic and Anaerobic Respiration: Getting Energy for Survival Ø Anaerobic respiration or fermentation: l l Some decomposers get energy by breaking down glucose (or other organic compounds) in the absence of oxygen. The end products vary based on the chemical reaction: • • Methane gas Ethyl alcohol Acetic acid Hydrogen sulfide

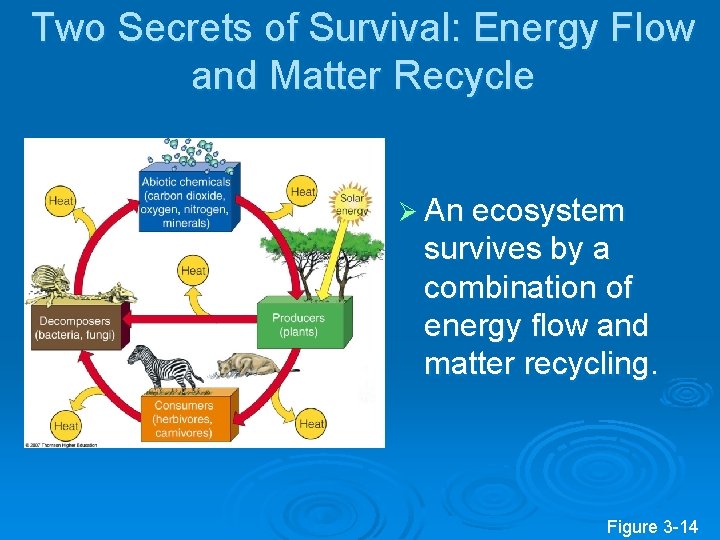
Two Secrets of Survival: Energy Flow and Matter Recycle Ø An ecosystem survives by a combination of energy flow and matter recycling. Figure 3 -14

LT 2

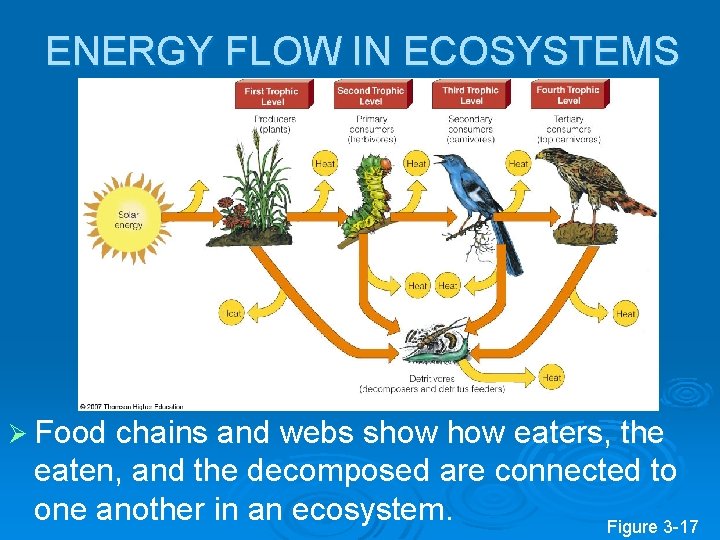
ENERGY FLOW IN ECOSYSTEMS Ø Food chains and webs show eaters, the eaten, and the decomposed are connected to one another in an ecosystem. Figure 3 -17

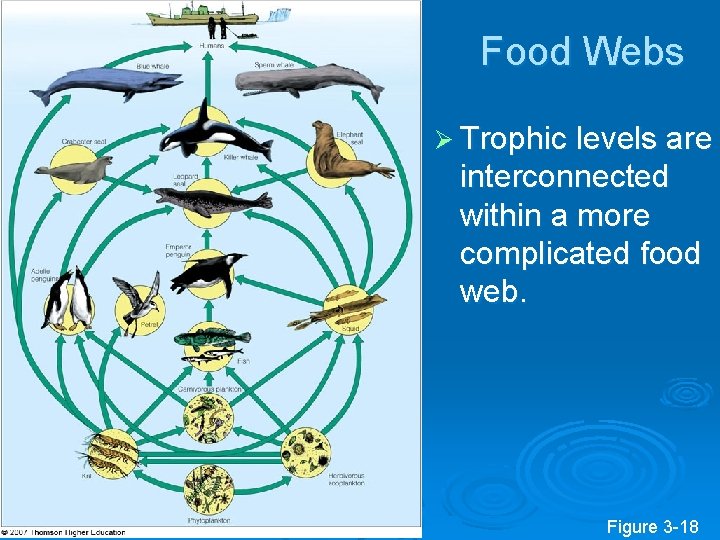
Food Webs Ø Trophic levels are interconnected within a more complicated food web. Figure 3 -18

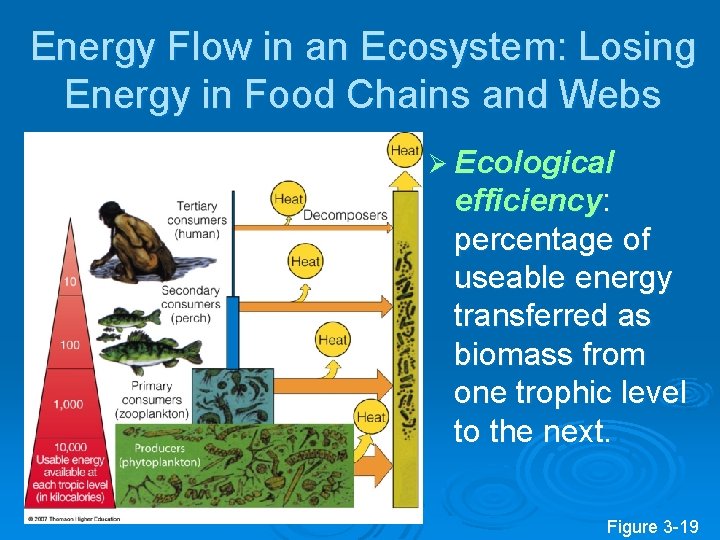
Energy Flow in an Ecosystem: Losing Energy in Food Chains and Webs Ø In accordance with the 2 nd law of thermodynamics, there is a decrease in the amount of energy available to each succeeding organism in a food chain or web.

Energy Flow in an Ecosystem: Losing Energy in Food Chains and Webs Ø Ecological efficiency: percentage of useable energy transferred as biomass from one trophic level to the next. Figure 3 -19

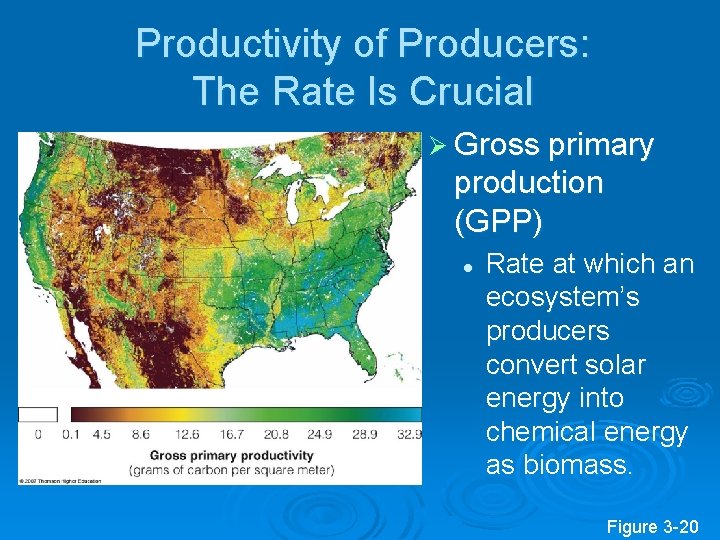
Productivity of Producers: The Rate Is Crucial Ø Gross primary production (GPP) l Rate at which an ecosystem’s producers convert solar energy into chemical energy as biomass. Figure 3 -20

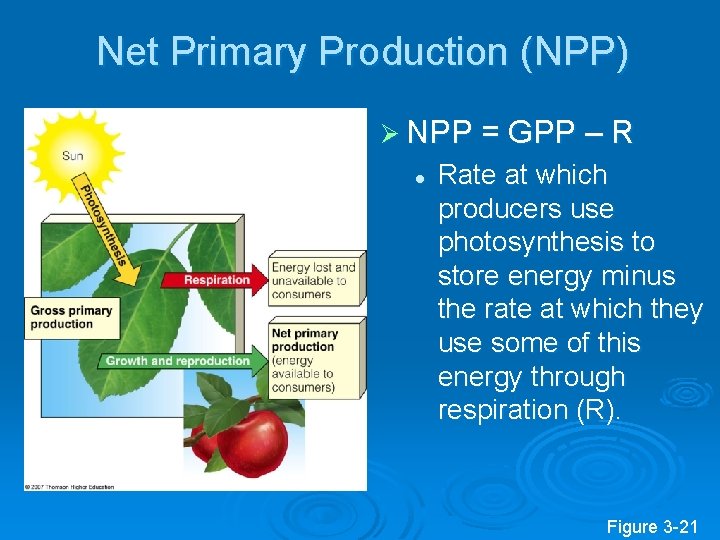
Net Primary Production (NPP) Ø NPP = GPP – R l Rate at which producers use photosynthesis to store energy minus the rate at which they use some of this energy through respiration (R). Figure 3 -21

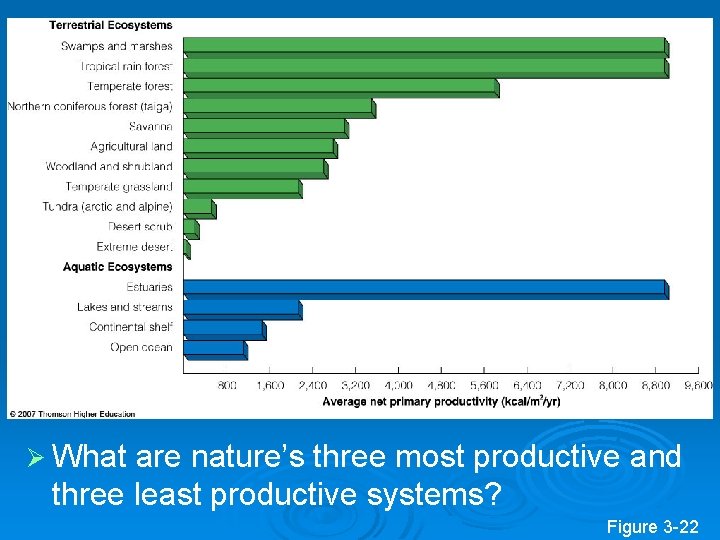
Ø What are nature’s three most productive and three least productive systems? Figure 3 -22

LT 9

MATTER CYCLING IN ECOSYSTEMS Ø Nutrient Cycles: Global Recycling l l l Global Cycles recycle nutrients through the earth’s air, land, water, and living organisms. Nutrients are the elements and compounds that organisms need to live, grow, and reproduce. Biogeochemical cycles move these substances through air, water, soil, rock and living organisms.

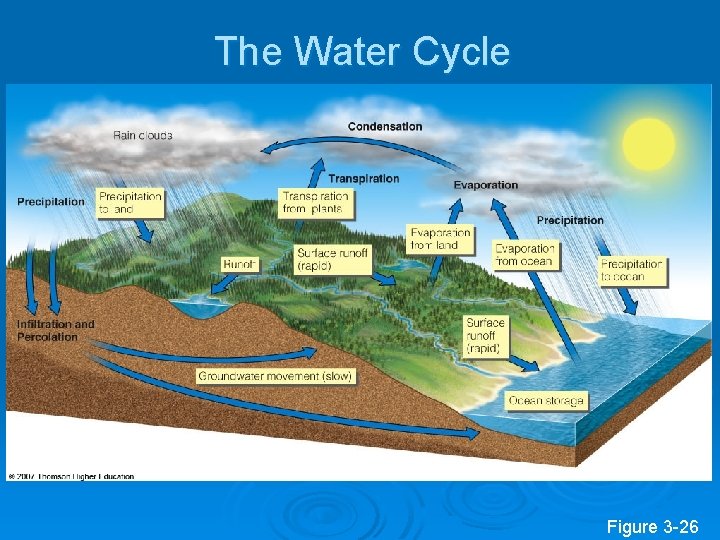
The Water Cycle Figure 3 -26

Water’ Unique Properties Ø There are strong forces of attraction between molecules of water. Ø Water exists as a liquid over a wide temperature range. Ø Liquid water changes temperature slowly. Ø It takes a large amount of energy for water to evaporate. Ø Liquid water can dissolve a variety of compounds. Ø Water expands when it freezes.

Effects of Human Activities on Water Cycle Ø We alter the water cycle by: l l Withdrawing large amounts of freshwater. Clearing vegetation and eroding soils. Polluting surface and underground water. Contributing to climate change.

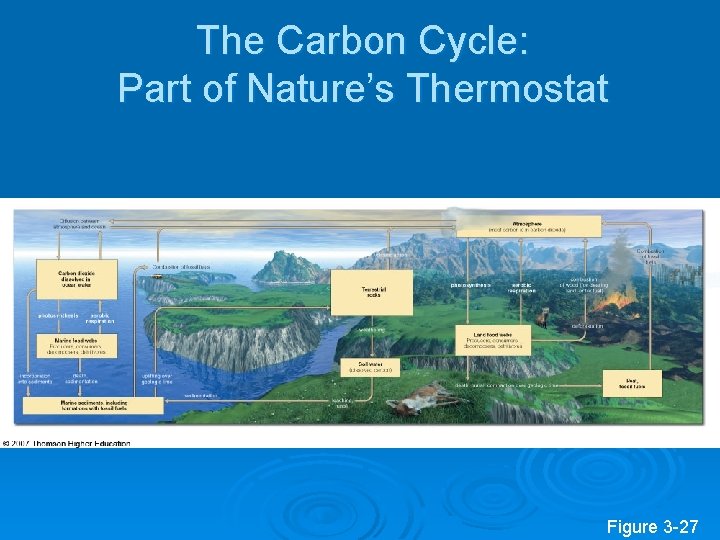
The Carbon Cycle: Part of Nature’s Thermostat Figure 3 -27

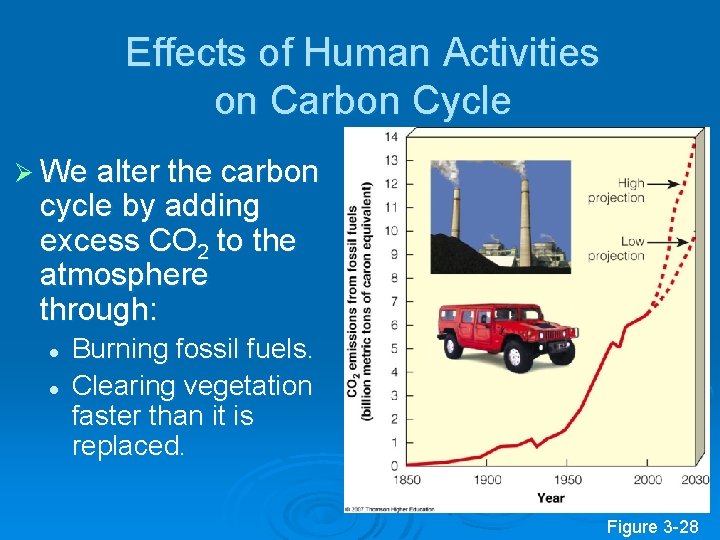
Effects of Human Activities on Carbon Cycle Ø We alter the carbon cycle by adding excess CO 2 to the atmosphere through: l l Burning fossil fuels. Clearing vegetation faster than it is replaced. Figure 3 -28

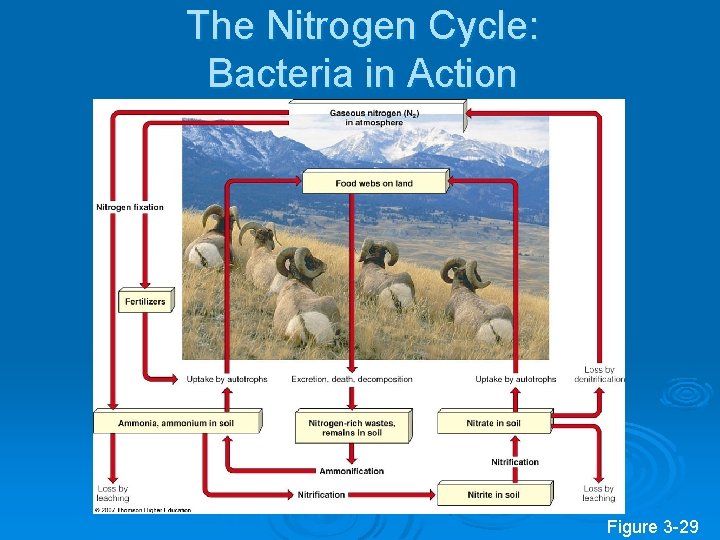
The Nitrogen Cycle: Bacteria in Action Figure 3 -29

Effects of Human Activities on the Nitrogen Cycle Ø We alter the nitrogen cycle by: l l Adding gases that contribute to acid rain. Adding nitrous oxide to the atmosphere through farming practices which can warm the atmosphere and deplete ozone. Contaminating ground water from nitrate ions in inorganic fertilizers. Releasing nitrogen into the troposphere through deforestation.

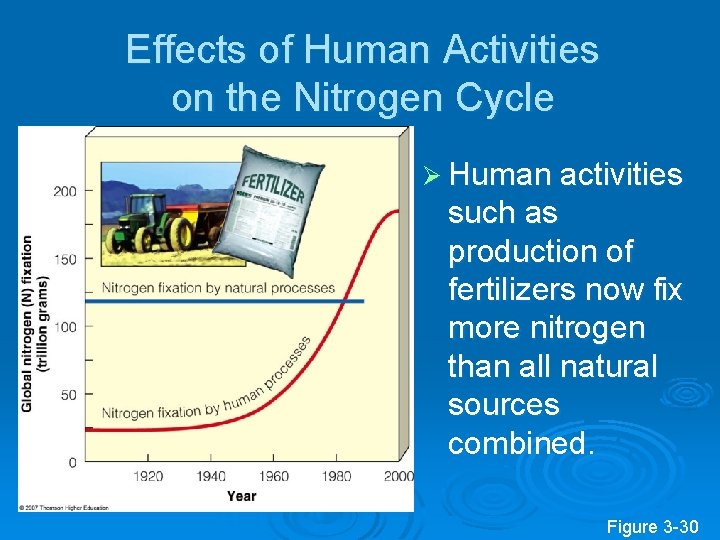
Effects of Human Activities on the Nitrogen Cycle Ø Human activities such as production of fertilizers now fix more nitrogen than all natural sources combined. Figure 3 -30

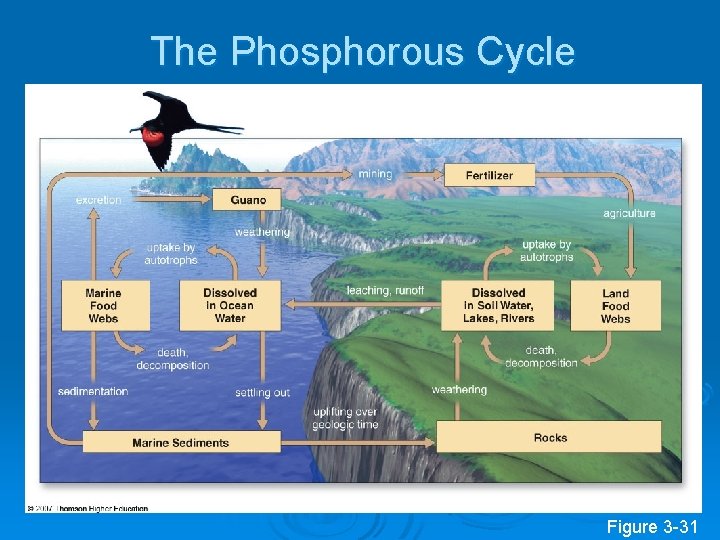
The Phosphorous Cycle Figure 3 -31

Effects of Human Activities on the Phosphorous Cycle Ø We remove large amounts of phosphate from the earth to make fertilizer. Ø We reduce phosphorous in tropical soils by clearing forests. Ø We add excess phosphates to aquatic systems from runoff of animal wastes and fertilizers.

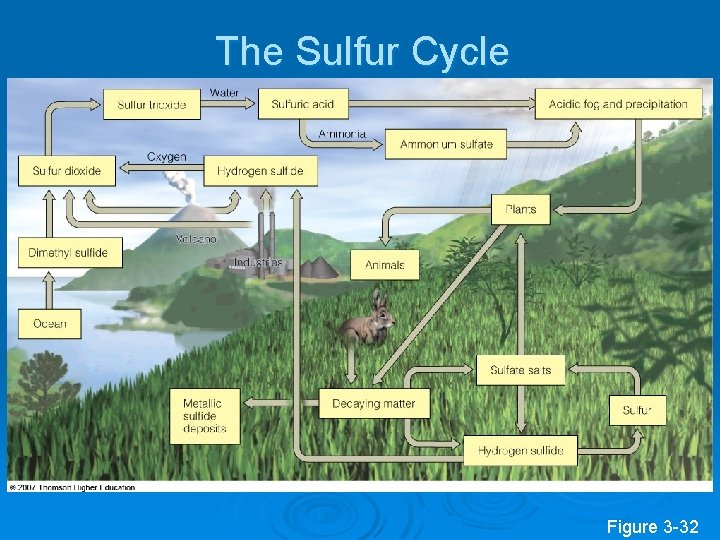
The Sulfur Cycle Figure 3 -32

Effects of Human Activities on the Sulfur Cycle Ø We add sulfur dioxide to the atmosphere by: l l l Burning coal and oil Refining sulfur containing petroleum. Convert sulfur-containing metallic ores into free metals such as copper, lead, and zinc releasing sulfur dioxide into the environment.

The Gaia Hypothesis: Is the Earth Alive? Ø Some have proposed that the earth’s various forms of life control or at least influence its chemical cycles and other earth-sustaining processes. l l The strong Gaia hypothesis: life controls the earth’s life-sustaining processes. The weak Gaia hypothesis: life influences the earth’s life-sustaining processes.