Chapter 3 Different forms of energy I WHAT




- Slides: 21

Chapter 3: Different forms of energy I. WHAT IS ENERGY? i. The law of conservation of energy ii. Energy efficiency iii. Thermal energy iv. Kinetic energy v. Potential energy vi. Mechanical energy

I. What is Energy? = the ability to do work or effect change �Unit : 1 Joules (J) = the energy transferred to an object when a force of one newton (N) acts on an object through a distance of one meter (m) �In many different forms

Forms of Energy 1. Elastic energy From compression or extension

Forms of Energy 2. Electrical Energy From the ordered movement of electrons from one atom to another

Forms of Energy 3. Thermal Energy From the random motion of all particles in a substance

Forms of Energy 4. Radiation energy o Transported by electromagnetic waves

Forms of Energy 5. Chemical Energy Stored in molecular bonds

Forms of Energy 6. Wind energy From the movement of air.

Forms of Energy 7. Sound energy Transported by sound waves

Forms of Energy 8. Hydraulic energy From the flow of water

Forms of Energy 9. Nuclear energy Stored in atomic nuclei

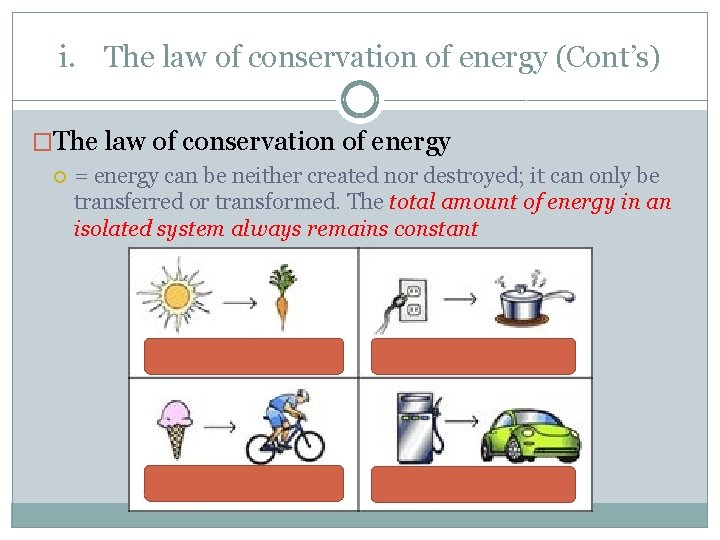
i. The law of conservation of energy (Cont’s) �The law of conservation of energy = energy can be neither created nor destroyed; it can only be transferred or transformed. The total amount of energy in an isolated system always remains constant

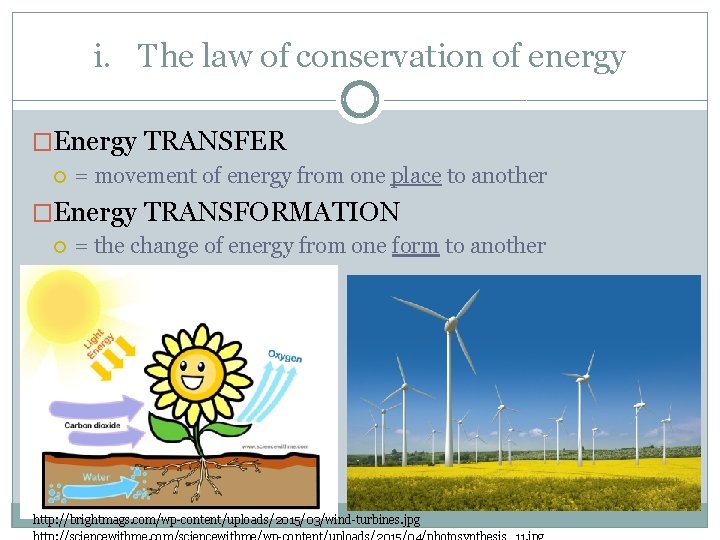
i. The law of conservation of energy �Energy TRANSFER = movement of energy from one place to another �Energy TRANSFORMATION = the change of energy from one form to another http: //brightmags. com/wp-content/uploads/2015/03/wind-turbines. jpg

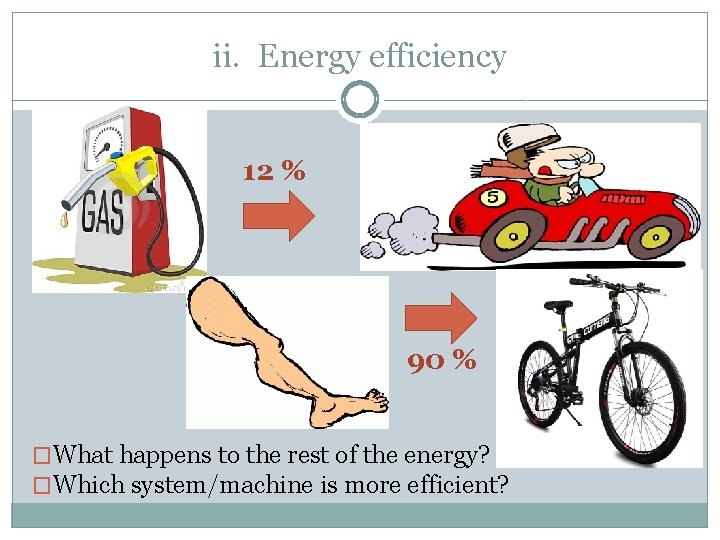
ii. Energy efficiency 12 % 90 % �What happens to the rest of the energy? �Which system/machine is more efficient?

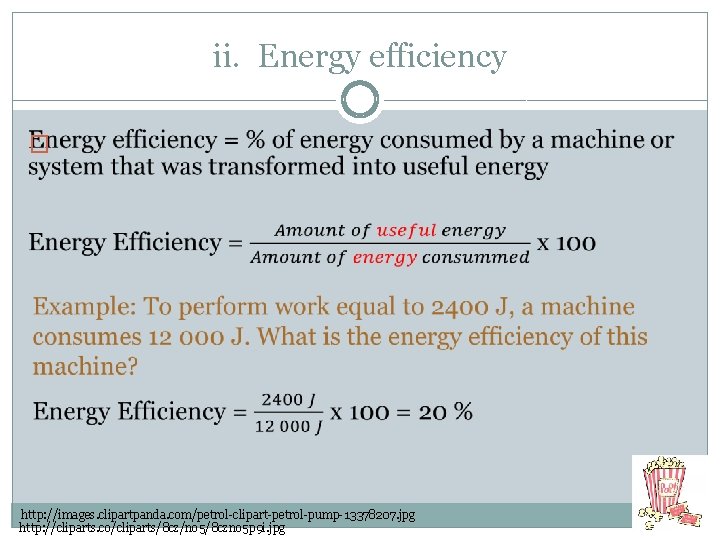
ii. Energy efficiency � http: //images. clipartpanda. com/petrol-clipart-petrol-pump-13378207. jpg http: //cliparts. co/cliparts/8 cz/no 5/8 czno 5 p 9 i. jpg

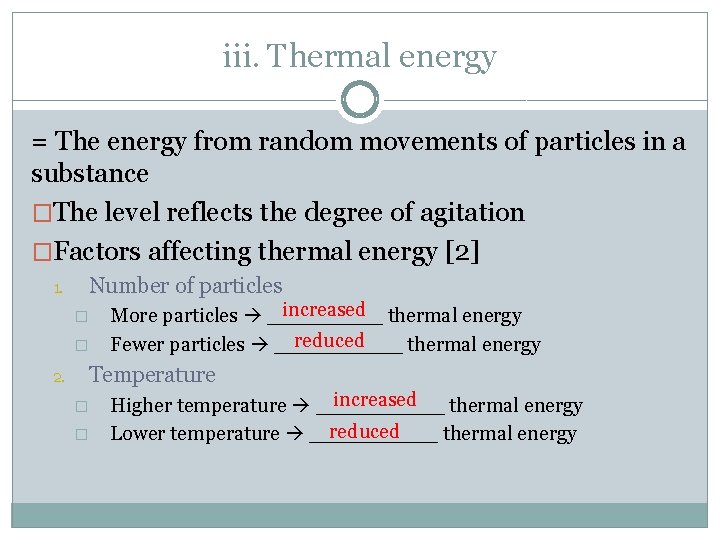
iii. Thermal energy = The energy from random movements of particles in a substance �The level reflects the degree of agitation �Factors affecting thermal energy [2] 1. Number of particles � � 2. increased thermal energy More particles _____ reduced Fewer particles _____ thermal energy Temperature � � increased Higher temperature _____ thermal energy reduced Lower temperature _____ thermal energy

iii. Thermal energy �Thermal energy can be transferred from an environment where the temperature is high (warmer) to an environment where the temperature is lower (colder)=Heat Symbol = Q (heat in J) Δ Et = variation in thermal energy in J Q = Δ Et �Heat vs. Temperature: speed of the particles in a substance (agitation) Heat: depends on BOTH the speed and the mass of particle (number) � Even heat. below freezing point, a substance retains the ability to give off

iv. Kinetic Energy = The energy an object possesses due to its motion �It depends on ; 1. 2. Mass Speed or Velocity (speed in given direction)

v. Potential Energy �Potential energy is considered an energy reserve It must first be transformed into another type of energy to do work When a hammer is lifted by a person (potential energy) when a hammer falls (energy transformation to kinetic energy) drives in the nails �Gravitational potential energy = the energy reserve of an object based on its mass and its height above a reference surface. Depends on; 1. 2. 3. Object’s mass Intensity of the gravitational field Height of the object above a reference surface

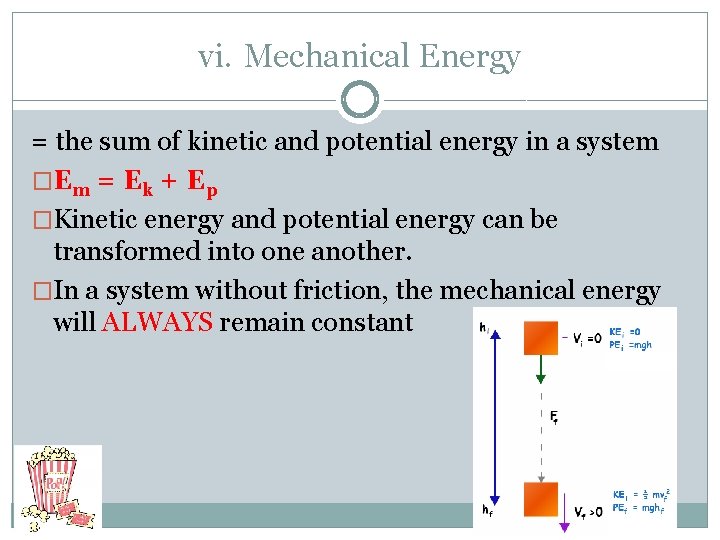
vi. Mechanical Energy = the sum of kinetic and potential energy in a system �Em = Ek + Ep �Kinetic energy and potential energy can be transformed into one another. �In a system without friction, the mechanical energy will ALWAYS remain constant

Homework �Review Package �Workbook Pg. 40