Chapter 3 Chemical Reactions Chemical and Physical Properties



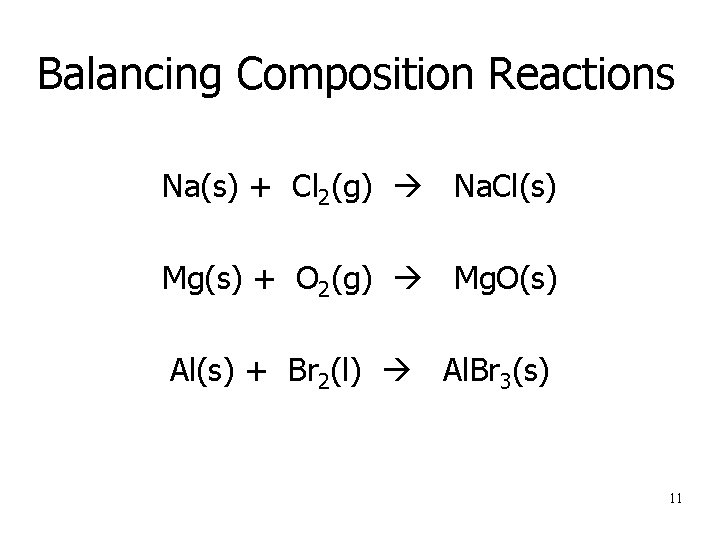
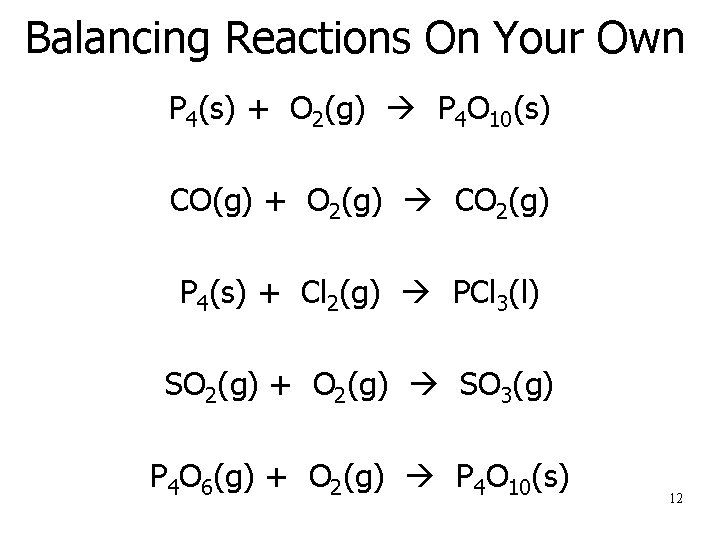













- Slides: 41

Chapter 3 Chemical Reactions

Chemical and Physical Properties • Chemical Changes – rusting or oxidation – chemical reactions • Physical Changes – changes of state – density, color, solubility, melting, boiling – Extensive Properties: depend on quantity – Intensive Properties: do not depend on quantity 2

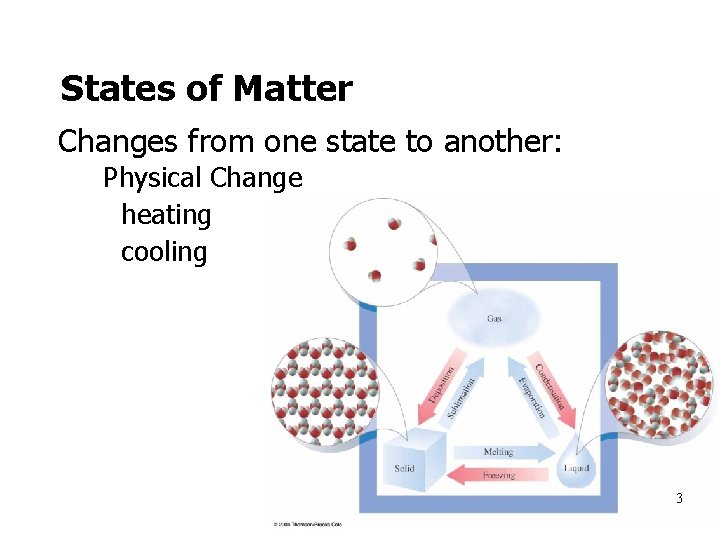
States of Matter • Changes from one state to another: Physical Change • heating • cooling 3

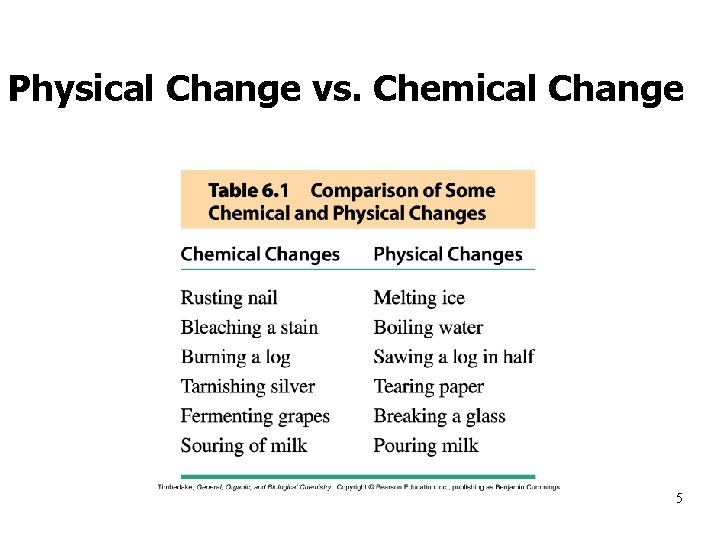
Physical Change vs. Chemical Change 4

Physical Change vs. Chemical Change 5

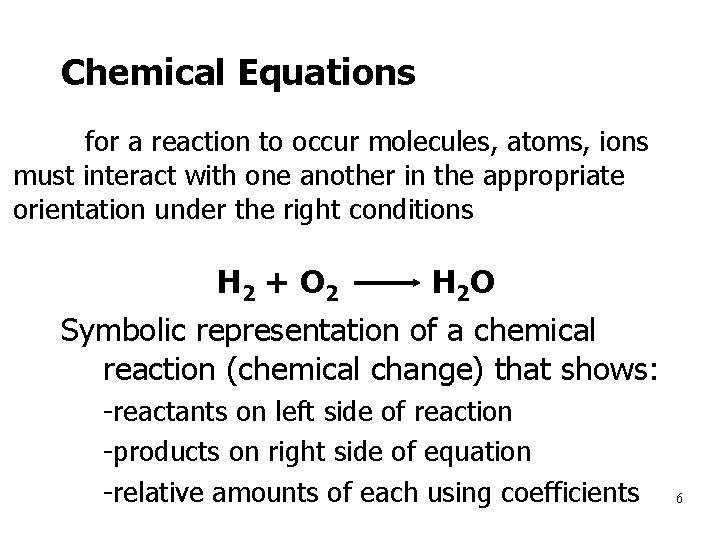
Chemical Equations for a reaction to occur molecules, atoms, ions must interact with one another in the appropriate orientation under the right conditions H 2 + O 2 H 2 O Symbolic representation of a chemical reaction (chemical change) that shows: 1. -reactants on left side of reaction 2. -products on right side of equation 3. -relative amounts of each using coefficients 6

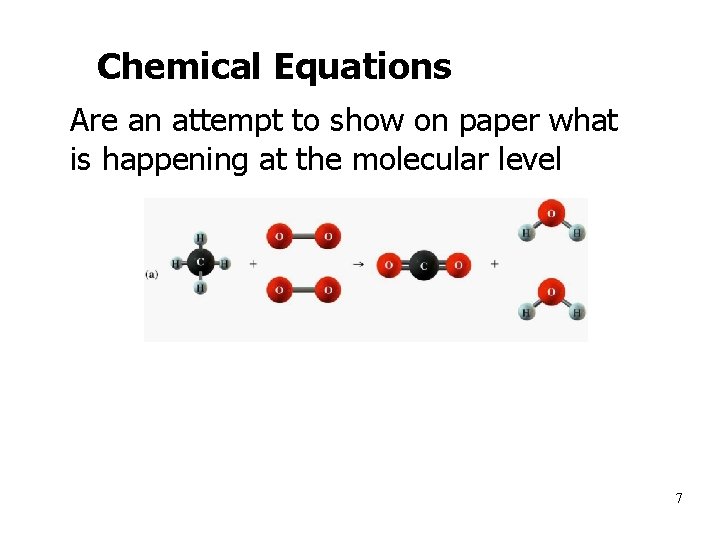
Chemical Equations • Are an attempt to show on paper what is happening at the molecular level 7

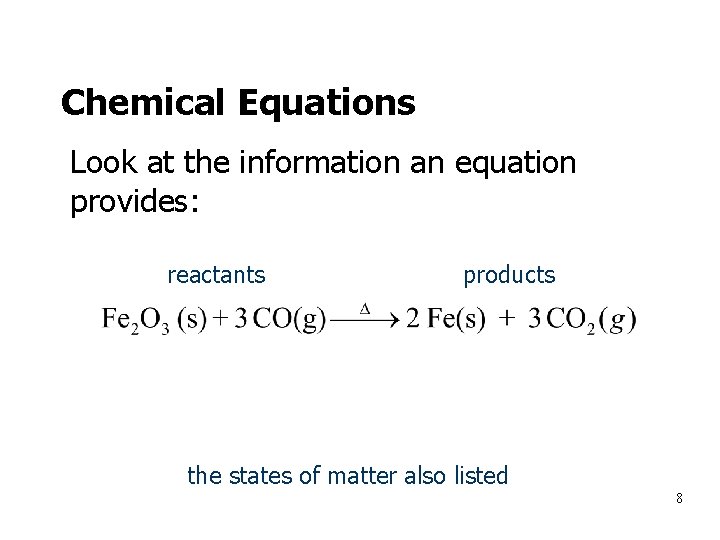
Chemical Equations • Look at the information an equation provides: • • reactants 1 formula unit (molecule/mole) 3 molecules (moles/f. u. ) products 2 atoms 3 moles (moles/f. u. ) (molecules. f. u. ) the states of matter also listed 8

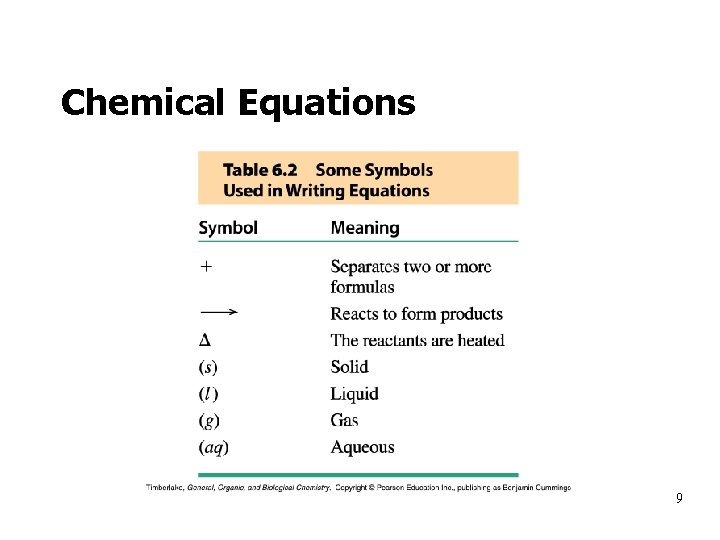
Chemical Equations 9

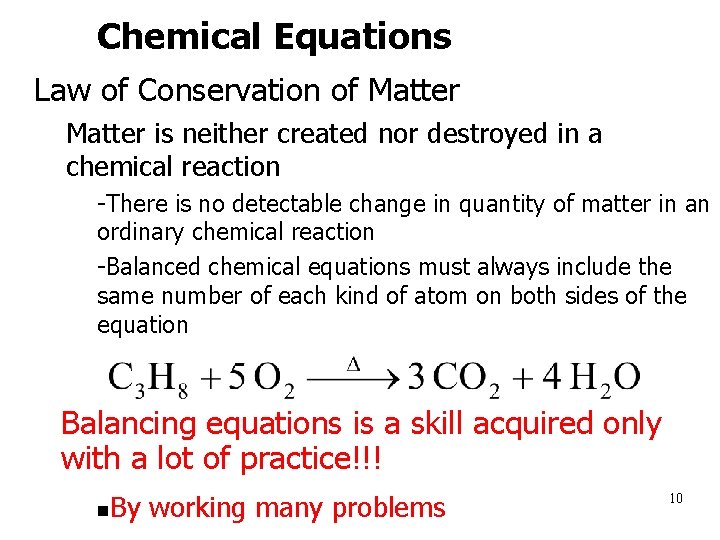
Chemical Equations • Law of Conservation of Matter – Matter is neither created nor destroyed in a chemical reaction • -There is no detectable change in quantity of matter in an ordinary chemical reaction • -Balanced chemical equations must always include the same number of each kind of atom on both sides of the equation Balancing equations is a skill acquired only with a lot of practice!!! n By working many problems 10
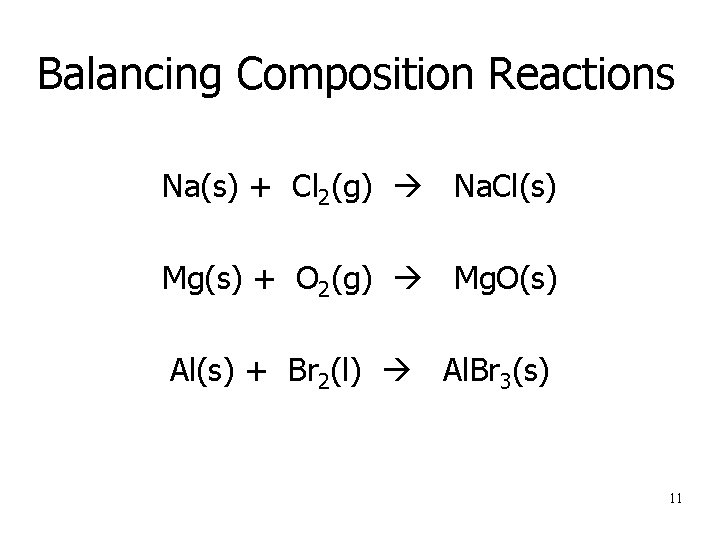
Balancing Composition Reactions Na(s) + Cl 2(g) Na. Cl(s) Mg(s) + O 2(g) Mg. O(s) Al(s) + Br 2(l) Al. Br 3(s) 11
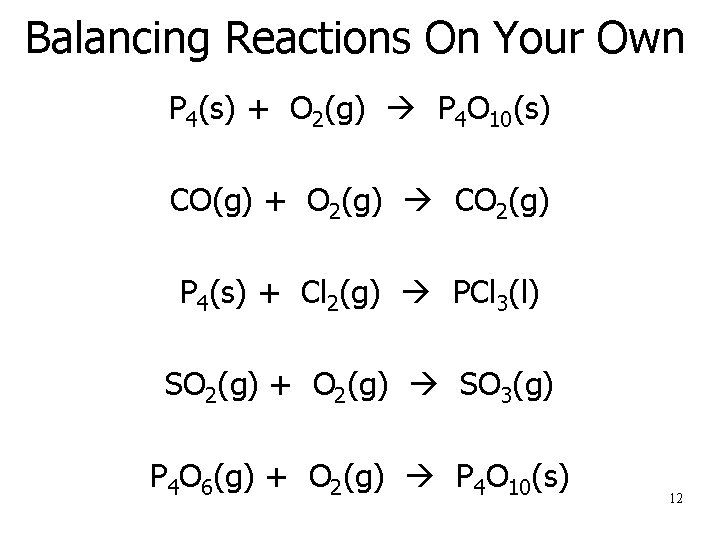
Balancing Reactions On Your Own P 4(s) + O 2(g) P 4 O 10(s) CO(g) + O 2(g) CO 2(g) P 4(s) + Cl 2(g) PCl 3(l) SO 2(g) + O 2(g) SO 3(g) P 4 O 6(g) + O 2(g) P 4 O 10(s) 12

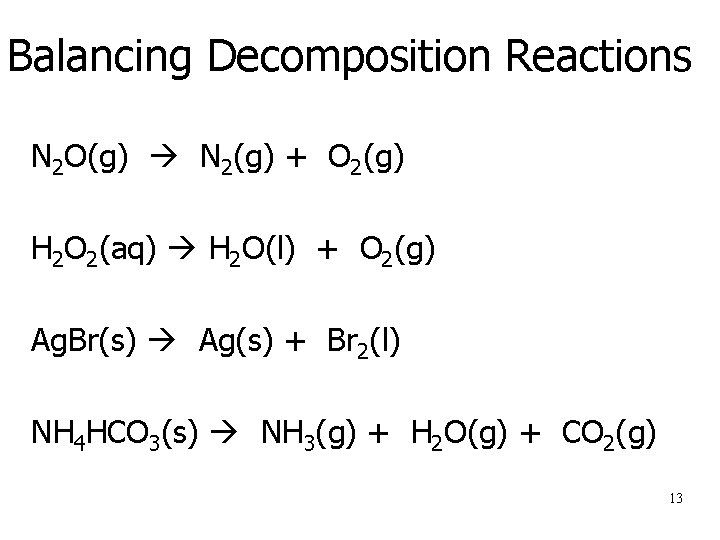
Balancing Decomposition Reactions N 2 O(g) N 2(g) + O 2(g) H 2 O 2(aq) H 2 O(l) + O 2(g) Ag. Br(s) Ag(s) + Br 2(l) NH 4 HCO 3(s) NH 3(g) + H 2 O(g) + CO 2(g) 13

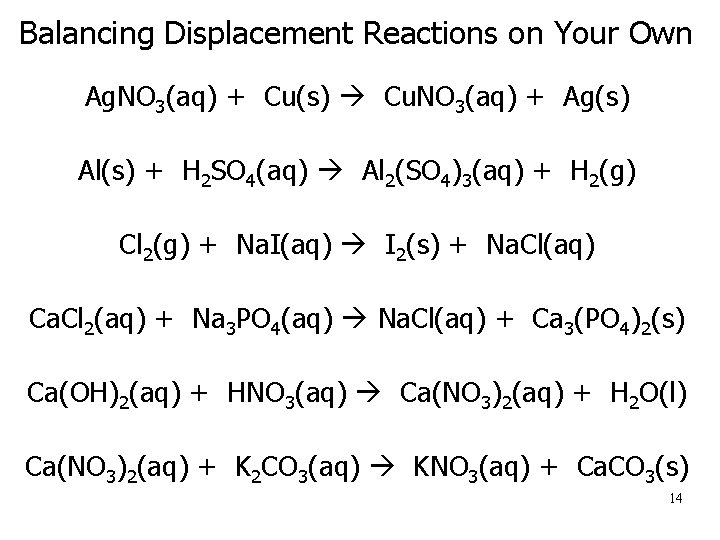
Balancing Displacement Reactions on Your Own Ag. NO 3(aq) + Cu(s) Cu. NO 3(aq) + Ag(s) Al(s) + H 2 SO 4(aq) Al 2(SO 4)3(aq) + H 2(g) Cl 2(g) + Na. I(aq) I 2(s) + Na. Cl(aq) Ca. Cl 2(aq) + Na 3 PO 4(aq) Na. Cl(aq) + Ca 3(PO 4)2(s) Ca(OH)2(aq) + HNO 3(aq) Ca(NO 3)2(aq) + H 2 O(l) Ca(NO 3)2(aq) + K 2 CO 3(aq) KNO 3(aq) + Ca. CO 3(s) 14

Law of Conservation of Matter Combustion reaction: the burning of a fuel in oxygen producing oxides or oxygen containing compounds – -NH 3 burns in oxygen to form nitrogen monoxide and water 15

Law of Conservation of Matter • C 7 H 16 burns in oxygen to form carbon dioxide and water. 16

Solutions a mixture of two or more substances dissolved in another Solute: substance present in the smaller amount that is dissolved by the solvent Solvent: substance present in the larger amount that dissolves the solute 17

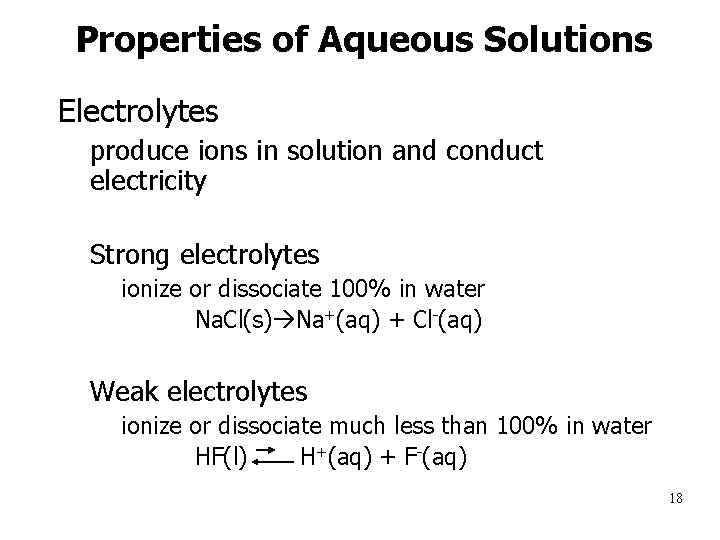
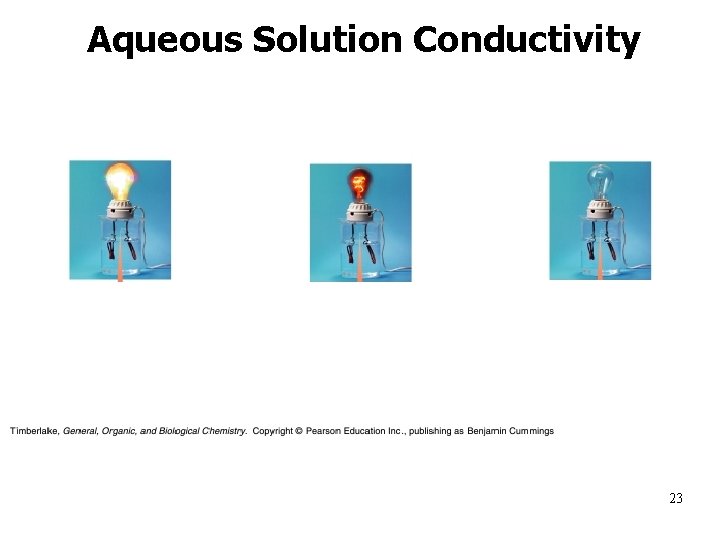
Properties of Aqueous Solutions • Electrolytes – produce ions in solution and conduct electricity – Strong electrolytes • ionize or dissociate 100% in water – Na. Cl(s) Na+(aq) + Cl-(aq) – Weak electrolytes • ionize or dissociate much less than 100% in water – HF(l) H+(aq) + F-(aq) 18

Strong Electrolytes conduct electricity extremely well in dilute aqueous solutions – -ionize in water 100% Examples: 1. HCl, HNO 3, etc • strong soluble acids 2. Na. OH, KOH, etc • strong soluble bases 3. Na. Cl, KBr, etc • soluble ionic salts 19

Strong Ionic Salts 20

Weak Electrolytes conduct electricity poorly in aqueous solutions -ionize much less than 100% in water Examples: 1. CH 3 COOH, (COOH)2 • weak acids 2. NH 3, Fe(OH)3 • weak bases 21

Properties of Aqueous Solutions Nonelectrolytes solutes that do not conduct electricity in water – do not “ionize” • • • Examples: C 2 H 5 OH – ethanol Sugars – glucose, sucrose, etc. 22

Aqueous Solution Conductivity 23

Solubility • maximum amount of solute that can dissolve in a given amount of solvent – -defined as the amount of solute that dissolves in 100 g solvent • • • Unsaturated Solution: contains less than the maximum amount that dissolves Saturated solution: contains the maximum amount that dissolves Supersaturated solution: contains more than the maximum amount that normally dissolves 24

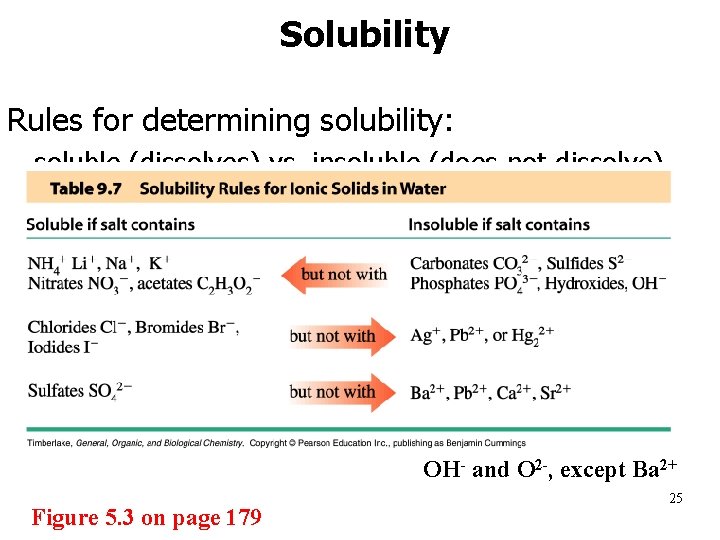
Solubility Rules for determining solubility: • soluble (dissolves) vs. insoluble (does not dissolve) OH- and O 2 -, except Ba 2+ Figure 5. 3 on page 179 25

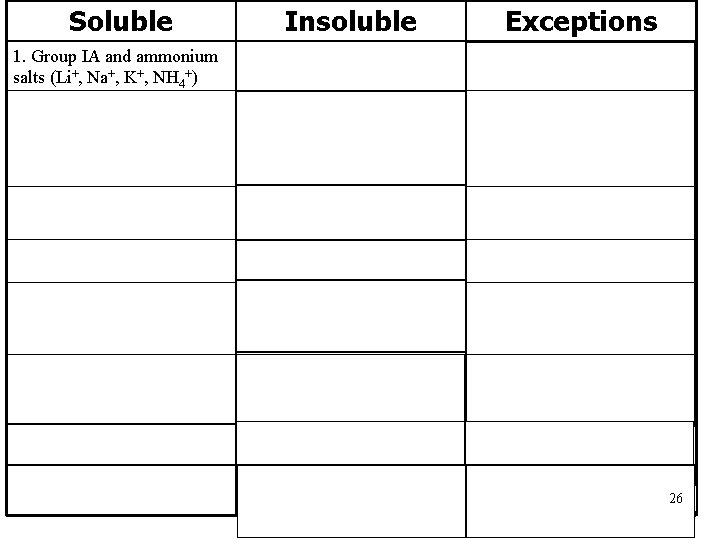
Soluble Insoluble 1. Group IA and ammonium salts (Li+, Na+, K+, NH 4+) Exceptions ___________ 2. Acetates, nitrates, chlorates, perchlorates (CH 3 COO-, NO 3 -, Cl. O 4 -) 3. most chlorides, bromides, and iodides (Cl-, Br -, I-) Salts formed with Ag+, Hg 2+, Pb 2+ 4. most fluorides (F-) Salts formed with Group IIA 5. most sulfates (SO 42 -) Salts formed with Group IIA (Ca 2+, Sr 2+, Ba 2+), Ag+, Hg 2+, Pb 2+ 6. most carbonates, phosphates, sulfides (CO 32 -, PO 43 -, S 2 -) Salts formed with Group IA and NH 4+ (rule #1) 7. most oxides (O 2 -) ____________ 8. most hydroxides (OH-) Salts formed with Group IA 26 and Ca 2+, Sr 2+

Solubility 27

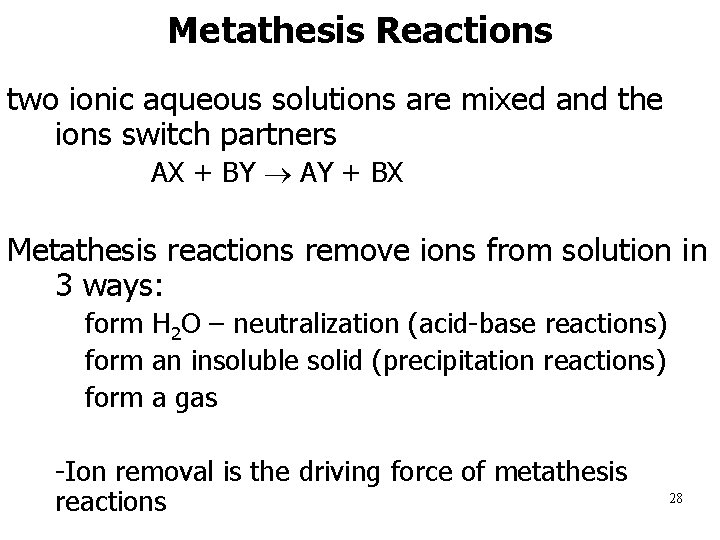
Metathesis Reactions two ionic aqueous solutions are mixed and the ions switch partners AX + BY AY + BX Metathesis reactions remove ions from solution in 3 ways: 1. form H 2 O – neutralization (acid-base reactions) 2. form an insoluble solid (precipitation reactions) 3. form a gas • -Ion removal is the driving force of metathesis reactions 28

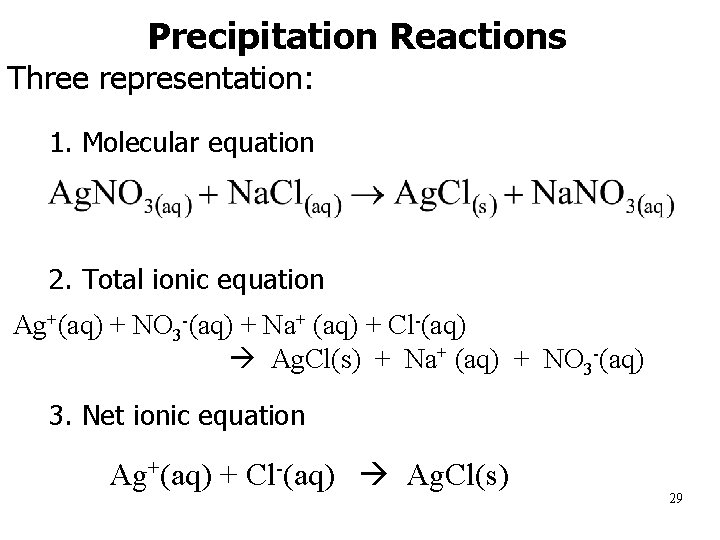
Precipitation Reactions Three representation: 1. 1. Molecular equation 2. 2. Total ionic equation Ag+(aq) + NO 3 -(aq) + Na+ (aq) + Cl-(aq) Ag. Cl(s) + Na+ (aq) + NO 3 -(aq) 3. Net ionic equation Ag+(aq) + Cl-(aq) Ag. Cl(s) 29

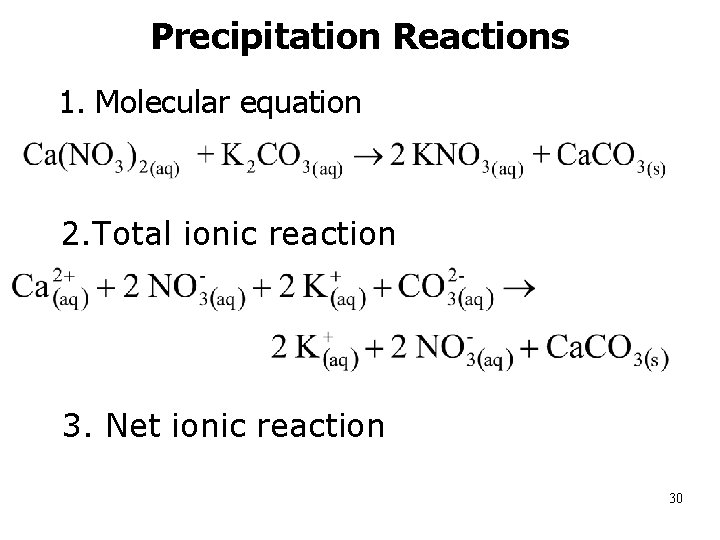
Precipitation Reactions • 1. Molecular equation n 2. Total ionic reaction n 3. Net ionic reaction 30

Arrhenius Acids substances that generate H 3 O+ (H+) in aqueous solutions -Strong acids ionize 100% in water (l) 31

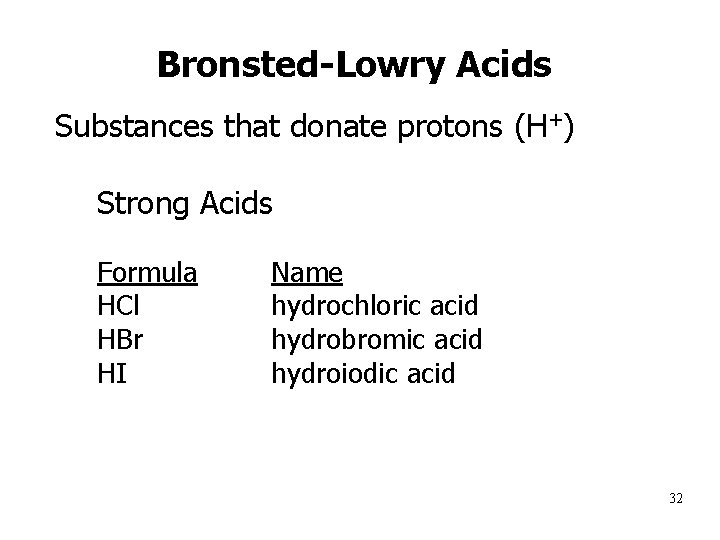
Bronsted-Lowry Acids Substances that donate protons (H+) • Strong Acids • 1. 2. 3. 4. 5. 6. 7. Formula HCl HBr HI HNO 3 H 2 SO 4 HCl. O 3 HCl. O 4 Name hydrochloric acid hydrobromic acid hydroiodic acid nitric acid sulfuric acid chloric acid perchloric acid 32

Acids • -Weak acids ionize <100% in water 33

Acids • Common Weak Acids • Formula Name 1. HF hydrofluoric acid 2. CH 3 COOH acetic acid (vinegar) 3. HCN hydrocyanic acid 4. HNO 2 nitrous acid 5. H 2 CO 3 carbonic acid (soda water) 6. H 3 PO 4 phosphoric acid 34

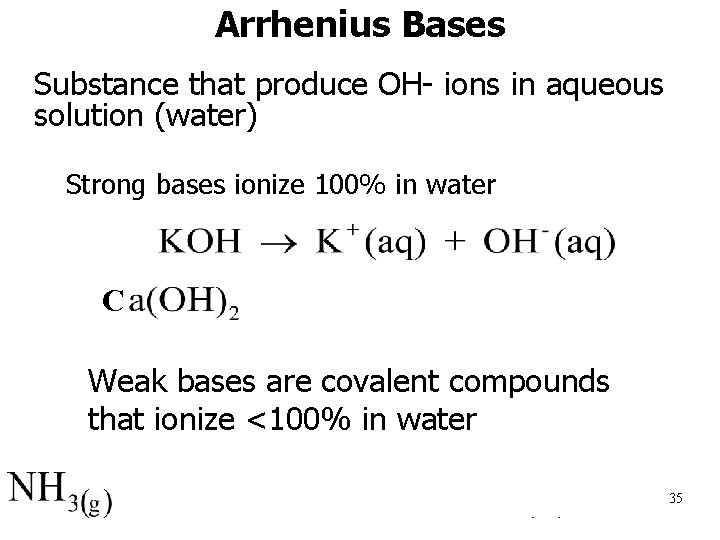
Arrhenius Bases • Substance that produce OH- ions in aqueous solution (water) – Strong bases ionize 100% in water C C • Weak bases are covalent compounds that ionize <100% in water (l) 35

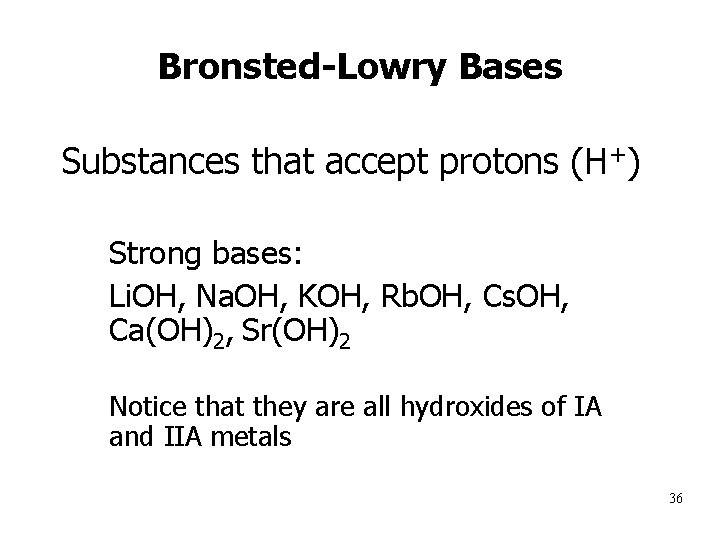
Bronsted-Lowry Bases Substances that accept protons (H+) • Strong bases: 1. Li. OH, Na. OH, KOH, Rb. OH, Cs. OH, Ca(OH)2, Sr(OH)2 2. Notice that they are all hydroxides of IA and IIA metals 36

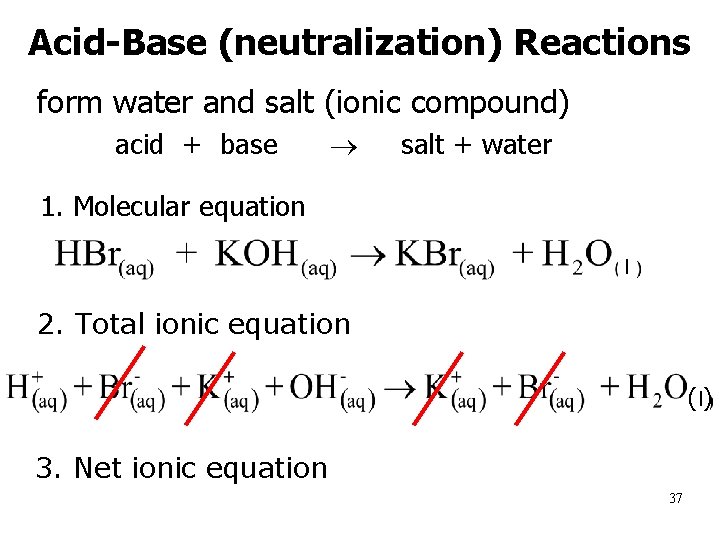
Acid-Base (neutralization) Reactions form water and salt (ionic compound) – acid + base salt + water • 1. Molecular equation l n 2. Total ionic equation (l) n 3. Net ionic equation 37

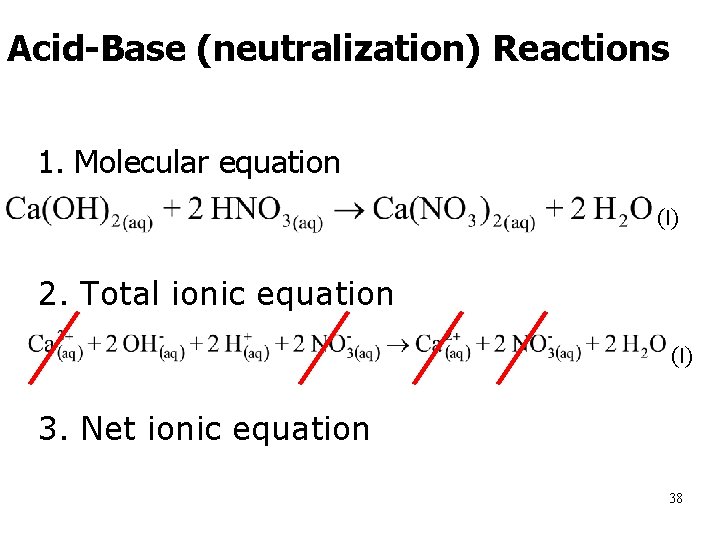
Acid-Base (neutralization) Reactions 1. Molecular equation (l) 2. Total ionic equation (l) 3. Net ionic equation 38

Acids and Bases There are four acid-base reaction combinations that are possible: 1. 2. 3. 4. strong acids – strong bases weak acids – strong bases strong acids – weak bases weak acids – weak bases 39

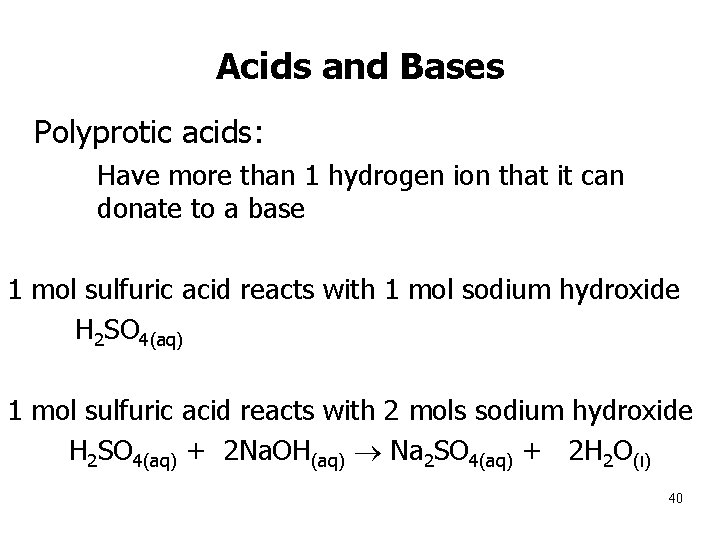
Acids and Bases • Polyprotic acids: • Have more than 1 hydrogen ion that it can donate to a base 1 mol sulfuric acid reacts with 1 mol sodium hydroxide H 2 SO 4(aq) + Na. OH(aq) Na. HSO 4(aq) + H 2 O(l) 1 mol sulfuric acid reacts with 2 mols sodium hydroxide H 2 SO 4(aq) + 2 Na. OH(aq) Na 2 SO 4(aq) + 2 H 2 O(l) 40

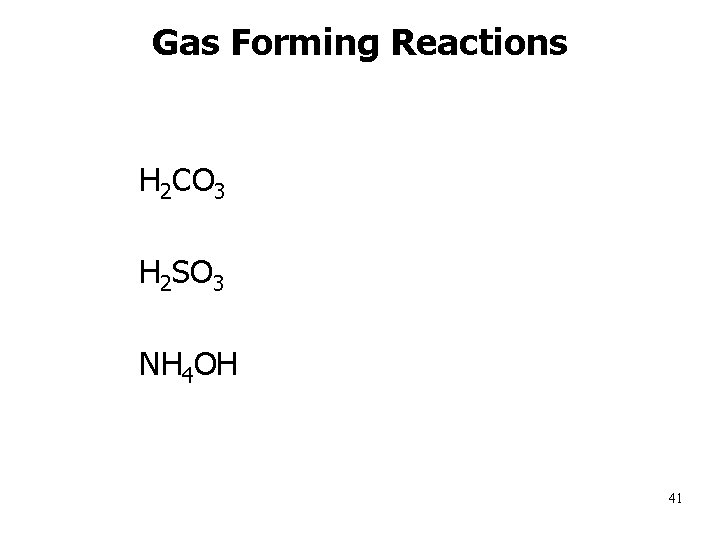
Gas Forming Reactions H 2 CO 3 H 2 O(l) + CO 2 (g) H 2 SO 3 H 2 O(l) + SO 2 (g) NH 4 OH NH 3(g) + H 2 O(l) 41