Chapter 3 Chemical Compounds Compounds combination of two

Chapter 3 Chemical Compounds
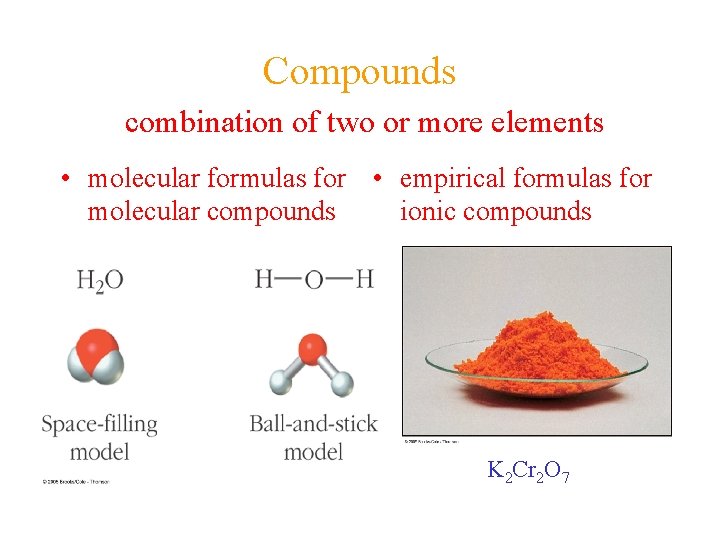
Compounds combination of two or more elements • molecular formulas for • empirical formulas for ionic compounds molecular compounds K 2 Cr 2 O 7
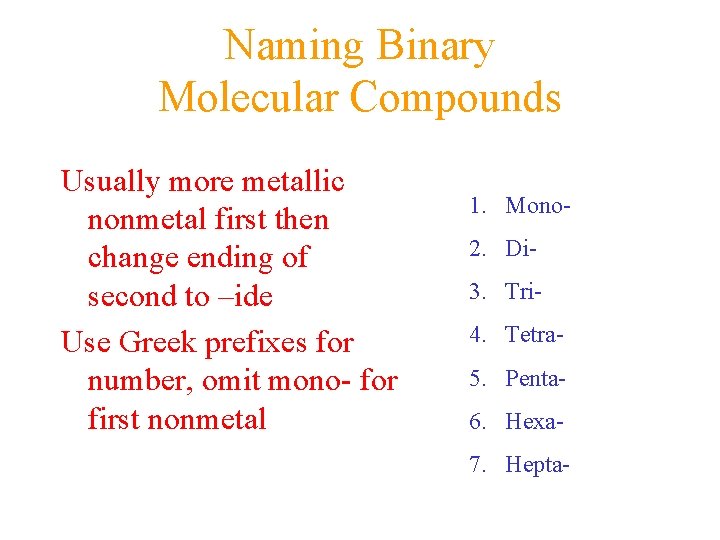
Naming Binary Molecular Compounds Usually more metallic nonmetal first then change ending of second to –ide Use Greek prefixes for number, omit mono- for first nonmetal 1. Mono 2. Di 3. Tri 4. Tetra 5. Penta 6. Hexa 7. Hepta-
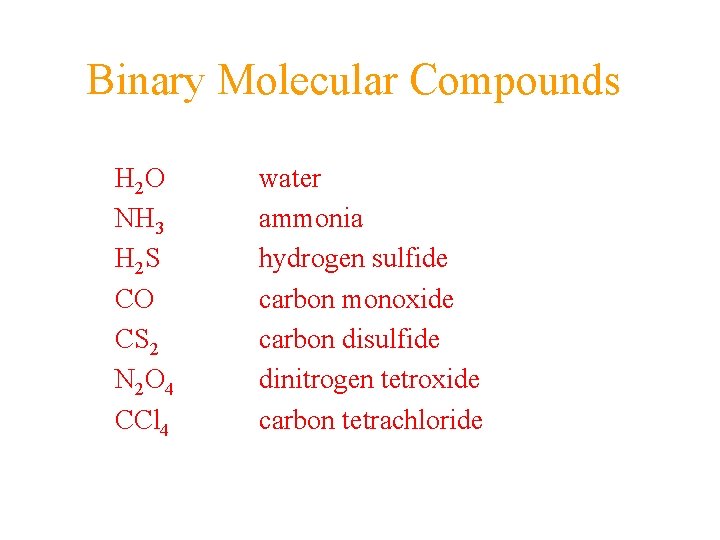
Binary Molecular Compounds H 2 O NH 3 H 2 S CO CS 2 N 2 O 4 CCl 4 water ammonia hydrogen sulfide carbon monoxide carbon disulfide dinitrogen tetroxide carbon tetrachloride
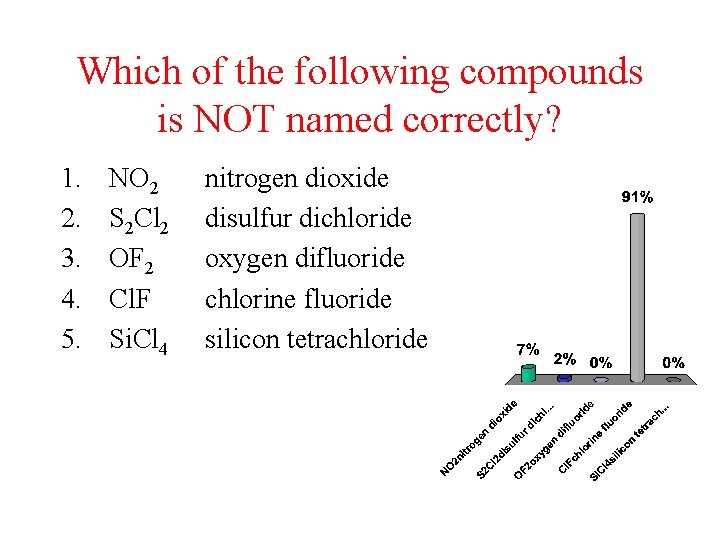
Which of the following compounds is NOT named correctly? 1. 2. 3. 4. 5. NO 2 S 2 Cl 2 OF 2 Cl. F Si. Cl 4 nitrogen dioxide disulfur dichloride oxygen difluoride chlorine fluoride silicon tetrachloride
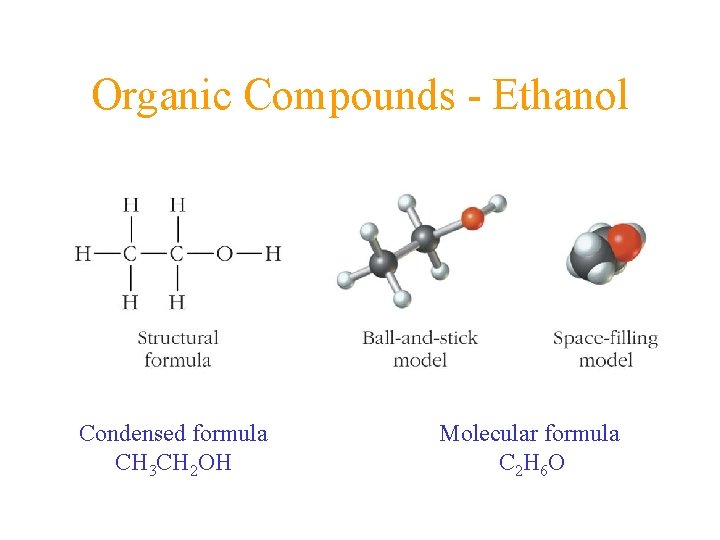
Organic Compounds - Ethanol Condensed formula CH 3 CH 2 OH Molecular formula C 2 H 6 O
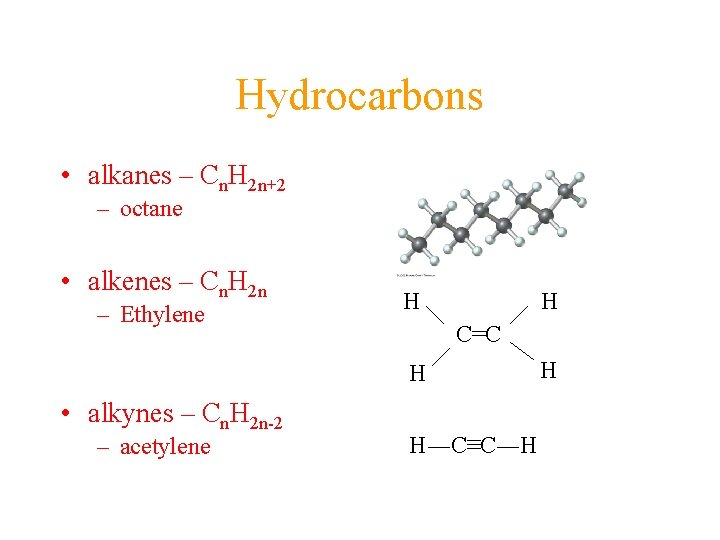
Hydrocarbons • alkanes – Cn. H 2 n+2 – octane • alkenes – Cn. H 2 n – Ethylene H H C=C H • alkynes – Cn. H 2 n-2 – acetylene H―C≡C―H H
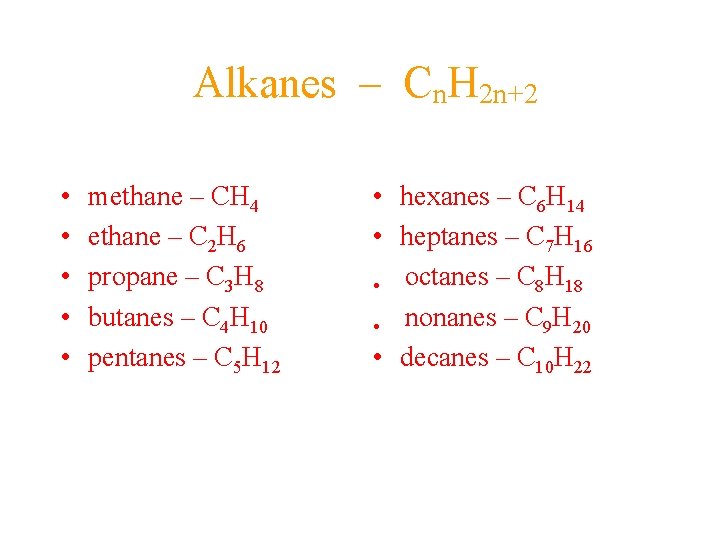
Alkanes – Cn. H 2 n+2 • • • methane – CH 4 ethane – C 2 H 6 propane – C 3 H 8 butanes – C 4 H 10 pentanes – C 5 H 12 • hexanes – C 6 H 14 • heptanes – C 7 H 16 • octanes – C 8 H 18 • nonanes – C 9 H 20 • decanes – C 10 H 22

Butane • Butane molecules are present in the liquid and gaseous states in the lighter
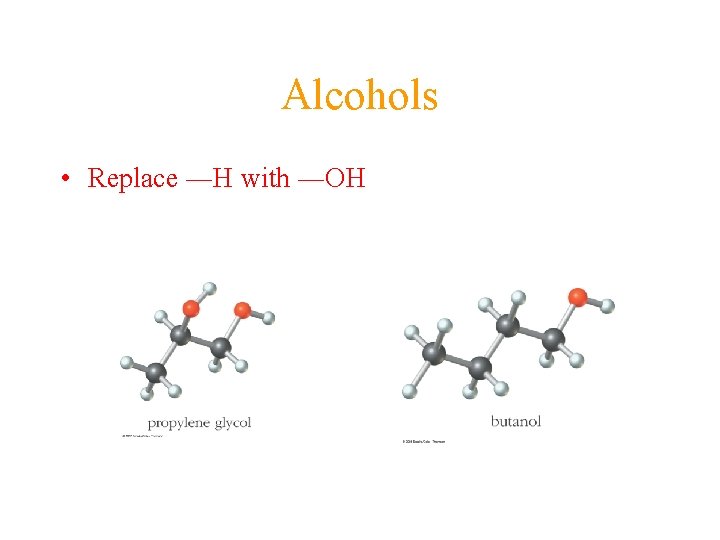
Alcohols • Replace ―H with ―OH
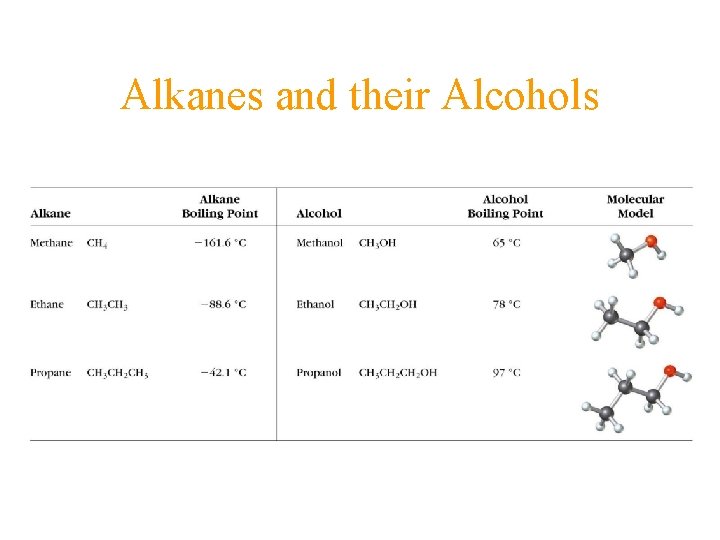
Alkanes and their Alcohols
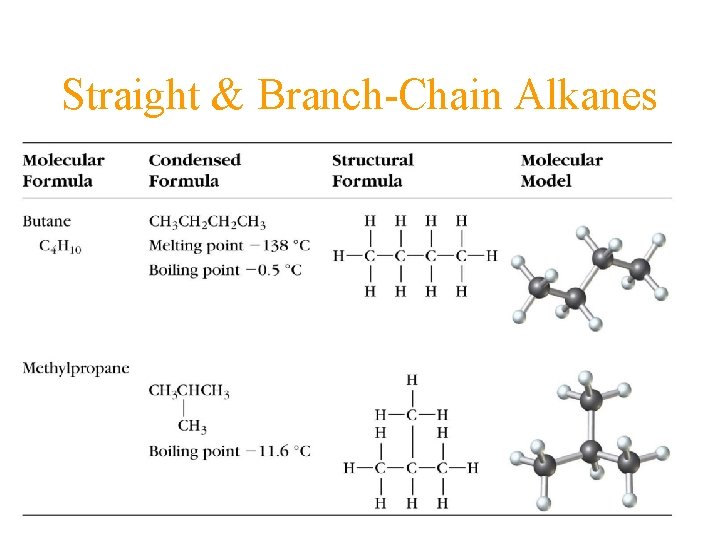
Straight & Branch-Chain Alkanes
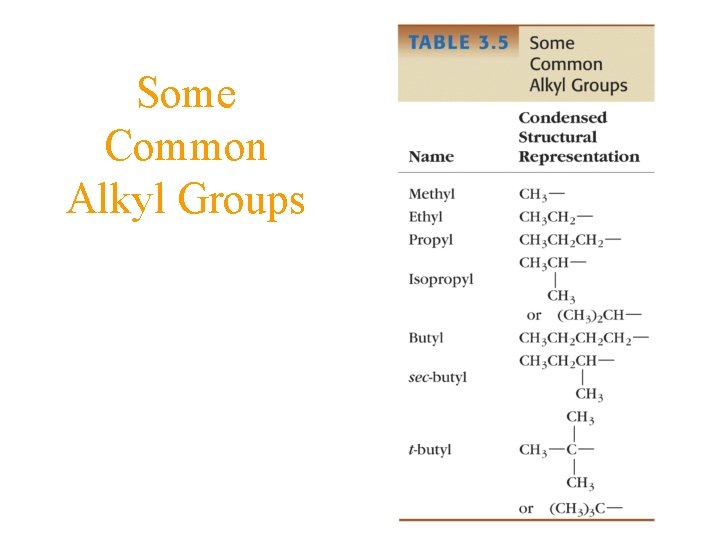
Some Common Alkyl Groups
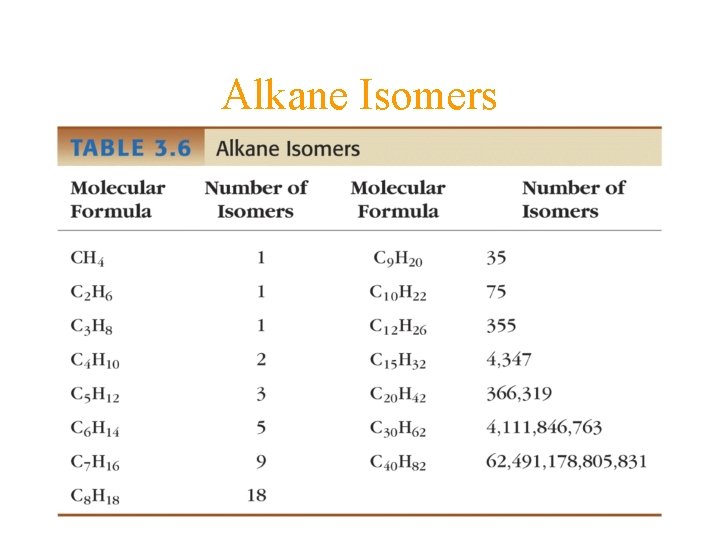
Alkane Isomers

Naming Branch-Chain Alkanes • select the longest chain alkane as the base name • determine the side chains and give them a number corresponding to the carbon number on the base chain • use Greek prefixes of mono-(1), bi-(2), tri(3), etc. for multiplicity of same side chain
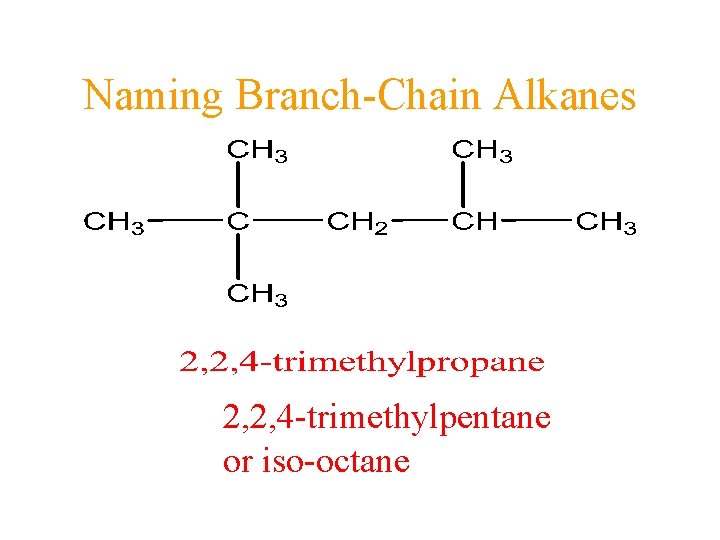
Naming Branch-Chain Alkanes 2, 2, 4 -trimethylpentane or iso-octane
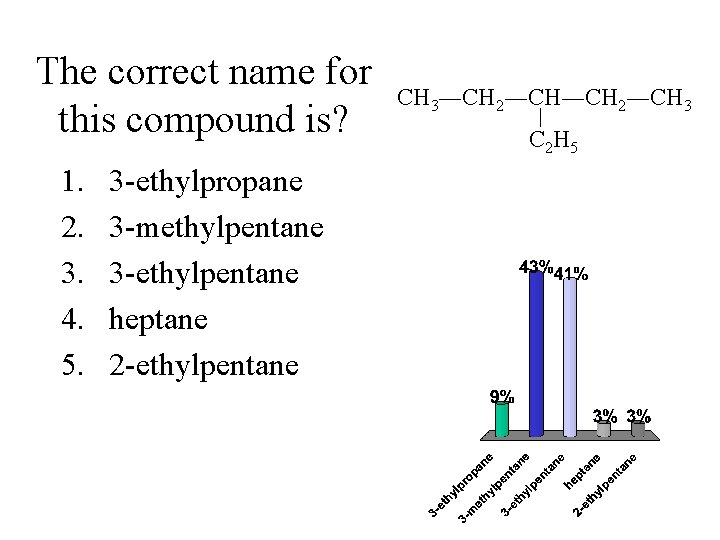
The correct name for this compound is? 1. 2. 3. 4. 5. 3 -ethylpropane 3 -methylpentane 3 -ethylpentane heptane 2 -ethylpentane CH 3―CH 2―CH 3 C 2 H 5
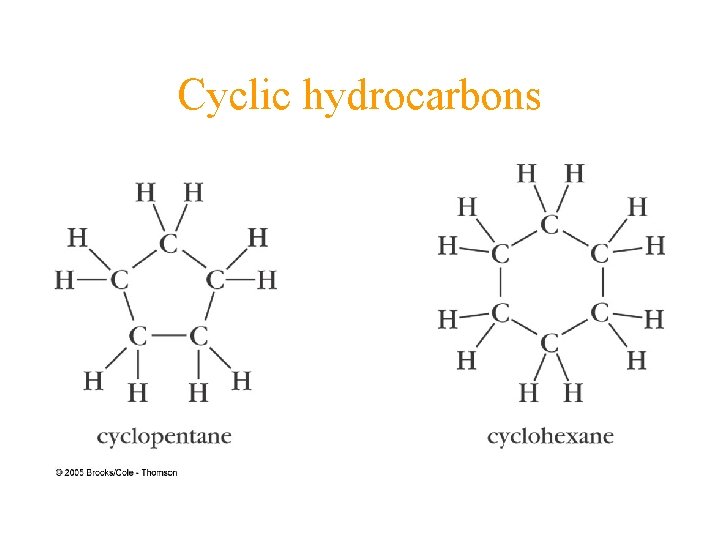
Cyclic hydrocarbons

Ionic. Compounds Characteristics of compounds with ionic bonding: • non-volatile, thus high melting points • solids do not conduct electricity, but melts (liquid state) do • many, but not all, are water soluble
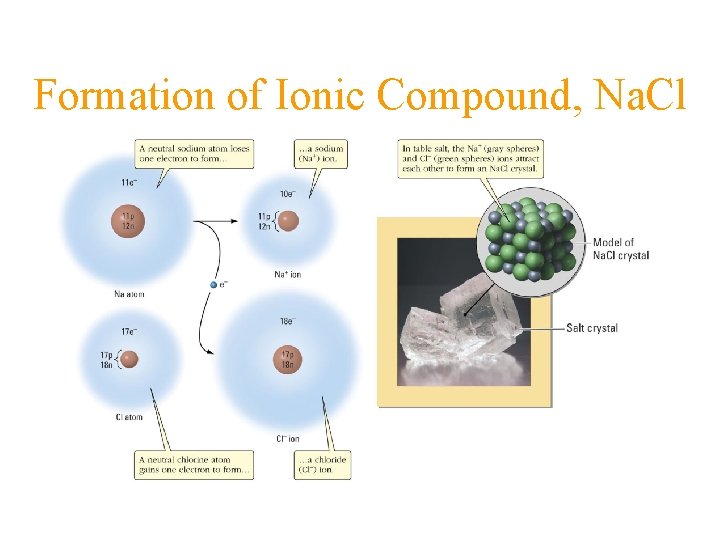
Formation of Ionic Compound, Na. Cl

Valance, Charge on Ions • • • compounds have electrical neutrality metals form positive monatomic ions non-metals form negative monatomic ions balance charges Al 3+ O 2 Al 2 O 3

Valence of Metal Ions Monatomic Ions Group IA +1 Group IIA +2 Maximum positive valence equals Group A #
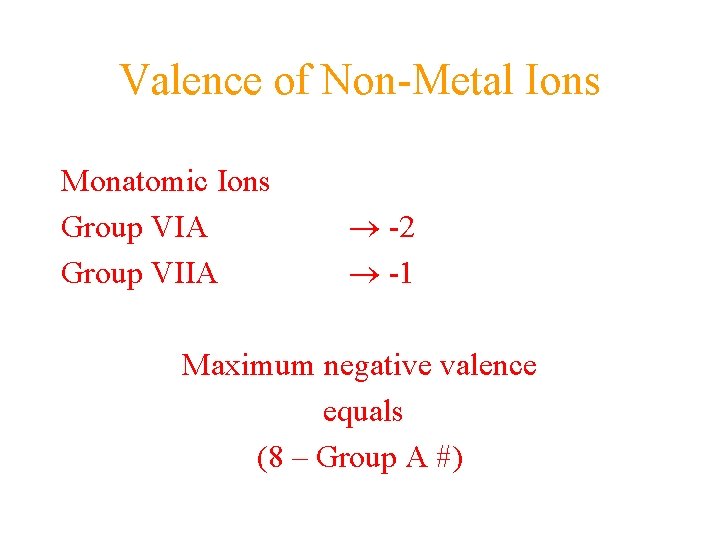
Valence of Non-Metal Ions Monatomic Ions Group VIA Group VIIA -2 -1 Maximum negative valence equals (8 – Group A #)
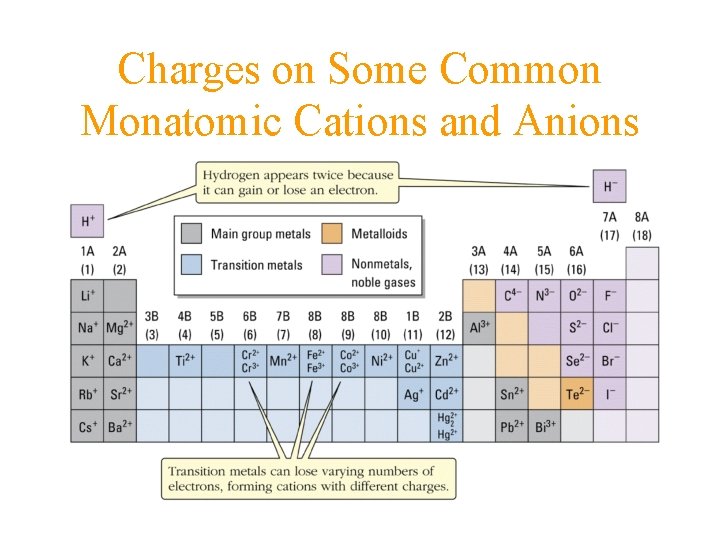
Charges on Some Common Monatomic Cations and Anions
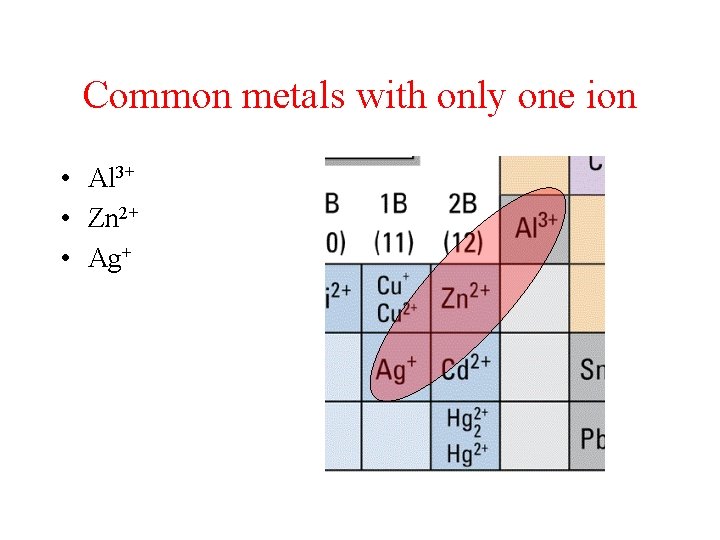
Common metals with only one ion • Al 3+ • Zn 2+ • Ag+
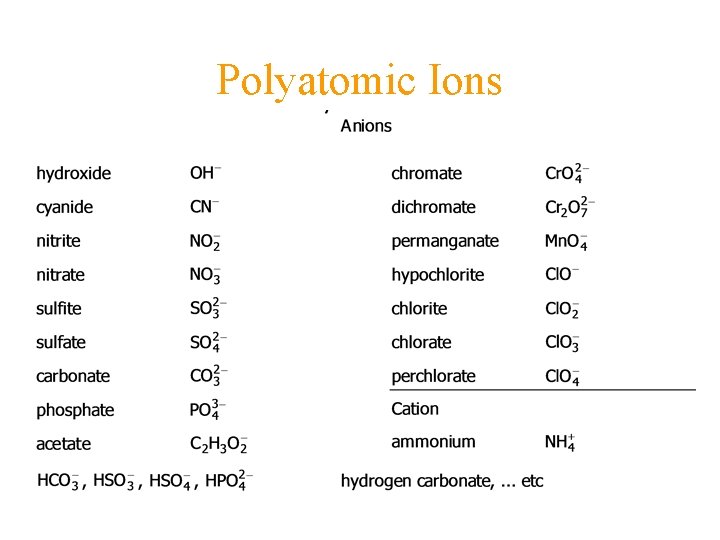
Polyatomic Ions

-ate and -ite • NO 3 nitrate • NO 2 nitrite • -ate is great, -ite is slight -ate has one more oxygen than –ite • hypo- under one less • percompletely one more

Name other ions by analogy with element above • • Se. O 42 Br. O 3 HSe. H 2 As. O 4 - selenate bromate hydrogen selenide dihydrogen arsenate
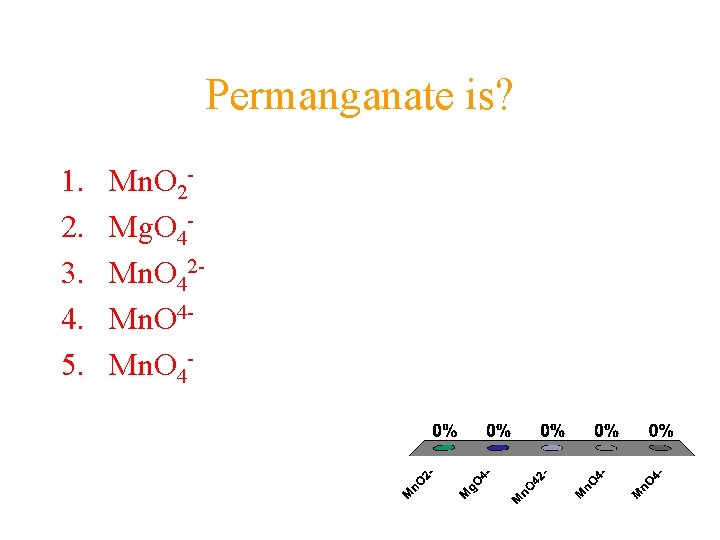
Permanganate is? 1. 2. 3. 4. 5. Mn. O 2 Mg. O 4 Mn. O 42 Mn. O 4 -
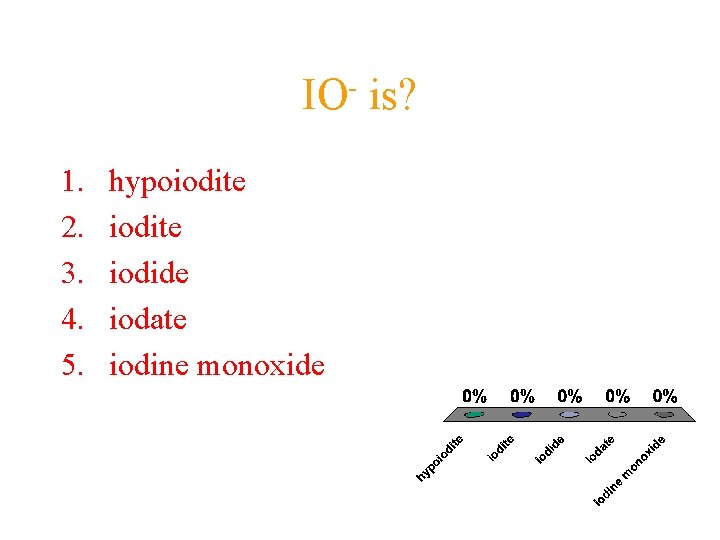
IO 1. 2. 3. 4. 5. hypoiodite iodide iodate iodine monoxide is?
- Slides: 30