Chapter 3 Chemical Bonds Octet Rule Naming anions

Chapter 3: Chemical Bonds • Octet Rule • Naming anions and cations • Ionic and Covalent bonds • Electronegativity • Drawing Lewis dot structures • Octet rule exceptions • Resonance • Bond Angles • Polarity of molecules

The Octet Rule Main group elements react in ways that achieve an electron configuration of eight valence electrons. – An atom that loses one or more electrons becomes a positively charged ion = cation. – An atom that gains one or more electrons becomes a negatively charged ion = anion.
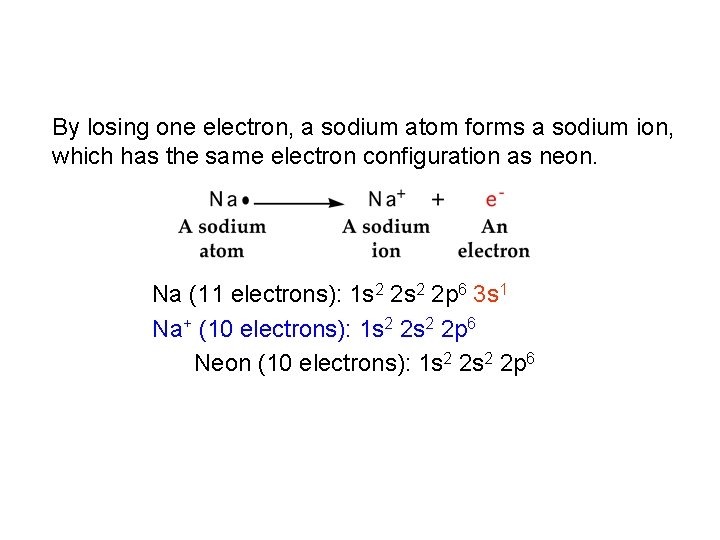
By losing one electron, a sodium atom forms a sodium ion, which has the same electron configuration as neon. Na (11 electrons): 1 s 2 2 p 6 3 s 1 Na+ (10 electrons): 1 s 2 2 p 6 Neon (10 electrons): 1 s 2 2 p 6
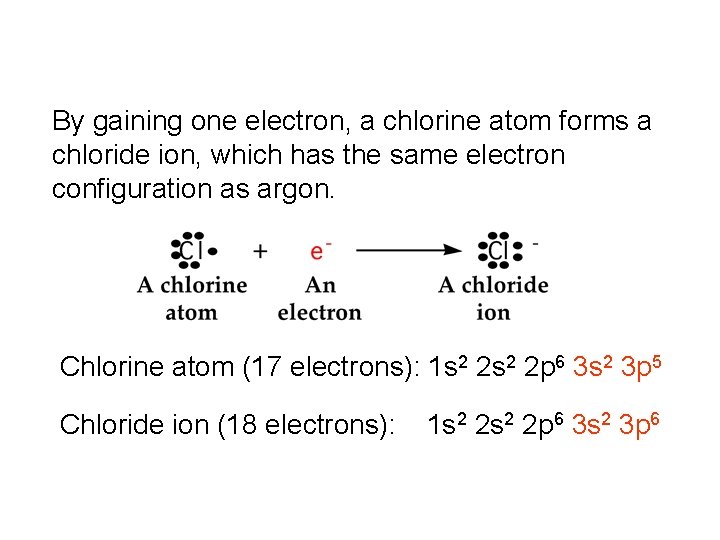
By gaining one electron, a chlorine atom forms a chloride ion, which has the same electron configuration as argon. Chlorine atom (17 electrons): 1 s 2 2 p 6 3 s 2 3 p 5 Chloride ion (18 electrons): 1 s 2 2 p 6 3 s 2 3 p 6

When an atom gains electrons, it has a ____ charge and is called a(n) ____. 1. 2. 3. 4. negative; anion negative; cation positive; anion positive; cation
The Octet Rule The octet rule gives us a good way to understand why Main Group elements form the ions they do: elements in group 1 always loss 1 electron group 2 always loss 2 electrons group 7 A (17) always gain 1 electron group 6 A (16) always gain 2 electrons but it is not perfect: – Ions of period 1 and 2 elements with charges greater than +2 (i. e. +3, +4) or smaller than -2 (i. e. -3, -4) are unstable. For example, boron does not lose its three valence electrons to become B 3+, nor does carbon lose its four valence electrons to become C 4+ or gain four valence electrons to become C 4– The octet rule does not apply to transition elements, most of which form ions with two or more different positive charges.
Forming Chemical Bonds An atom may lose or gain enough electrons to acquire a filled valence shell and become an ion. An ionic bond is the result of the force of attraction between a cation and an anion. An atom may share electrons with one or more other atoms to acquire a filled valence shell. A covalent bond results from two atoms that share one or more pairs of electrons.
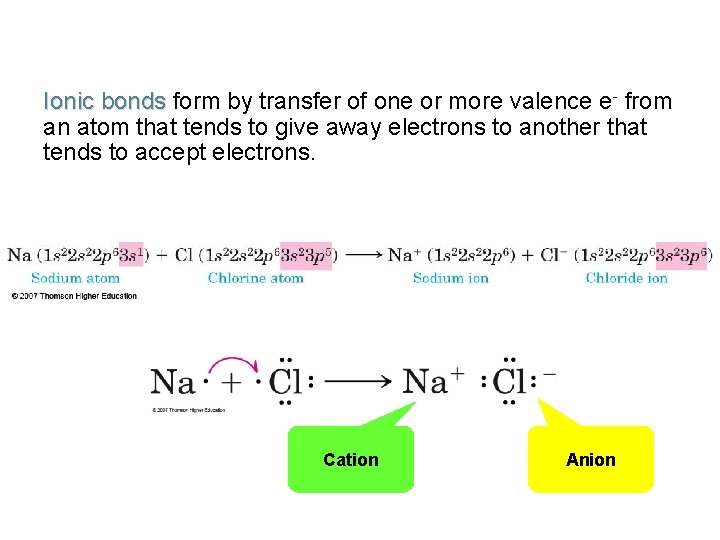
Ionic bonds form by transfer of one or more valence e- from an atom that tends to give away electrons to another that tends to accept electrons. Cation Anion
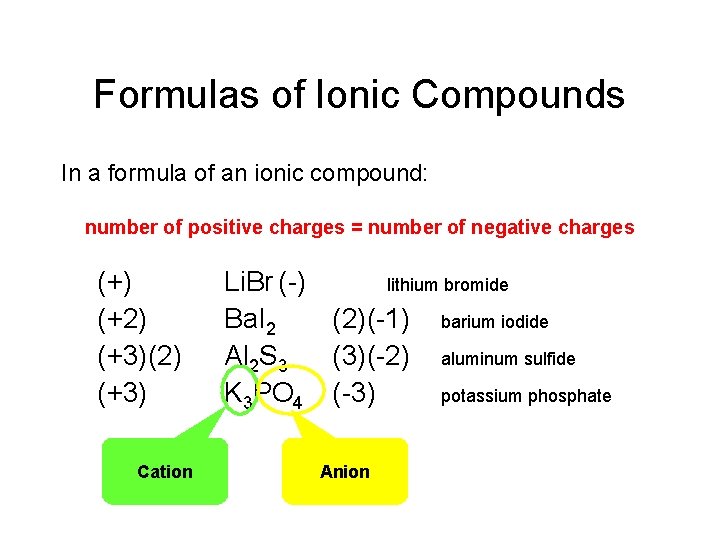
Formulas of Ionic Compounds In a formula of an ionic compound: number of positive charges = number of negative charges (+) (+2) (+3)(2) (+3) Cation Li. Br (-) lithium bromide Ba. I 2 (2)(-1) barium iodide Al 2 S 3 (3)(-2) aluminum sulfide K 3 PO 4 (-3) potassium phosphate Anion
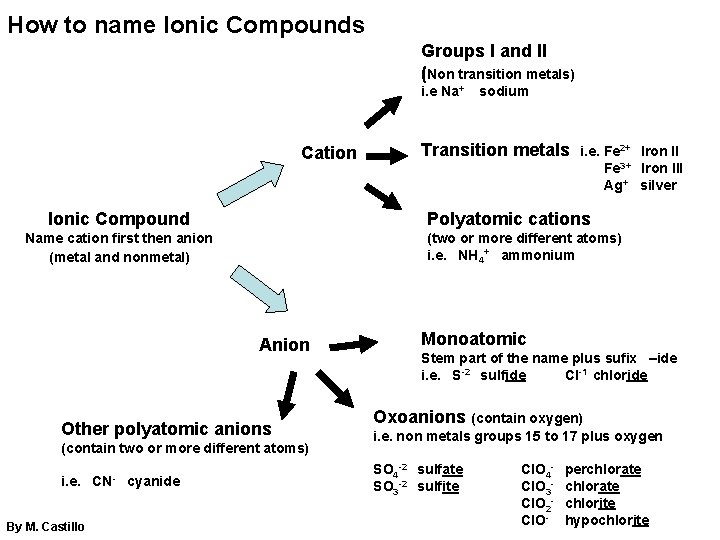
How to name Ionic Compounds Groups I and II (Non transition metals) i. e Na+ Cation Ionic Compound Other polyatomic anions (contain two or more different atoms) By M. Castillo cyanide i. e. Fe 2+ Iron II Fe 3+ Iron III Ag+ silver (two or more different atoms) i. e. NH 4+ ammonium Anion i. e. Transition metals Polyatomic cations Name cation first then anion (metal and nonmetal) CN- sodium Monoatomic Stem part of the name plus sufix –ide i. e. S-2 sulfide Cl-1 chloride Oxoanions (contain oxygen) i. e. non metals groups 15 to 17 plus oxygen SO 4 -2 sulfate SO 3 -2 sulfite Cl. O 4 Cl. O 3 Cl. O 2 Cl. O- perchlorate chlorite hypochlorite
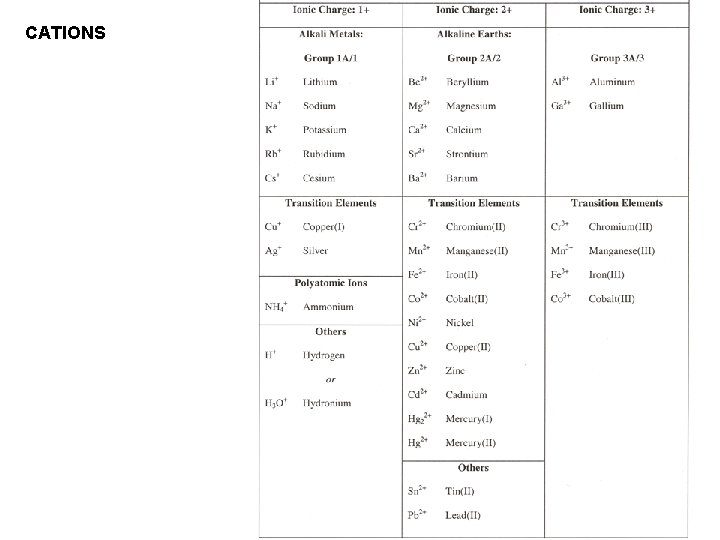
CATIONS
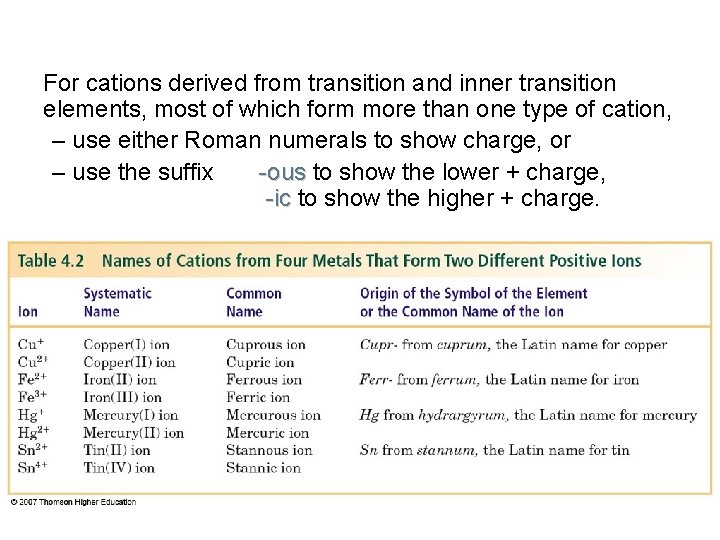
For cations derived from transition and inner transition elements, most of which form more than one type of cation, – use either Roman numerals to show charge, or – use the suffix -ous to show the lower + charge, -ic to show the higher + charge.

Naming Anions For monatomic (containing only one atom) anions, add “ide” to the stem part of the name.
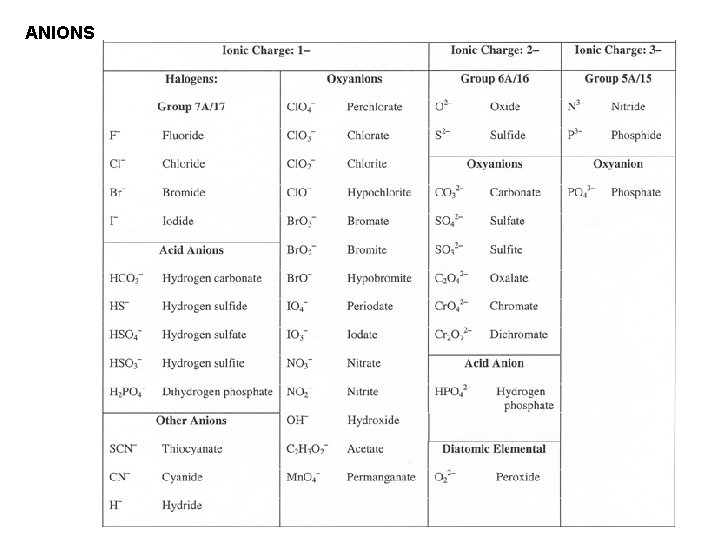
ANIONS
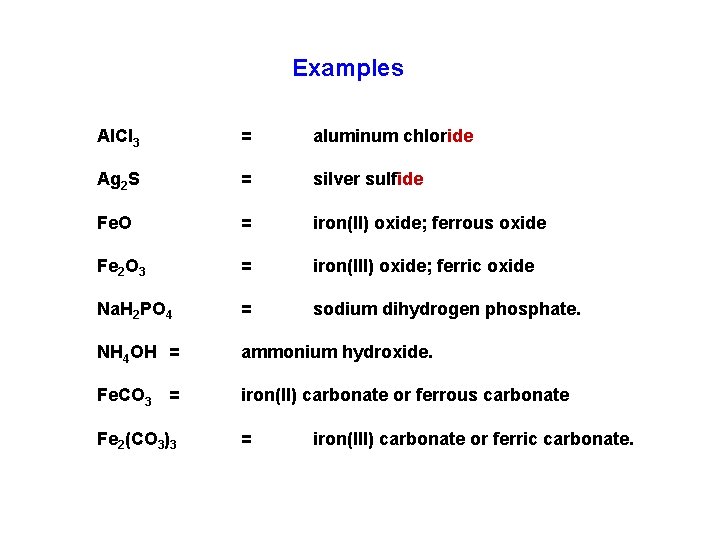
Examples Al. Cl 3 = aluminum chloride Ag 2 S = silver sulfide Fe. O = iron(II) oxide; ferrous oxide Fe 2 O 3 = iron(III) oxide; ferric oxide Na. H 2 PO 4 = sodium dihydrogen phosphate. NH 4 OH = ammonium hydroxide. Fe. CO 3 = iron(II) carbonate or ferrous carbonate Fe 2(CO 3)3 = iron(III) carbonate or ferric carbonate.

What is the systematic name for Mn. O? 1. 2. 3. 4. manganese oxide manganese trioxide manganese (III) oxide manganese (II) oxide
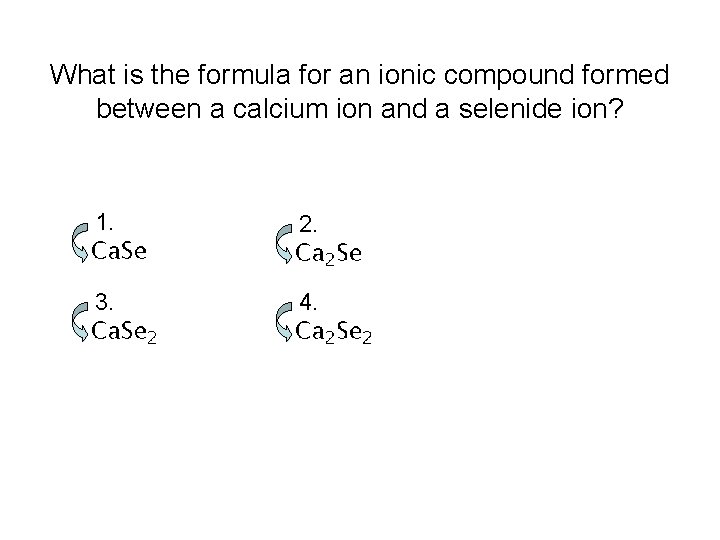
What is the formula for an ionic compound formed between a calcium ion and a selenide ion? 1. 2. 3. 4.
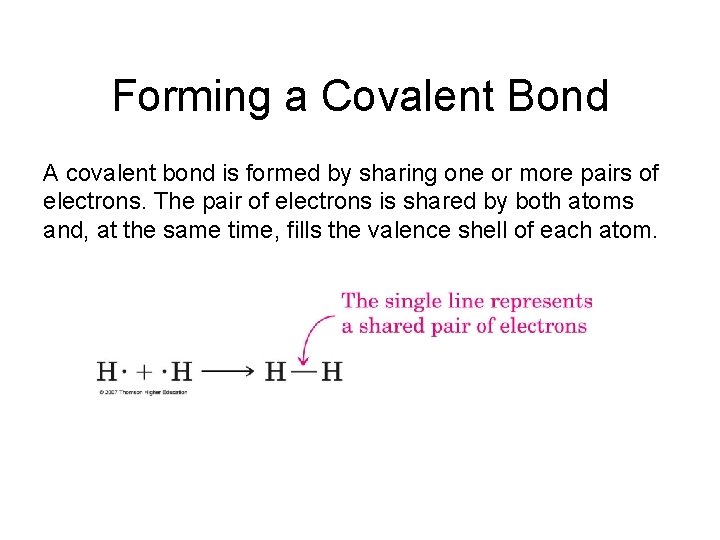
Forming a Covalent Bond A covalent bond is formed by sharing one or more pairs of electrons. The pair of electrons is shared by both atoms and, at the same time, fills the valence shell of each atom.
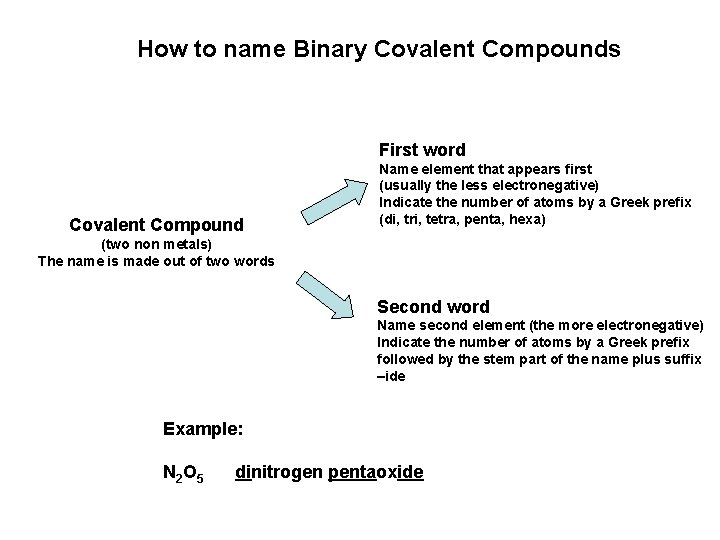
How to name Binary Covalent Compounds First word Covalent Compound Name element that appears first (usually the less electronegative) Indicate the number of atoms by a Greek prefix (di, tri, tetra, penta, hexa) (two non metals) The name is made out of two words Second word Name second element (the more electronegative) Indicate the number of atoms by a Greek prefix followed by the stem part of the name plus suffix –ide Example: N 2 O 5 dinitrogen pentaoxide

NO is nitrogen oxide (nitric oxide) SF 2 is sulfur difluoride N 2 O is dinitrogen oxide (laughing gas)

What is the systematic name for N 2 O 4? 1. 2. 3. 4. dinitrogen tetroxide dinitrogen tetroxygen nitrogen dioxide nitrogen (IV) oxide
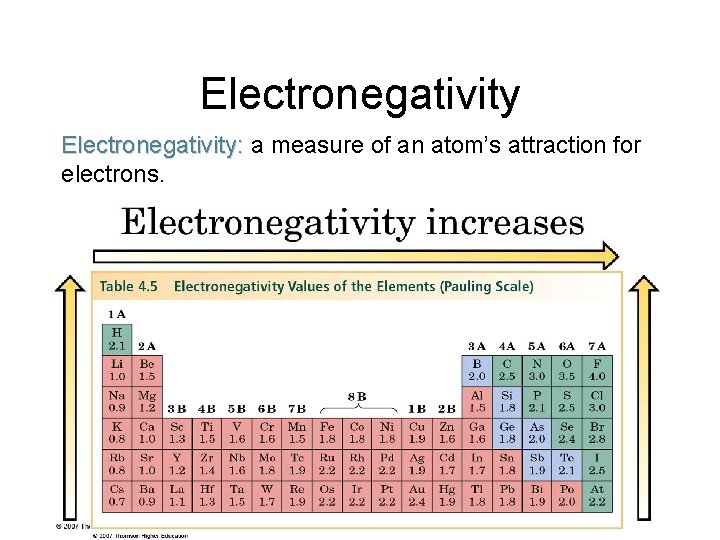
Electronegativity: a measure of an atom’s attraction for electrons.
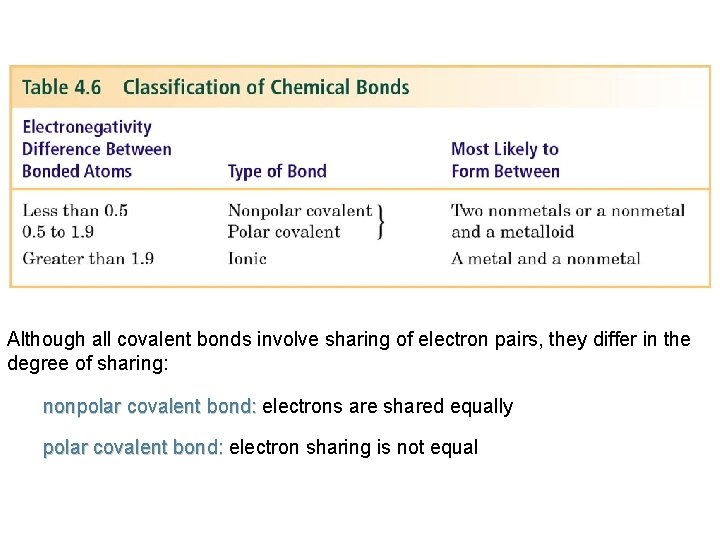
Although all covalent bonds involve sharing of electron pairs, they differ in the degree of sharing: nonpolar covalent bond: electrons are shared equally polar covalent bond: electron sharing is not equal
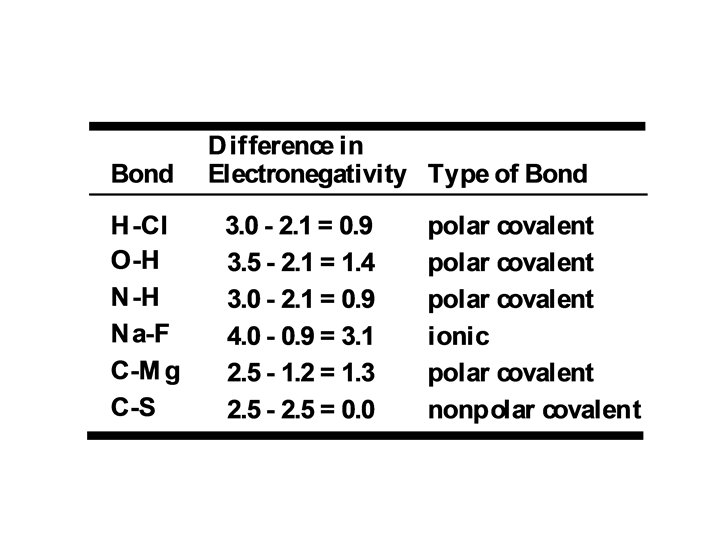
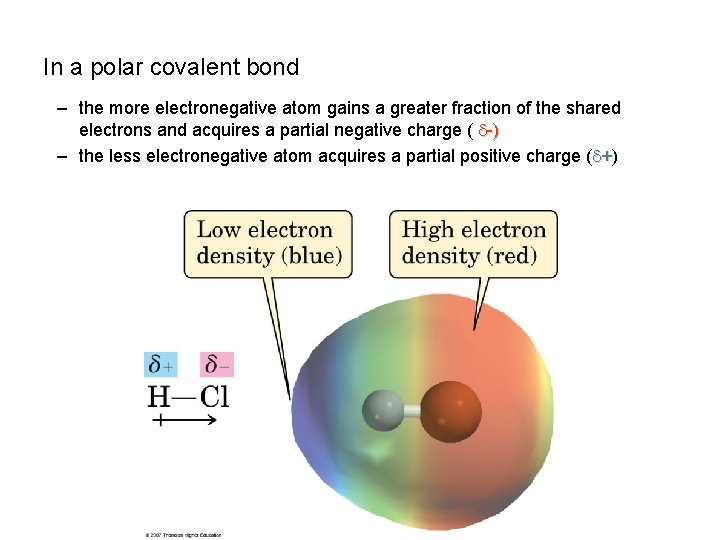
In a polar covalent bond – the more electronegative atom gains a greater fraction of the shared electrons and acquires a partial negative charge ( -) – the less electronegative atom acquires a partial positive charge ( +)

An ionic bond has a ____ electronegativity difference between atoms than a covalent bond almost always involves ____. 1. 2. 3. 4. greater; a metal and a nonmetal greater; two nonmetals lesser; a metal and a nonmetal lesser; two nonmetals
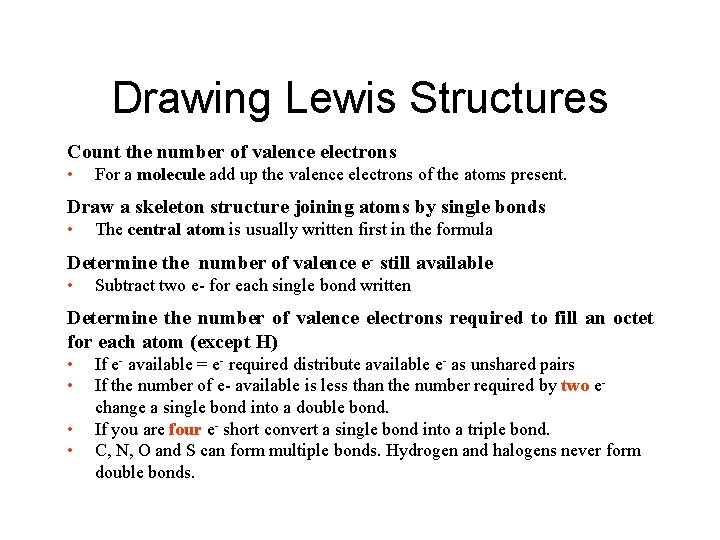
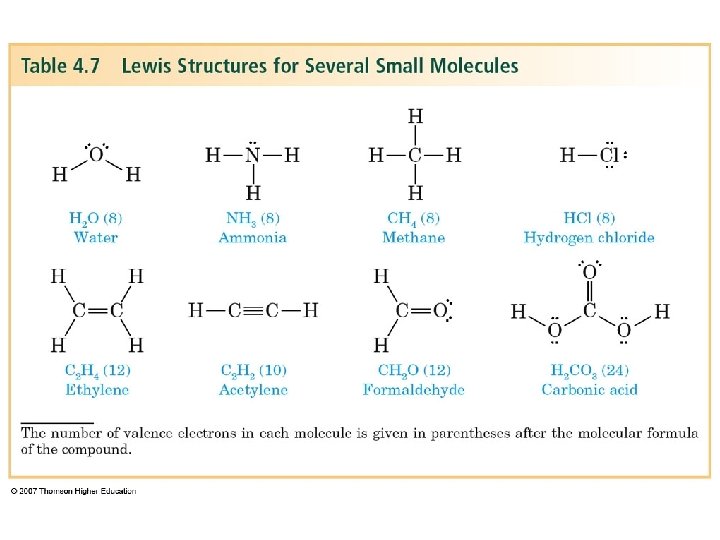
Drawing Lewis Structures Count the number of valence electrons • For a molecule add up the valence electrons of the atoms present. Draw a skeleton structure joining atoms by single bonds • The central atom is usually written first in the formula Determine the number of valence e- still available • Subtract two e- for each single bond written Determine the number of valence electrons required to fill an octet for each atom (except H) • • If e- available = e- required distribute available e- as unshared pairs If the number of e- available is less than the number required by two echange a single bond into a double bond. If you are four e- short convert a single bond into a triple bond. C, N, O and S can form multiple bonds. Hydrogen and halogens never form double bonds.
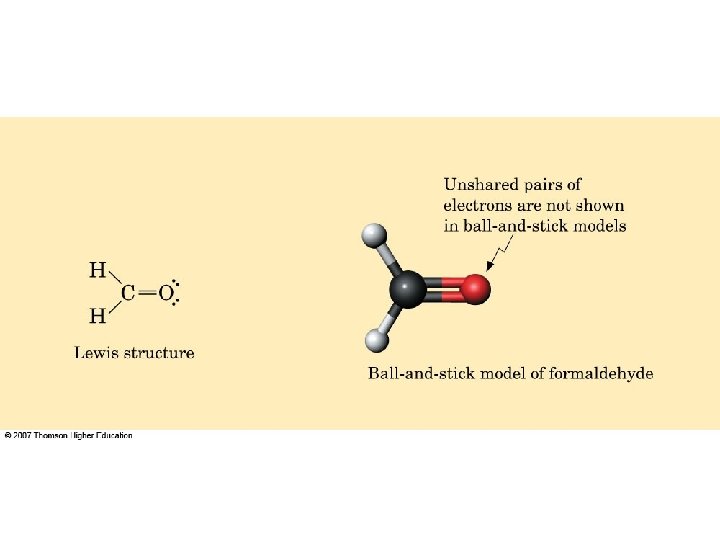
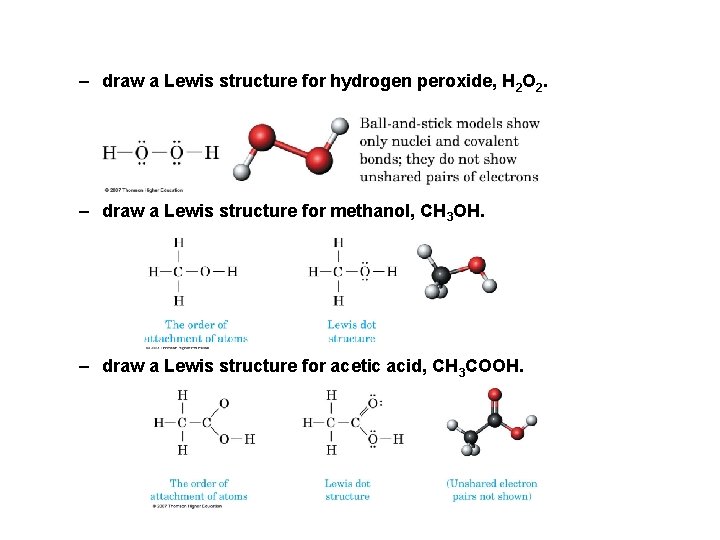
– draw a Lewis structure for hydrogen peroxide, H 2 O 2. – draw a Lewis structure for methanol, CH 3 OH. – draw a Lewis structure for acetic acid, CH 3 COOH.
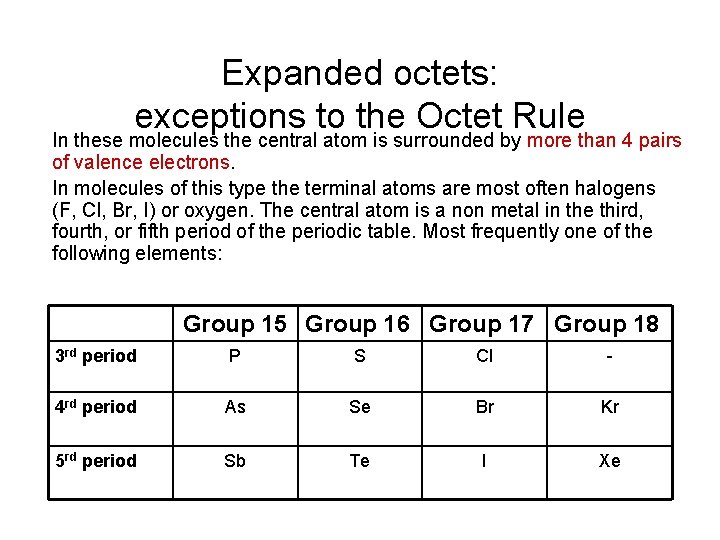
Expanded octets: exceptions to the Octet Rule In these molecules the central atom is surrounded by more than 4 pairs of valence electrons. In molecules of this type the terminal atoms are most often halogens (F, Cl, Br, I) or oxygen. The central atom is a non metal in the third, fourth, or fifth period of the periodic table. Most frequently one of the following elements: Group 15 Group 16 Group 17 Group 18 3 rd period P S Cl - 4 rd period As Se Br Kr 5 rd period Sb Te I Xe
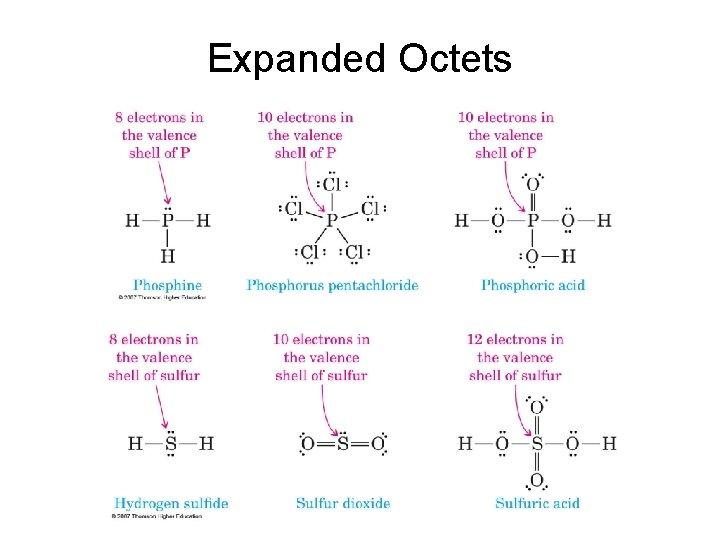
Expanded Octets
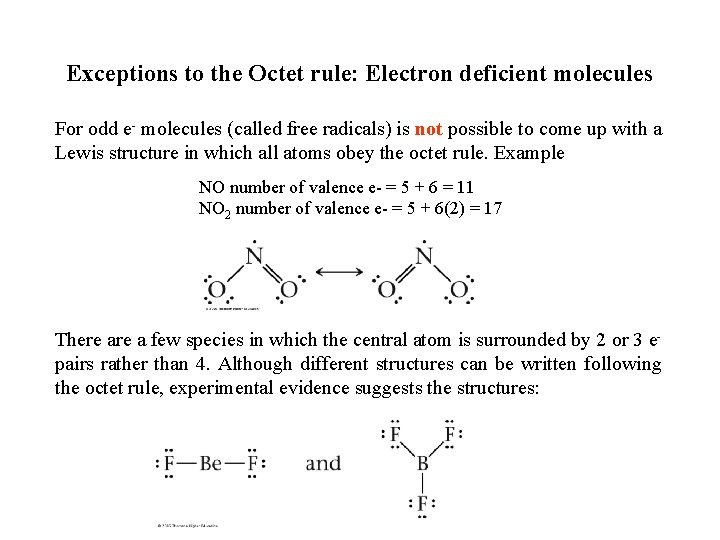
Exceptions to the Octet rule: Electron deficient molecules For odd e- molecules (called free radicals) is not possible to come up with a Lewis structure in which all atoms obey the octet rule. Example NO number of valence e- = 5 + 6 = 11 NO 2 number of valence e- = 5 + 6(2) = 17 There a few species in which the central atom is surrounded by 2 or 3 epairs rather than 4. Although different structures can be written following the octet rule, experimental evidence suggests the structures:
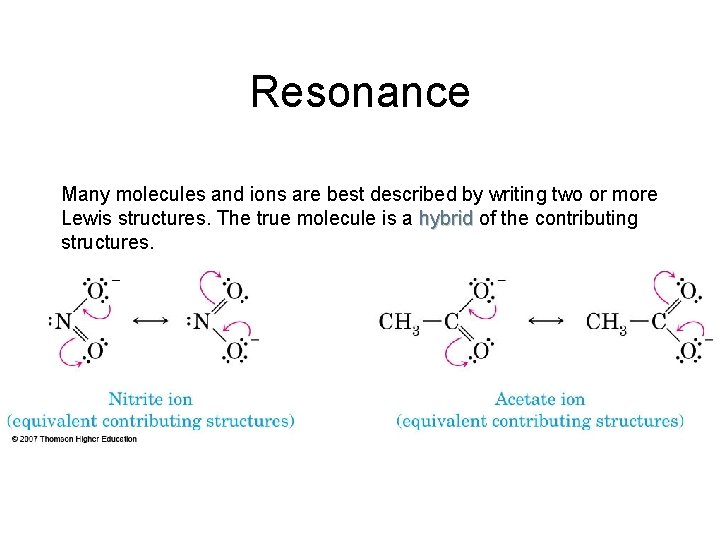
Resonance Many molecules and ions are best described by writing two or more Lewis structures. The true molecule is a hybrid of the contributing structures.
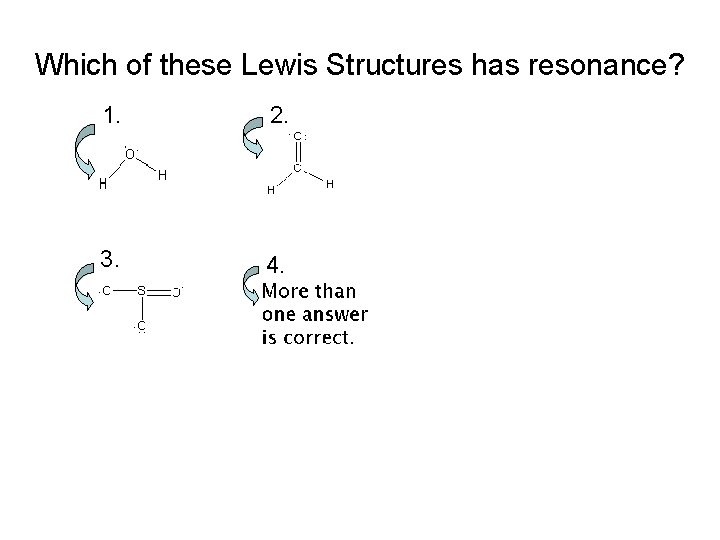
Which of these Lewis Structures has resonance? 1. 2. 3. 4.

Valence-Shell Electron-Pair Repulsion (VSEPR) Model – valence electrons of an atom may be involved in forming bonds or may be unshared. – each combination creates a negatively charged region of electrons around the nucleus. – because like charges repel each other, the various regions of electron density around an atom spread so that each is as far away from the others as possible.
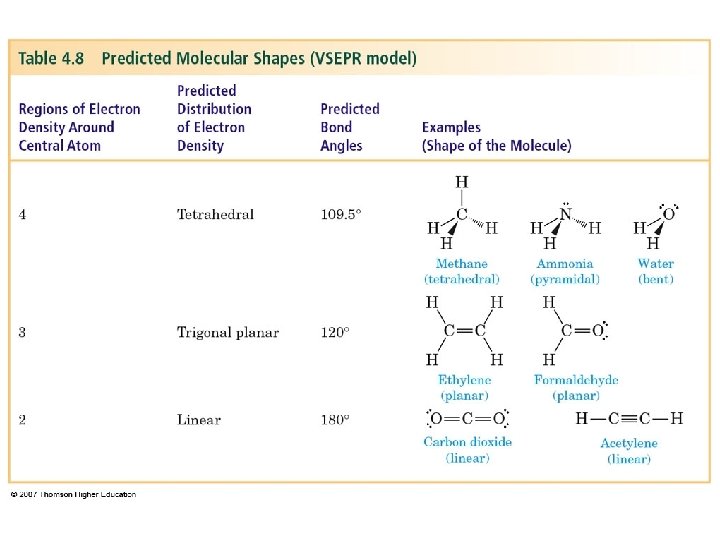
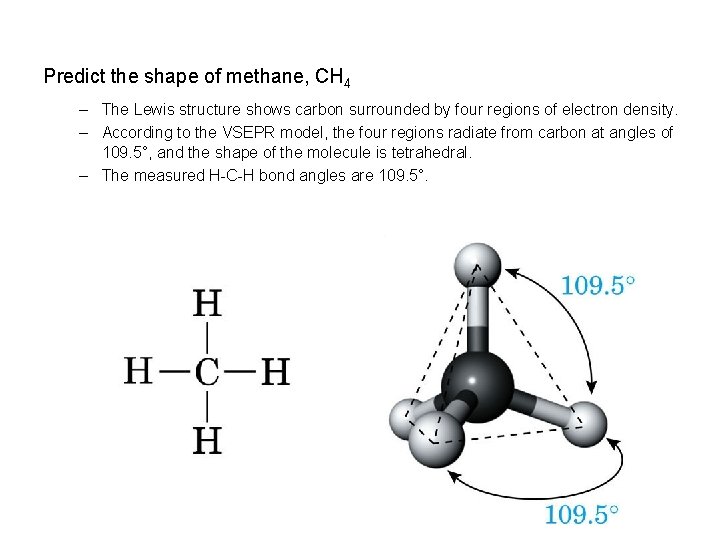
Predict the shape of methane, CH 4 – The Lewis structure shows carbon surrounded by four regions of electron density. – According to the VSEPR model, the four regions radiate from carbon at angles of 109. 5°, and the shape of the molecule is tetrahedral. – The measured H-C-H bond angles are 109. 5°.
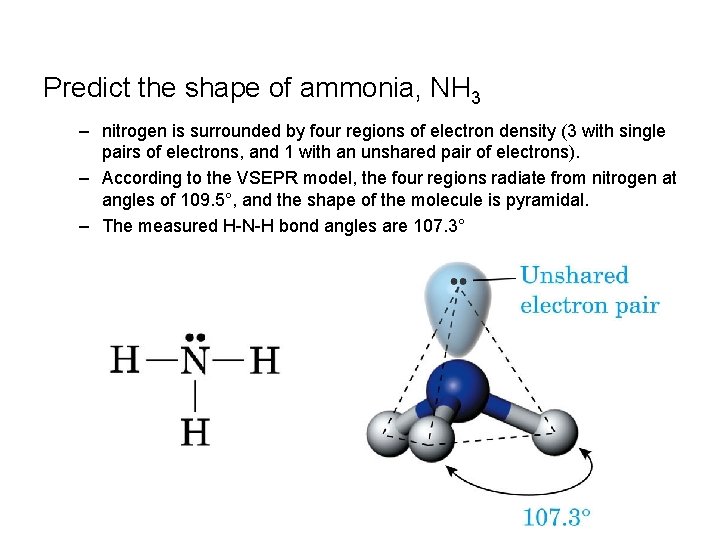
Predict the shape of ammonia, NH 3 – nitrogen is surrounded by four regions of electron density (3 with single pairs of electrons, and 1 with an unshared pair of electrons). – According to the VSEPR model, the four regions radiate from nitrogen at angles of 109. 5°, and the shape of the molecule is pyramidal. – The measured H-N-H bond angles are 107. 3°
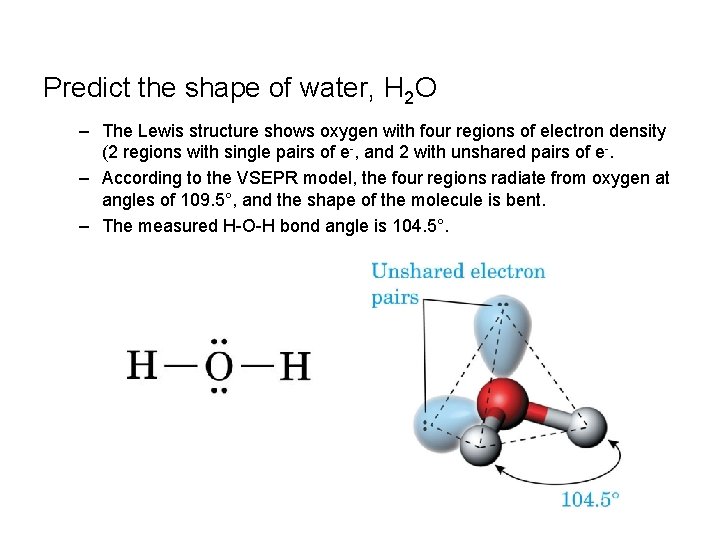
Predict the shape of water, H 2 O – The Lewis structure shows oxygen with four regions of electron density (2 regions with single pairs of e-, and 2 with unshared pairs of e-. – According to the VSEPR model, the four regions radiate from oxygen at angles of 109. 5°, and the shape of the molecule is bent. – The measured H-O-H bond angle is 104. 5°.
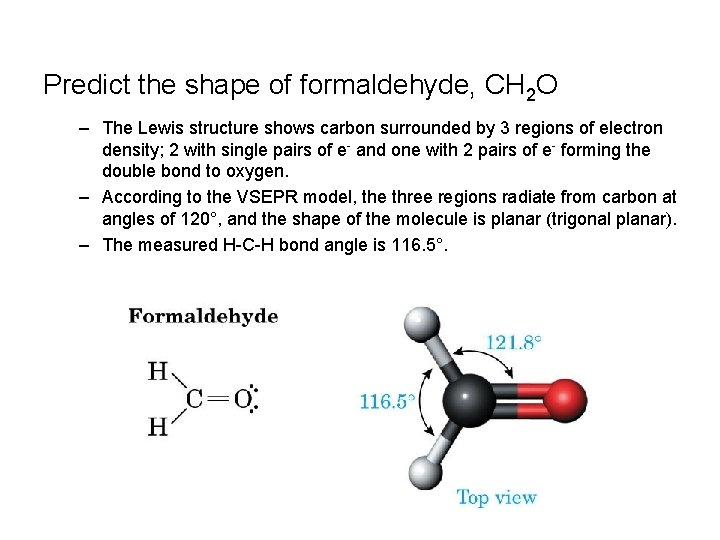
Predict the shape of formaldehyde, CH 2 O – The Lewis structure shows carbon surrounded by 3 regions of electron density; 2 with single pairs of e- and one with 2 pairs of e- forming the double bond to oxygen. – According to the VSEPR model, the three regions radiate from carbon at angles of 120°, and the shape of the molecule is planar (trigonal planar). – The measured H-C-H bond angle is 116. 5°.
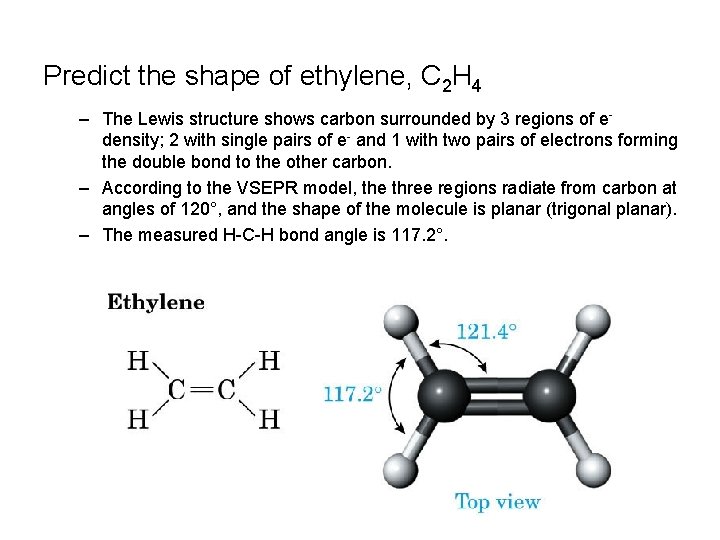
Predict the shape of ethylene, C 2 H 4 – The Lewis structure shows carbon surrounded by 3 regions of edensity; 2 with single pairs of e- and 1 with two pairs of electrons forming the double bond to the other carbon. – According to the VSEPR model, the three regions radiate from carbon at angles of 120°, and the shape of the molecule is planar (trigonal planar). – The measured H-C-H bond angle is 117. 2°.
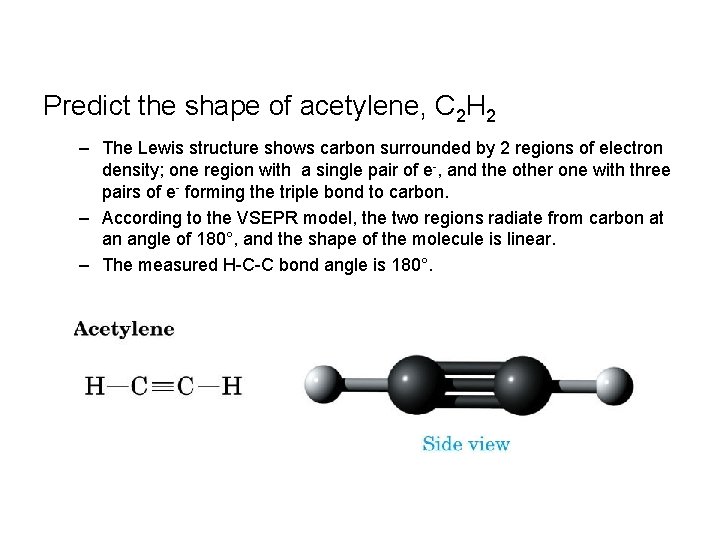
Predict the shape of acetylene, C 2 H 2 – The Lewis structure shows carbon surrounded by 2 regions of electron density; one region with a single pair of e-, and the other one with three pairs of e- forming the triple bond to carbon. – According to the VSEPR model, the two regions radiate from carbon at an angle of 180°, and the shape of the molecule is linear. – The measured H-C-C bond angle is 180°.

Polarity of Molecules A molecule will be polar if: – it has polar bonds, and – its centers of partial positive and partial negative charges lie at different places within the molecule.
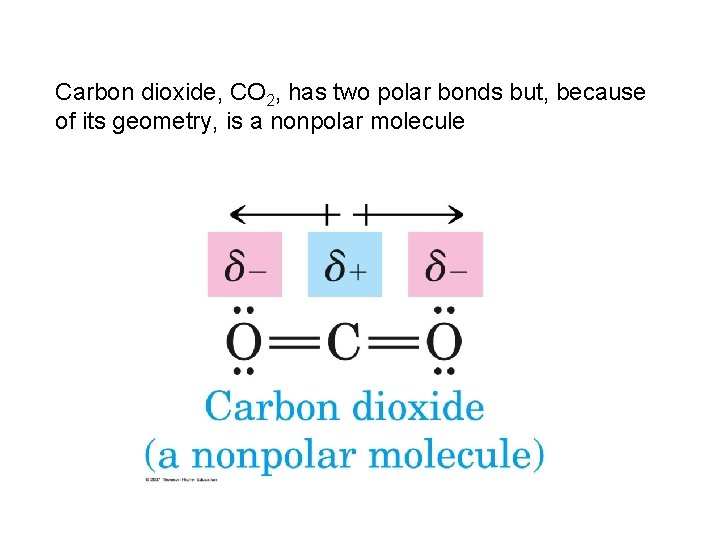
Carbon dioxide, CO 2, has two polar bonds but, because of its geometry, is a nonpolar molecule
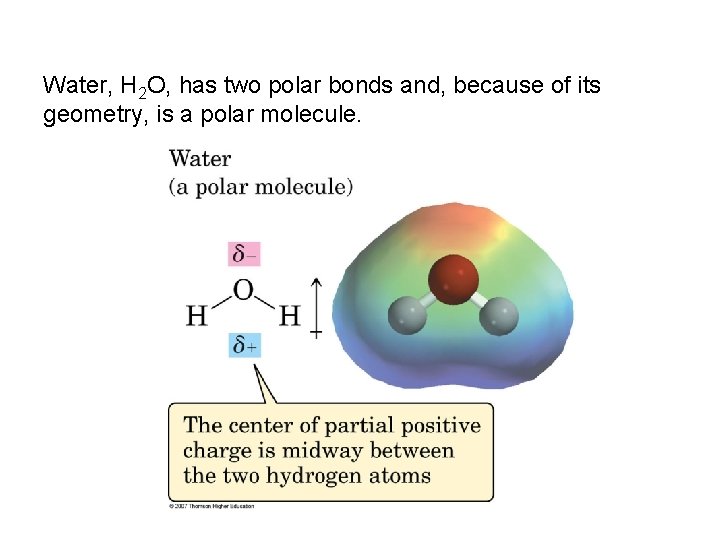
Water, H 2 O, has two polar bonds and, because of its geometry, is a polar molecule.
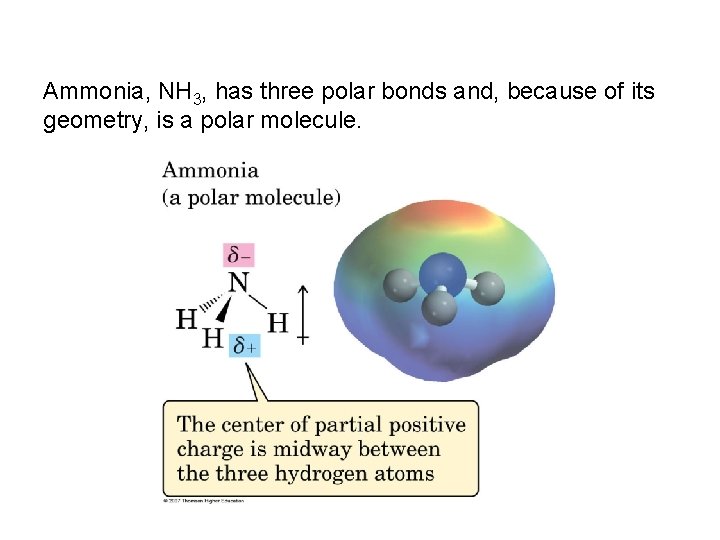
Ammonia, NH 3, has three polar bonds and, because of its geometry, is a polar molecule.
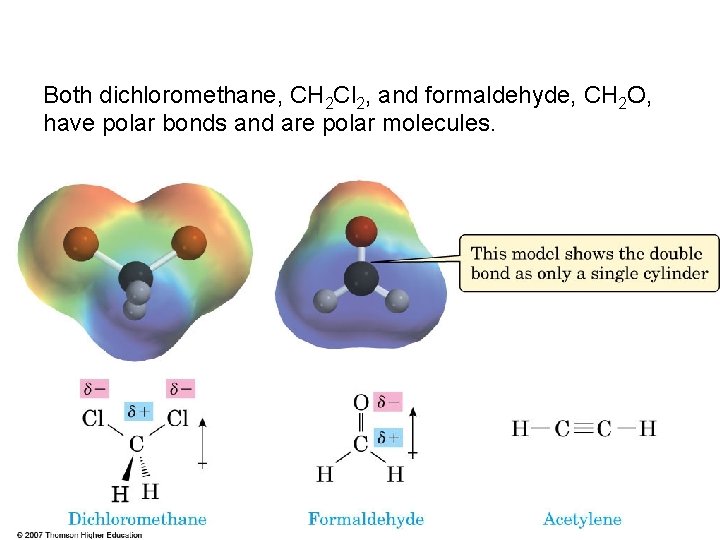
Both dichloromethane, CH 2 Cl 2, and formaldehyde, CH 2 O, have polar bonds and are polar molecules.
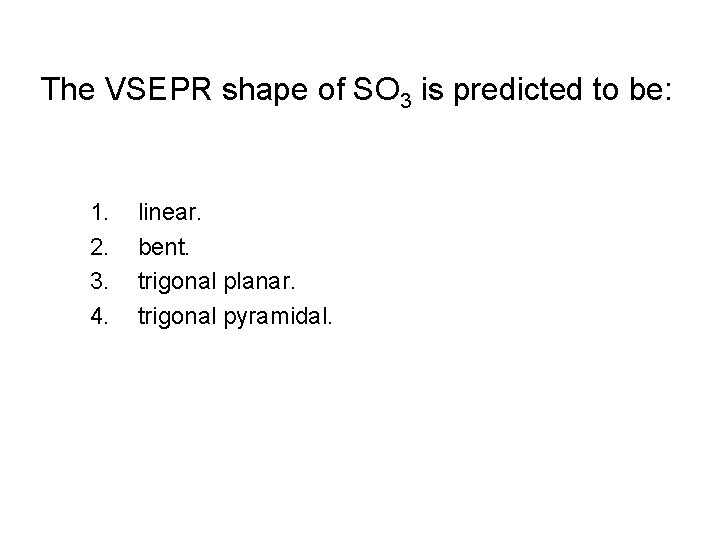
The VSEPR shape of SO 3 is predicted to be: 1. 2. 3. 4. linear. bent. trigonal planar. trigonal pyramidal.
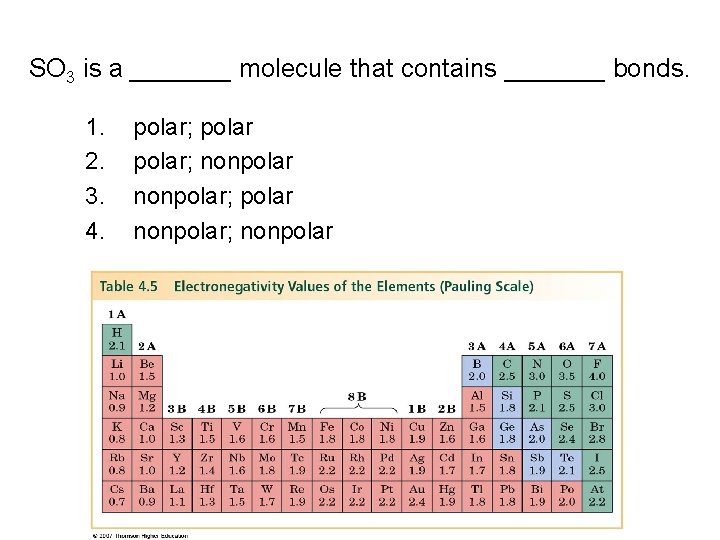
SO 3 is a _______ molecule that contains _______ bonds. 1. 2. 3. 4. polar; nonpolar; polar nonpolar; nonpolar

Chapter 4 Review Questions 1. Write the ion magnesium forms. What is the name? 2. Write the ion does chlorine forms. What is the name? 3. Write the symbols for the following ions. a) Silver(I) ion b) Iron (III) ion c) Cuprous ion 4. Name the following polyatomic ions. a) NO 3 b) CO 3 -2 c) OHd) PO 4 -3

5. Name the following compounds, using Roman numerals to indicate the charges on the cations where necessary. a) KF b) Mg. Cl 2 c) (NH 4)2 CO 3 d) Mg. SO 4 e) Fe 2 O 3 6. Write the formula for the following compounds. 5. a) sodium hydroxide 6. b) Magnesium chloride 7. c) copper(II) carbonate 8. d) calcium phosphate • Draw a Lewis structure for vinyl chloride, C 2 H 3 Cl, a substance used in making PVC plastic.
9. What shape do you expect for the hydronium ion, H 3 O+? 10. Predict the geometry of an acetaldehyde molecule, CH 3 CHO?
12. Use electronegativity differences to classify bonds between the following pairs of atoms as ionic, nonpolar covalent, or polar covalent. a) I (2. 7) and Cl (3. 2) b) Li (1. 0) and O (3. 4) c) Br (3. 0) and Br (3. 0) d) P (2. 2) and Br (3. 0) 13. Use the symbols + and - to identify the location of the partial charges on the polar covalent bonds formed between the following: a) Fluorine and sulfur b) phosphorus and oxygen 14. Name the following compounds. a) N 2 O 3 b) PCl 5 c) Se. F 4
- Slides: 54