Chapter 3 Calculations with Chemical Formulas and Equations
























- Slides: 78

Chapter 3 Calculations with Chemical Formulas and Equations

Contents and concepts Mass and Moles of Substances Molecular Mass and Formula Mass The Mole Concept, Molar Mass Determining Chemical Formulas 3. Mass Percentages from the Formula 4. Elemental Analysis: Percentages of C, H, and O 5. Determining Empirical, Molecular Formulas Stoichiometry: Quantitative Relations in Chemical Reactions 6. Molar Interpretation of a Chemical Equation 7. Amounts of Substances in a Chemical Equation 8. Limiting Reactant: Theoretical and Percentage Yield

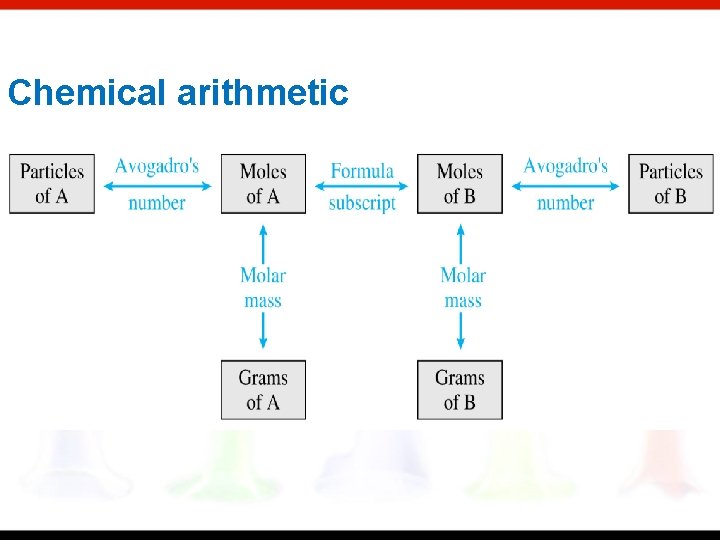
Chemical arithmetic

The law of definite proportions *The law states that: In a pure compound, the elements are always present in the same definite proportion by mass *Verifying the law of constant composition 1. Decomposition of a weighed amount of the compound into its individual elements. 2. Determination of the mass of a compound formed by the combination of known masses of its elements

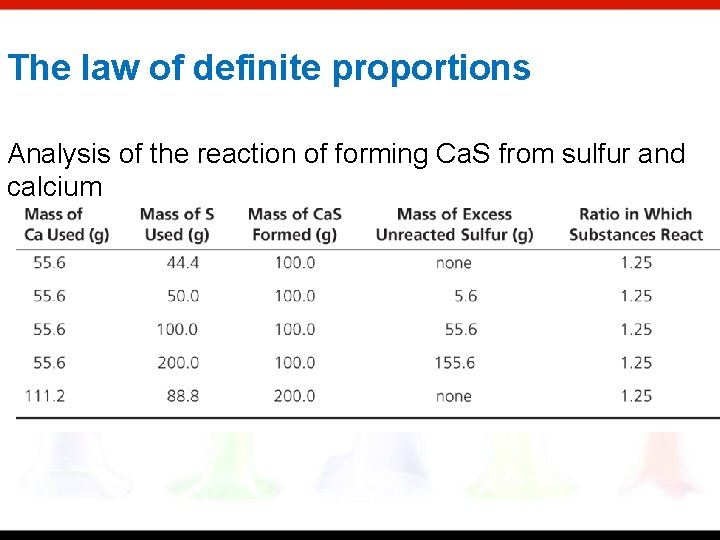
The law of definite proportions Analysis of the reaction of forming Ca. S from sulfur and calcium

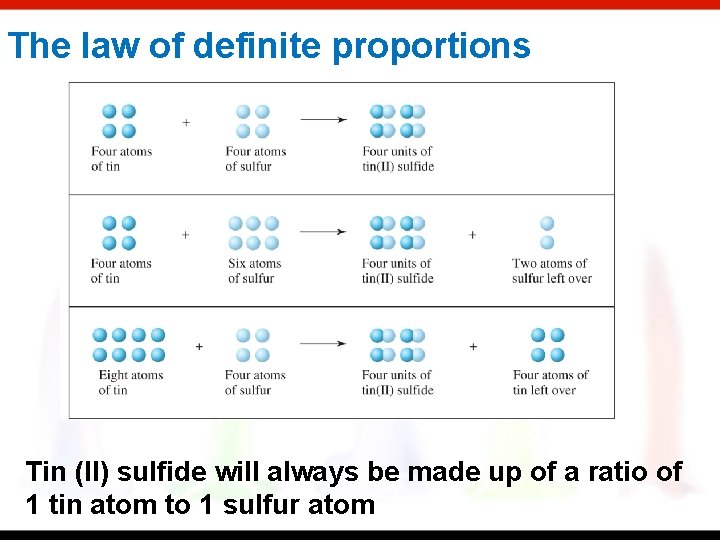
The law of definite proportions Tin (II) sulfide will always be made up of a ratio of 1 tin atom to 1 sulfur atom

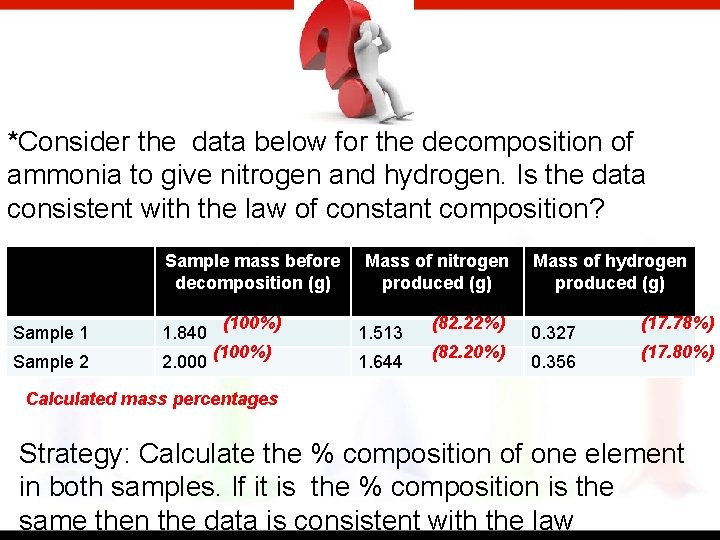
*Consider the data below for the decomposition of ammonia to give nitrogen and hydrogen. Is the data consistent with the law of constant composition? Sample mass before decomposition (g) Sample 1 1. 840 Sample 2 2. 000 (100%) Mass of nitrogen produced (g) 1. 513 1. 644 (82. 22%) (82. 20%) Mass of hydrogen produced (g) 0. 327 0. 356 (17. 78%) (17. 80%) Calculated mass percentages Strategy: Calculate the % composition of one element in both samples. If it is the % composition is the same then the data is consistent with the law

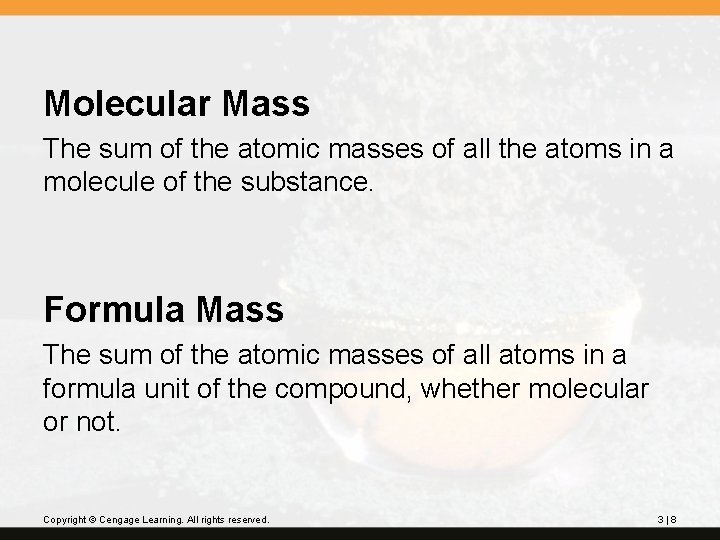
Molecular Mass The sum of the atomic masses of all the atoms in a molecule of the substance. Formula Mass The sum of the atomic masses of all atoms in a formula unit of the compound, whether molecular or not. Copyright © Cengage Learning. All rights reserved. 3|8

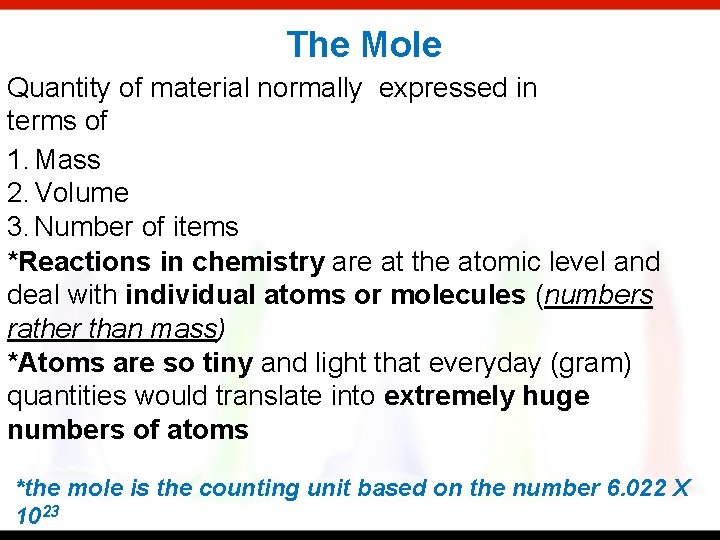
The Mole Quantity of material normally expressed in terms of 1. Mass 2. Volume 3. Number of items *Reactions in chemistry are at the atomic level and deal with individual atoms or molecules (numbers rather than mass) *Atoms are so tiny and light that everyday (gram) quantities would translate into extremely huge numbers of atoms *the mole is the counting unit based on the number 6. 022 X 1023

The Mole *Why 6. 02 X 1023? *Why not 1. 00 X 1023 or 1. 00 X 1025 or 1010? *The Mole is also equivalent to the number of atoms in exactly 12. 00 g of C-12 IN OTHER WORDS…. . WHEN EXPRESSED IN GRAMS; THE ATOMIC MASS of any atom (OR FORMULA MASS of a compound) contains 6. 02 x 1023 ATOMS (OR FORMULA UNITS). …. mole↔mass conversions are simplified by simply using atomic masses from the Periodic table

Counting numbers and the mole

Mole, mol The quantity of a given amount of substance that contains as many molecules or formula units as the number of atoms in exactly 12 g of carbon-12. Avogadro’s Number, NA The number of atoms in exactly 12 g of carbon-12 NA = 6. 022 × 1023 (to four significant figures). Copyright © Cengage Learning. All rights reserved. 3 | 12

Significant figures q Always follow sig. figs. Rules every time you preform a mathematical operation q Avogadro’s number = 6. 022 X 1023 particles ( do not consider it in determining appropriate sig figs ) q. All atomic and molar masses will be recorded to 2 decimal places q Remember : 1 amu = 1. 661 × 10− 27 kg ( do not consider it in determining appropriate sig figs )

? Calculate the formula weight of the following compounds from their formulas. Report your answers to three significant figures. calcium hydroxide, Ca(OH)2 methylamine, CH 3 NH 2

Ca(OH)2 1 Ca 1(40. 08) = 40. 08 amu 2 O 2(16. 00) = 32. 00 amu 2 H 2(1. 008) = 2. 02 amu Total 74. 10 amu CH 3 NH 2 1 C 1(12. 01) = 12. 01 amu 1 N 1(14. 01) = 14. 01 amu 5 H 5(1. 008) = 5. 04 amu Total 31. 060 31. 06 amu

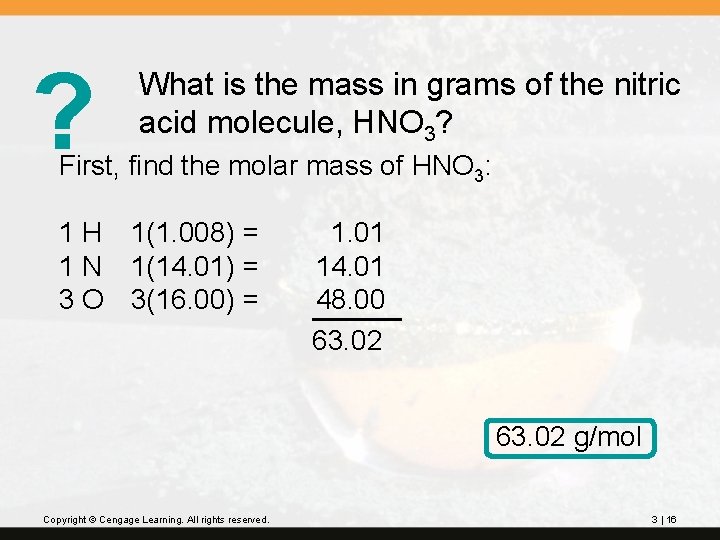
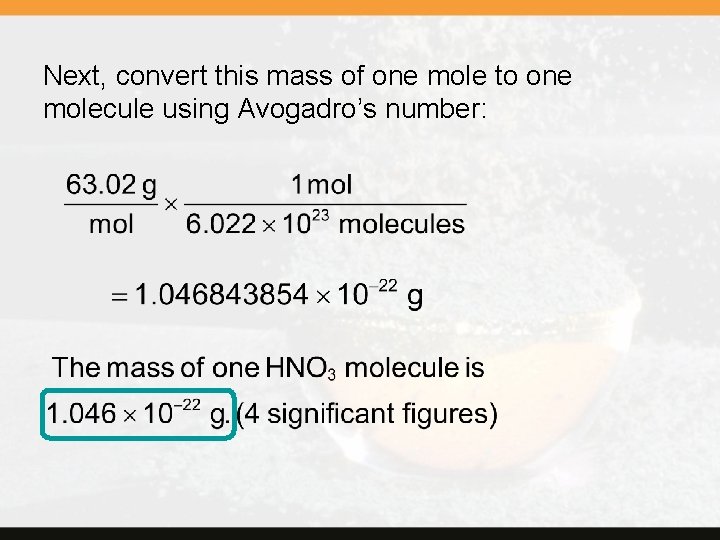
? What is the mass in grams of the nitric acid molecule, HNO 3? First, find the molar mass of HNO 3: 1 H 1(1. 008) = 1 N 1(14. 01) = 3 O 3(16. 00) = 1. 01 14. 01 48. 00 63. 02 g/mol Copyright © Cengage Learning. All rights reserved. 3 | 16

Next, convert this mass of one mole to one molecule using Avogadro’s number:

Molar Mass The mass of one mole of substance. For example: Carbon-12 has a molar mass of 12 g/mol

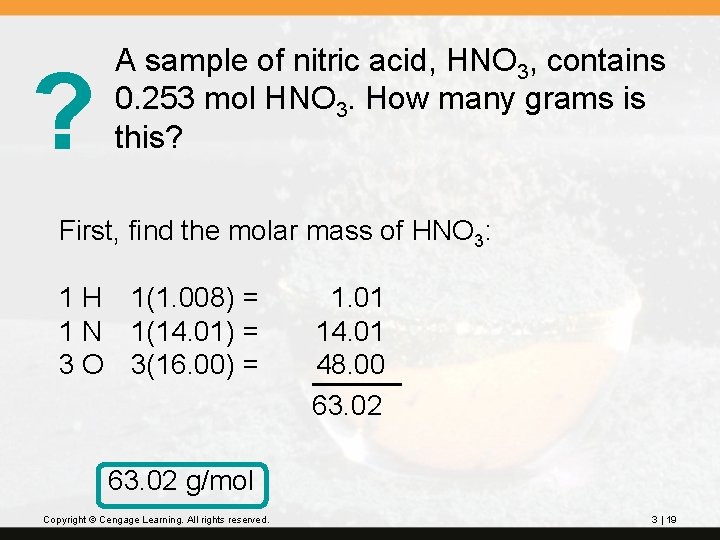
? A sample of nitric acid, HNO 3, contains 0. 253 mol HNO 3. How many grams is this? First, find the molar mass of HNO 3: 1 H 1(1. 008) = 1 N 1(14. 01) = 3 O 3(16. 00) = 1. 01 14. 01 48. 00 63. 02 g/mol Copyright © Cengage Learning. All rights reserved. 3 | 19

Next, using the molar mass, find the mass of 0. 253 mole: = 15. 94406 g Copyright © Cengage Learning. All rights reserved. 3 | 20

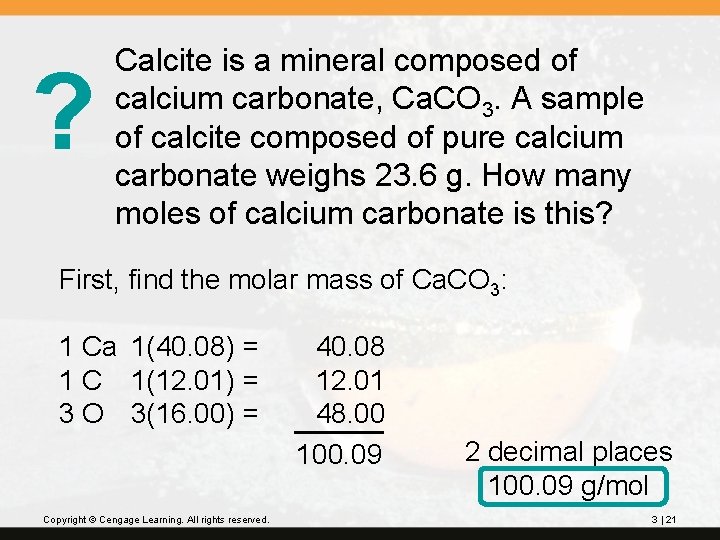
? Calcite is a mineral composed of calcium carbonate, Ca. CO 3. A sample of calcite composed of pure calcium carbonate weighs 23. 6 g. How many moles of calcium carbonate is this? First, find the molar mass of Ca. CO 3: 1 Ca 1(40. 08) = 1 C 1(12. 01) = 3 O 3(16. 00) = Copyright © Cengage Learning. All rights reserved. 40. 08 12. 01 48. 00 100. 09 2 decimal places 100. 09 g/mol 3 | 21

Next, find the number of moles in 23. 6 g: Copyright © Cengage Learning. All rights reserved. 3 | 22

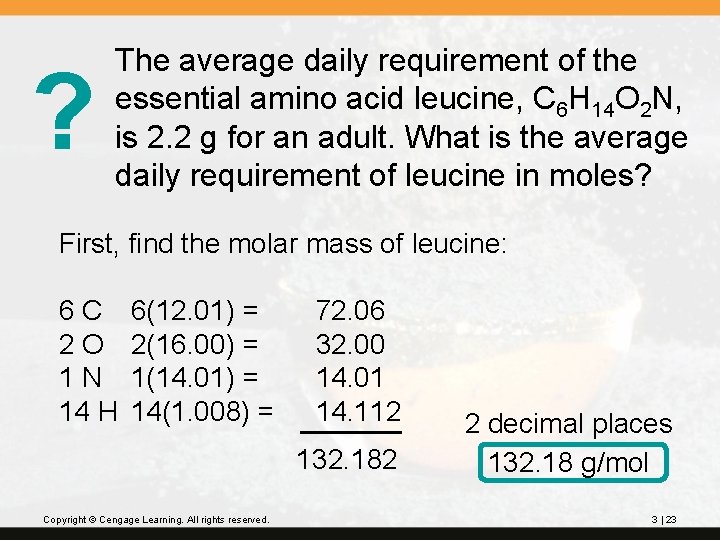
? The average daily requirement of the essential amino acid leucine, C 6 H 14 O 2 N, is 2. 2 g for an adult. What is the average daily requirement of leucine in moles? First, find the molar mass of leucine: 6 C 2 O 1 N 14 H 6(12. 01) = 2(16. 00) = 1(14. 01) = 14(1. 008) = 72. 06 32. 00 14. 01 14. 112 132. 182 Copyright © Cengage Learning. All rights reserved. 2 decimal places 132. 18 g/mol 3 | 23

Next, find the number of moles in 2. 2 g: Copyright © Cengage Learning. All rights reserved. 3 | 24

? The daily requirement of chromium in the human diet is 1. 0 × 10 -6 g. How many atoms of chromium does this represent? Copyright © Cengage Learning. All rights reserved. 3 | 25

First, find the molar mass of Cr: 1 Cr 1(51. 996) = 51. 996 Now, convert 1. 0 x 10 -6 grams to moles: =1. 158166 1016 atoms 1. 2 1016 atoms (2 significant figures)

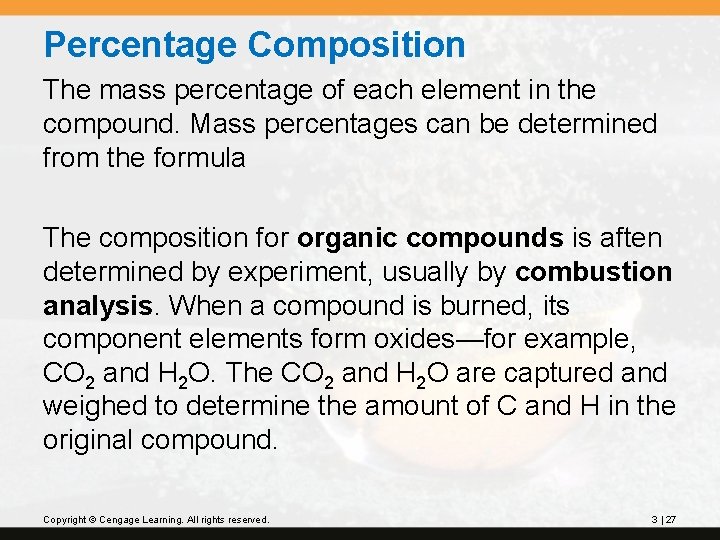
Percentage Composition The mass percentage of each element in the compound. Mass percentages can be determined from the formula The composition for organic compounds is aften determined by experiment, usually by combustion analysis. When a compound is burned, its component elements form oxides—for example, CO 2 and H 2 O. The CO 2 and H 2 O are captured and weighed to determine the amount of C and H in the original compound. Copyright © Cengage Learning. All rights reserved. 3 | 27

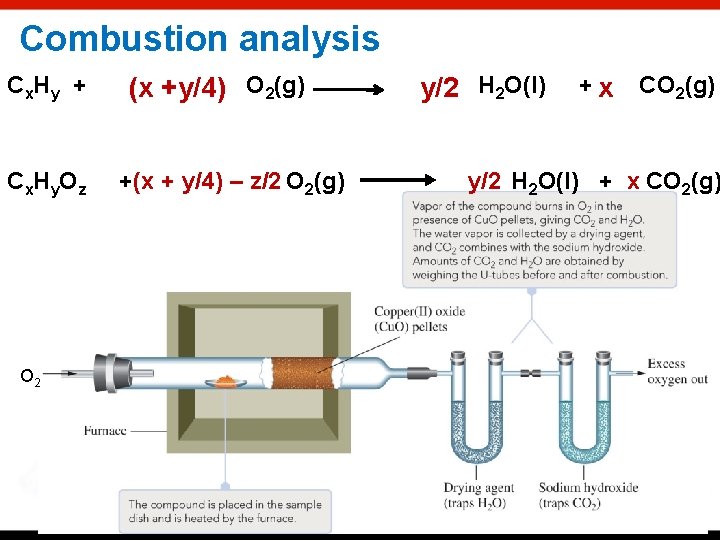
Combustion analysis C x. H y + C x. H y. O z O 2 (x +y/4) O 2(g) +(x + y/4) – z/2 O 2(g) y/2 H 2 O(l) +x CO 2(g) y/2 H 2 O(l) + x CO 2(g)

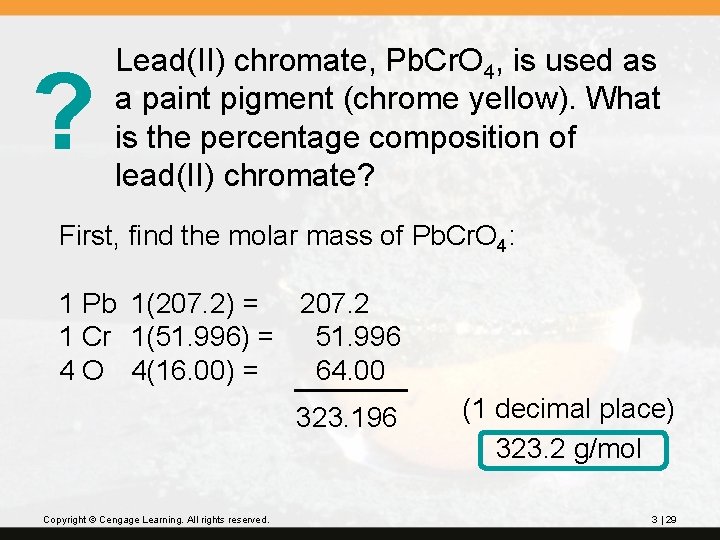
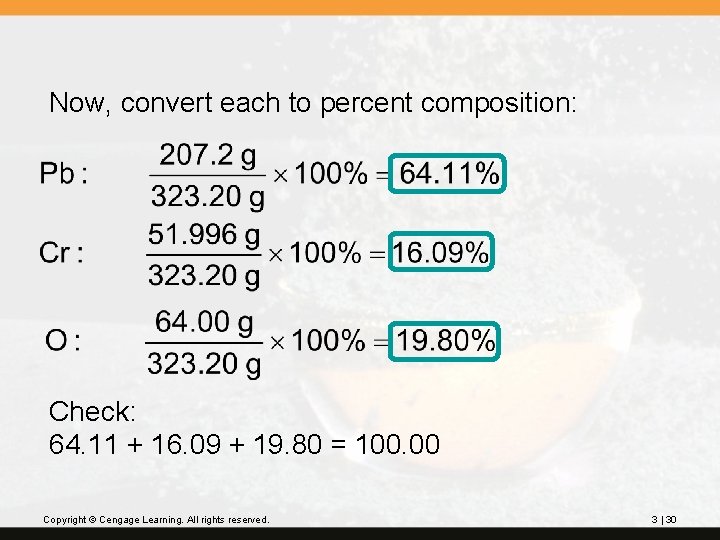
? Lead(II) chromate, Pb. Cr. O 4, is used as a paint pigment (chrome yellow). What is the percentage composition of lead(II) chromate? First, find the molar mass of Pb. Cr. O 4: 1 Pb 1(207. 2) = 207. 2 1 Cr 1(51. 996) = 51. 996 4 O 4(16. 00) = 64. 00 323. 196 Copyright © Cengage Learning. All rights reserved. (1 decimal place) 323. 2 g/mol 3 | 29

Now, convert each to percent composition: Check: 64. 11 + 16. 09 + 19. 80 = 100. 00 Copyright © Cengage Learning. All rights reserved. 3 | 30

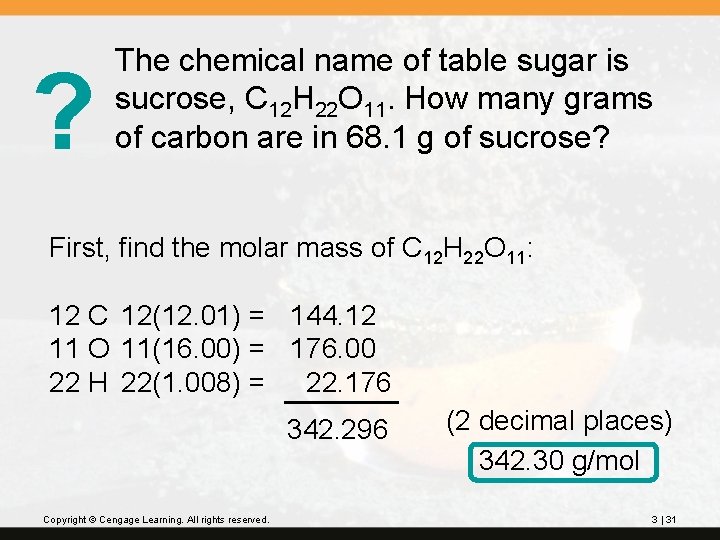
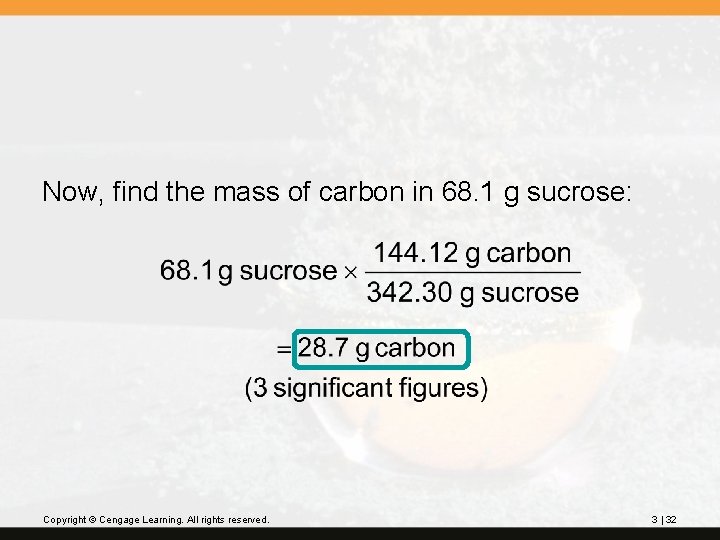
? The chemical name of table sugar is sucrose, C 12 H 22 O 11. How many grams of carbon are in 68. 1 g of sucrose? First, find the molar mass of C 12 H 22 O 11: 12 C 12(12. 01) = 144. 12 11 O 11(16. 00) = 176. 00 22 H 22(1. 008) = 22. 176 342. 296 Copyright © Cengage Learning. All rights reserved. (2 decimal places) 342. 30 g/mol 3 | 31

Now, find the mass of carbon in 68. 1 g sucrose: Copyright © Cengage Learning. All rights reserved. 3 | 32

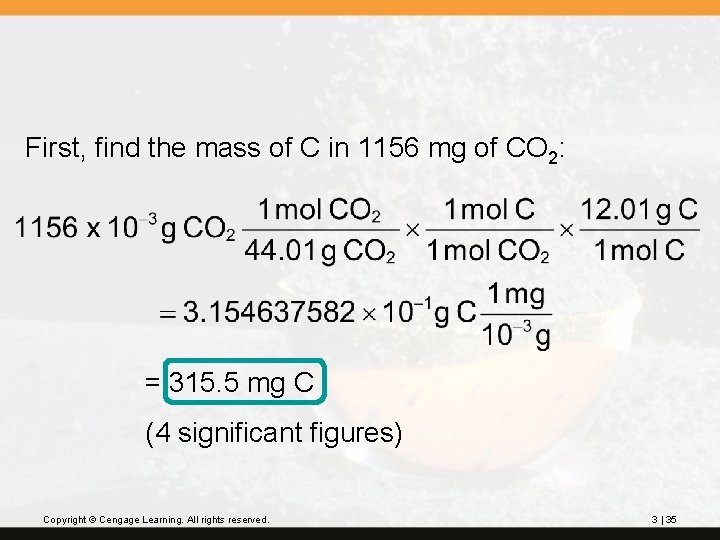
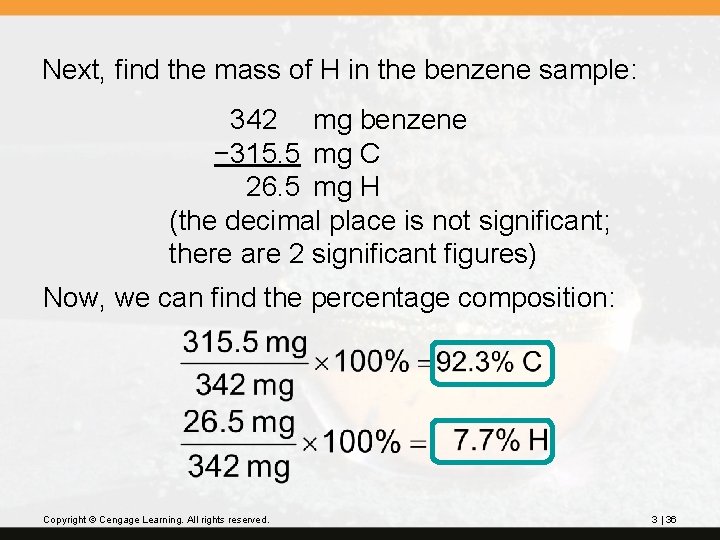
? Benzene is a liquid compound composed of carbon and hydrogen; it is used in the preparation of polystyrene plastic. A sample of benzene weighing 342 mg is burned in oxygen and forms 1156 mg of carbon dioxide. What is the percentage composition of benzene? Copyright © Cengage Learning. All rights reserved. 3 | 33

Strategy 1. Use the mass of CO 2 to find the mass of carbon from the benzene. 2. Use the mass of benzene and the mass of carbon to find the mass of hydrogen. 3. Use these two masses to find the percent composition. Copyright © Cengage Learning. All rights reserved. 3 | 34

First, find the mass of C in 1156 mg of CO 2: = 315. 5 mg C (4 significant figures) Copyright © Cengage Learning. All rights reserved. 3 | 35

Next, find the mass of H in the benzene sample: 342 mg benzene − 315. 5 mg C 26. 5 mg H (the decimal place is not significant; there are 2 significant figures) Now, we can find the percentage composition: Copyright © Cengage Learning. All rights reserved. 3 | 36

Chemical arithmetic…… …. ……. how many conversions do I need to perform? ……. . which conversions do I need to perform?

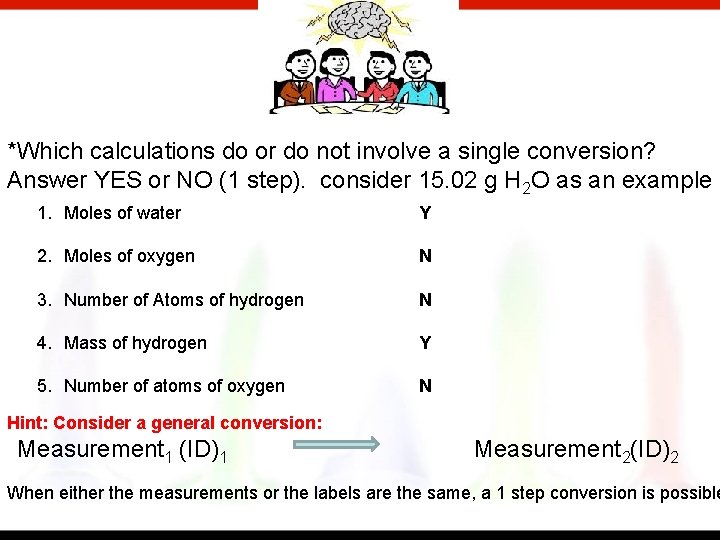
*Which calculations do or do not involve a single conversion? Answer YES or NO (1 step). consider 15. 02 g H 2 O as an example 1. Moles of water Y 2. Moles of oxygen N 3. Number of Atoms of hydrogen N 4. Mass of hydrogen Y 5. Number of atoms of oxygen N Hint: Consider a general conversion: Measurement 1 (ID)1 Measurement 2(ID)2 When either the measurements or the labels are the same, a 1 step conversion is possible

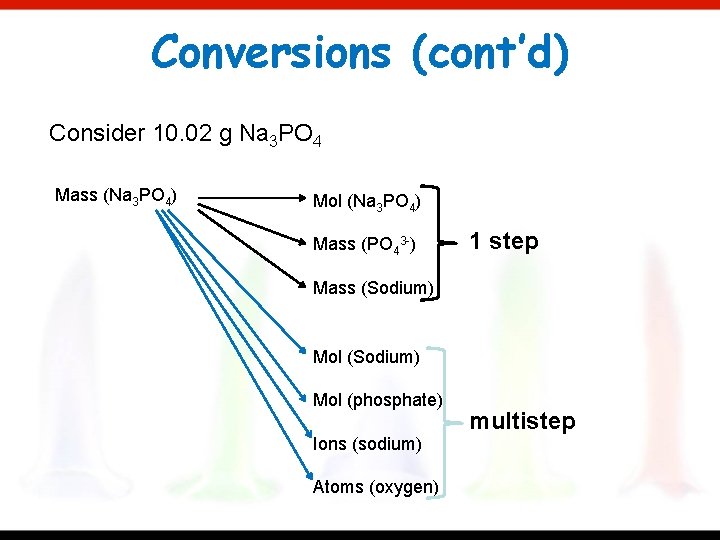
Conversions (cont’d) Consider 10. 02 g Na 3 PO 4 Mass (Na 3 PO 4) Mol (Na 3 PO 4) Mass (PO 43 -) 1 step Mass (Sodium) Mol (phosphate) Ions (sodium) Atoms (oxygen) multistep

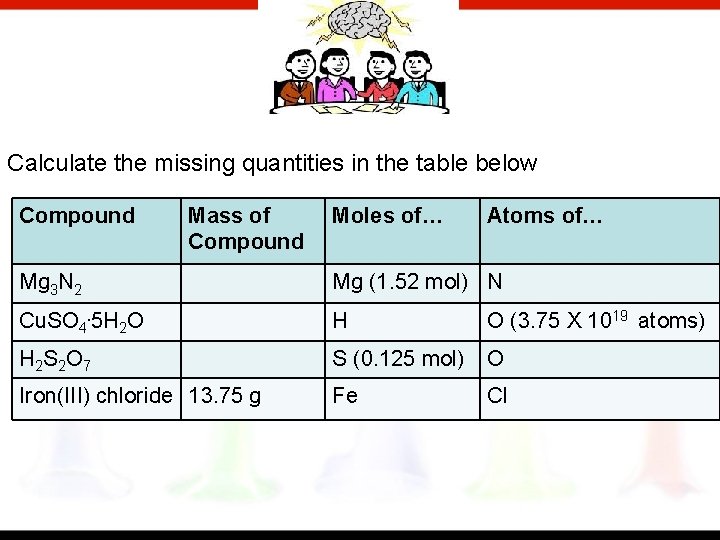
Calculate the missing quantities in the table below Compound Mass of Compound Moles of… Atoms of… Mg 3 N 2 Mg (1. 52 mol) N Cu. SO 4. 5 H 2 O H O (3. 75 X 1019 atoms) H 2 S 2 O 7 S (0. 125 mol) O Iron(III) chloride 13. 75 g Fe Cl

Empirical Formula (Simplest Formula) The formula of a substance written with the smallest integer subscripts. For example: The empirical formula for N 2 O 4 is NO 2. The empirical formula for H 2 O 2 is HO. Copyright © Cengage Learning. All rights reserved. 3 | 41

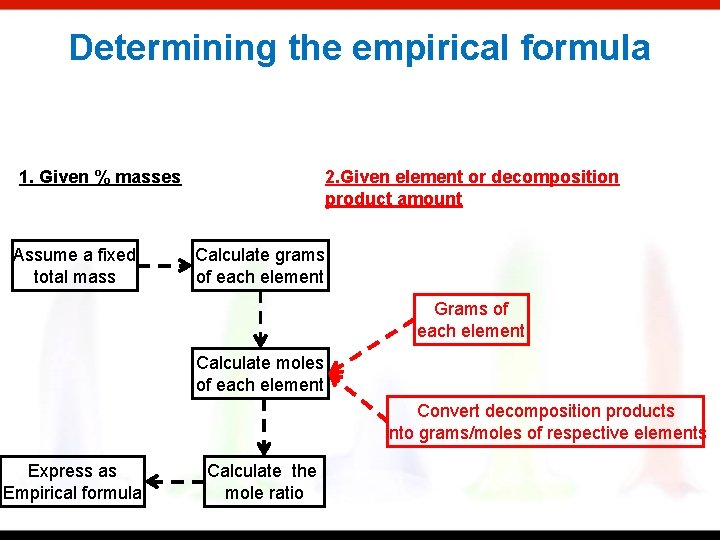
Determining the empirical formula 1. Given % masses Assume a fixed total mass 2. Given element or decomposition product amount Calculate grams of each element Grams of each element Calculate moles of each element Convert decomposition products into grams/moles of respective elements Express as Empirical formula Calculate the mole ratio

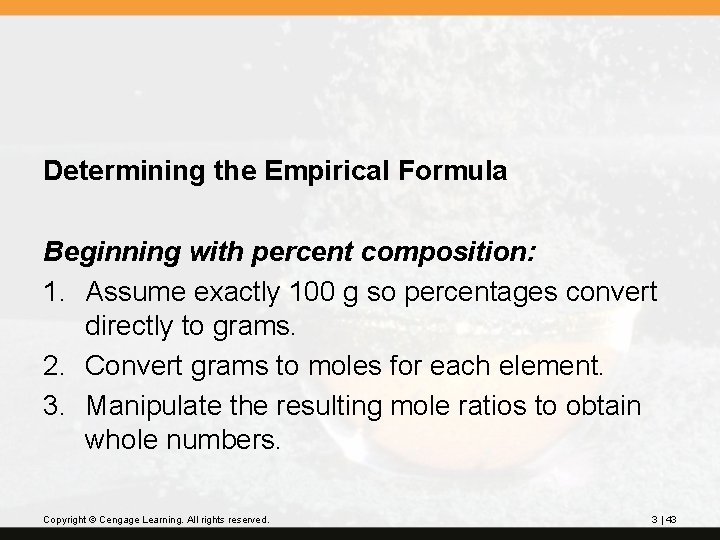
Determining the Empirical Formula Beginning with percent composition: 1. Assume exactly 100 g so percentages convert directly to grams. 2. Convert grams to moles for each element. 3. Manipulate the resulting mole ratios to obtain whole numbers. Copyright © Cengage Learning. All rights reserved. 3 | 43

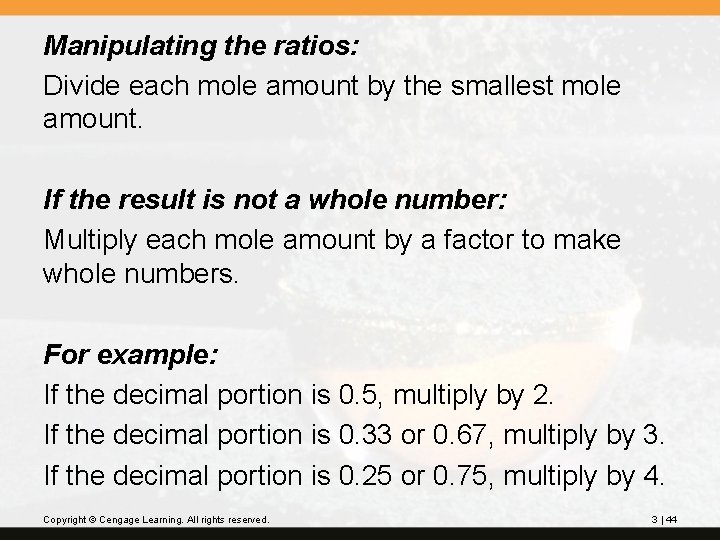
Manipulating the ratios: Divide each mole amount by the smallest mole amount. If the result is not a whole number: Multiply each mole amount by a factor to make whole numbers. For example: If the decimal portion is 0. 5, multiply by 2. If the decimal portion is 0. 33 or 0. 67, multiply by 3. If the decimal portion is 0. 25 or 0. 75, multiply by 4. Copyright © Cengage Learning. All rights reserved. 3 | 44

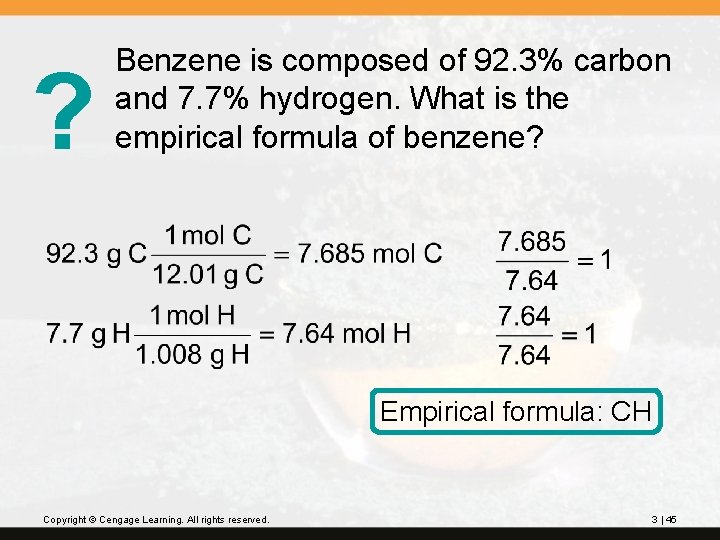
? Benzene is composed of 92. 3% carbon and 7. 7% hydrogen. What is the empirical formula of benzene? Empirical formula: CH Copyright © Cengage Learning. All rights reserved. 3 | 45

Molecular Formula A formula for a molecule in which the subscripts are whole-number multiples of the subscripts in the empirical formula. Copyright © Cengage Learning. All rights reserved. 3 | 46

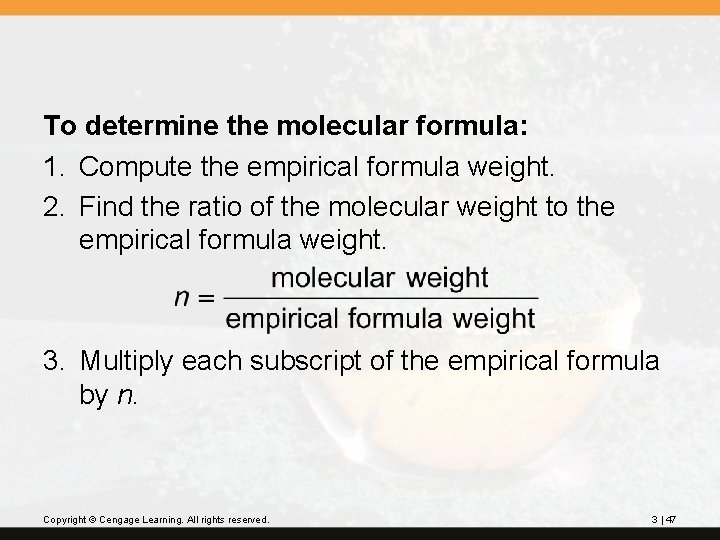
To determine the molecular formula: 1. Compute the empirical formula weight. 2. Find the ratio of the molecular weight to the empirical formula weight. 3. Multiply each subscript of the empirical formula by n. Copyright © Cengage Learning. All rights reserved. 3 | 47

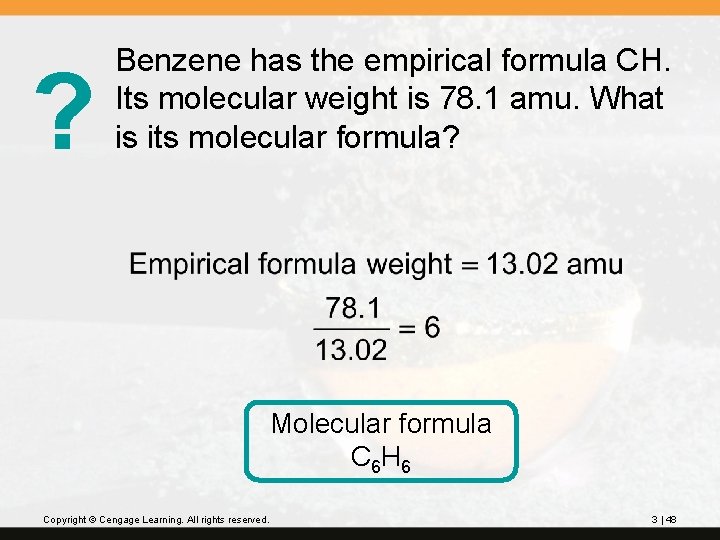
? Benzene has the empirical formula CH. Its molecular weight is 78. 1 amu. What is its molecular formula? Molecular formula C 6 H 6 Copyright © Cengage Learning. All rights reserved. 3 | 48

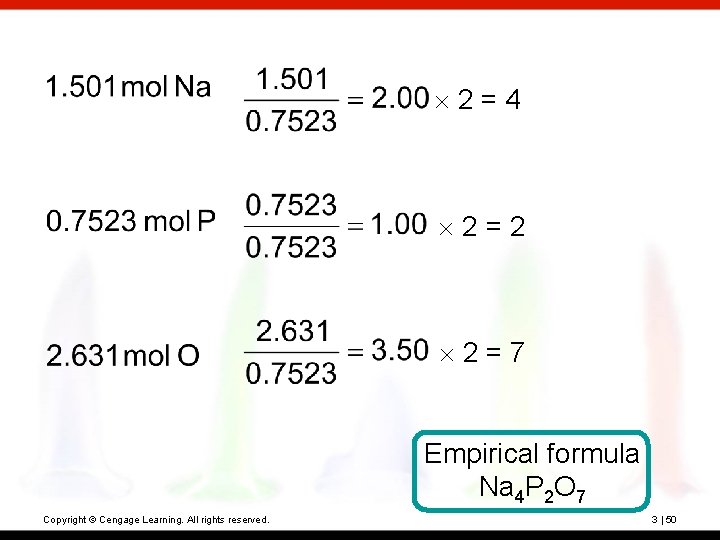
? Sodium pyrophosphate is used in detergent preparations. It is composed of 34. 5% Na, 23. 3% P, and 42. 1% O. What is its empirical formula? Copyright © Cengage Learning. All rights reserved. 3 | 49

2=4 2=2 2=7 Empirical formula Na 4 P 2 O 7 Copyright © Cengage Learning. All rights reserved. 3 | 50

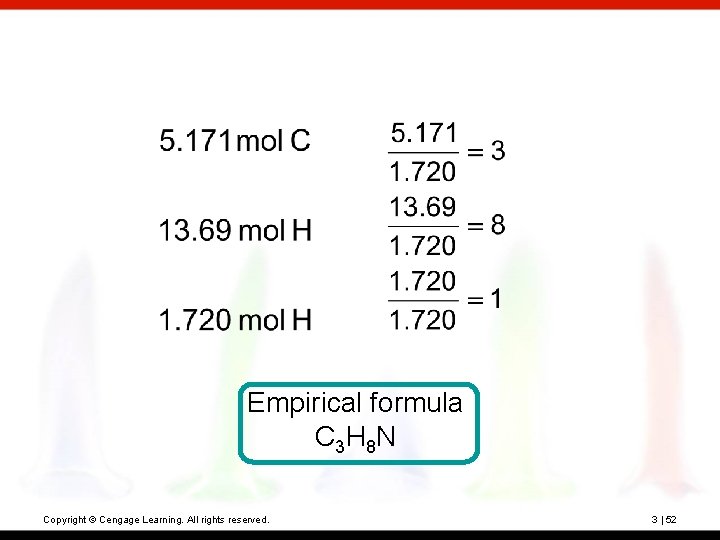
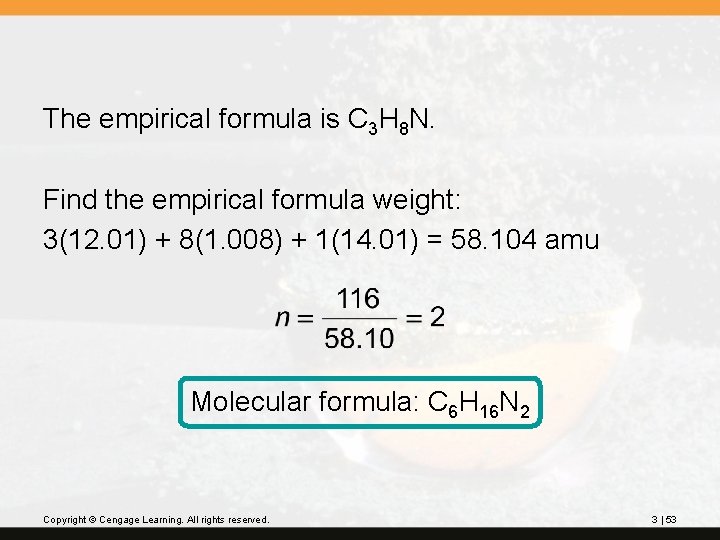
? Hexamethylene is one of the materials used to produce a type of nylon. It is composed of 62. 1% C, 13. 8% H, and 24. 1% N. Its molecular weight is 116 amu. What is its molecular formula? Copyright © Cengage Learning. All rights reserved. 3 | 51

Empirical formula C 3 H 8 N Copyright © Cengage Learning. All rights reserved. 3 | 52

The empirical formula is C 3 H 8 N. Find the empirical formula weight: 3(12. 01) + 8(1. 008) + 1(14. 01) = 58. 104 amu Molecular formula: C 6 H 16 N 2 Copyright © Cengage Learning. All rights reserved. 3 | 53

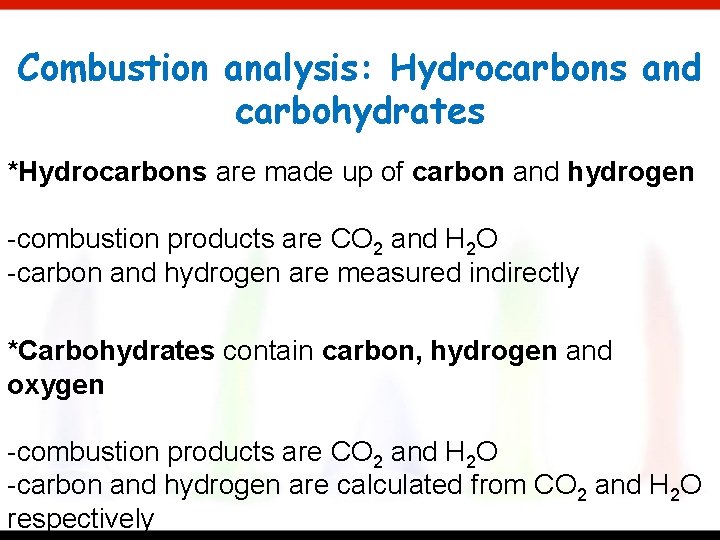
Combustion analysis: Hydrocarbons and carbohydrates *Hydrocarbons are made up of carbon and hydrogen -combustion products are CO 2 and H 2 O -carbon and hydrogen are measured indirectly *Carbohydrates contain carbon, hydrogen and oxygen -combustion products are CO 2 and H 2 O -carbon and hydrogen are calculated from CO 2 and H 2 O respectively

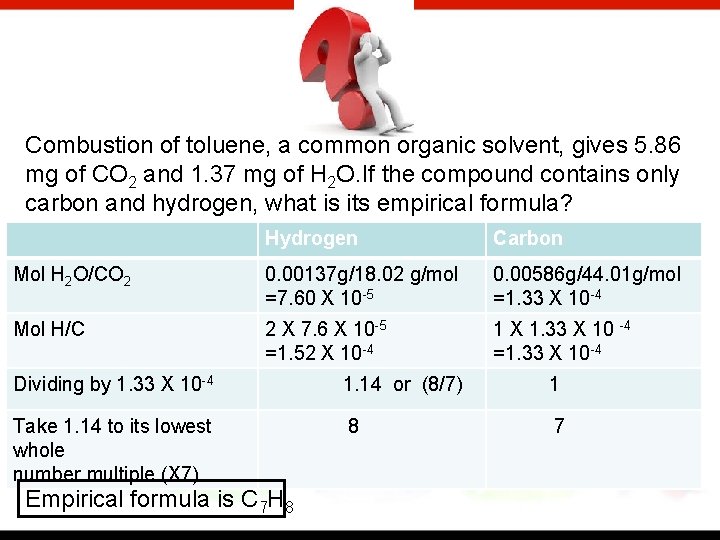
Combustion of toluene, a common organic solvent, gives 5. 86 mg of CO 2 and 1. 37 mg of H 2 O. If the compound contains only carbon and hydrogen, what is its empirical formula? Hydrogen Carbon Mol H 2 O/CO 2 0. 00137 g/18. 02 g/mol =7. 60 X 10 -5 0. 00586 g/44. 01 g/mol =1. 33 X 10 -4 Mol H/C 2 X 7. 6 X 10 -5 =1. 52 X 10 -4 1 X 1. 33 X 10 -4 =1. 33 X 10 -4 Dividing by 1. 33 X 10 -4 1. 14 or (8/7) 1 Take 1. 14 to its lowest whole number multiple (X 7) 8 7 Empirical formula is C 7 H 8

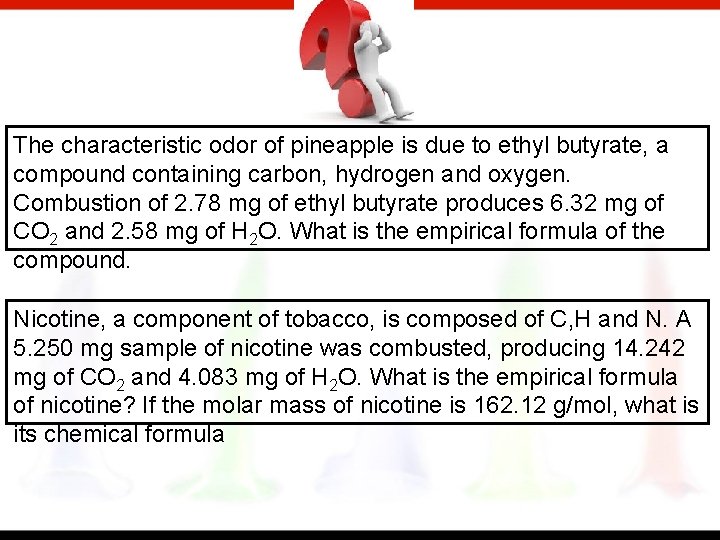
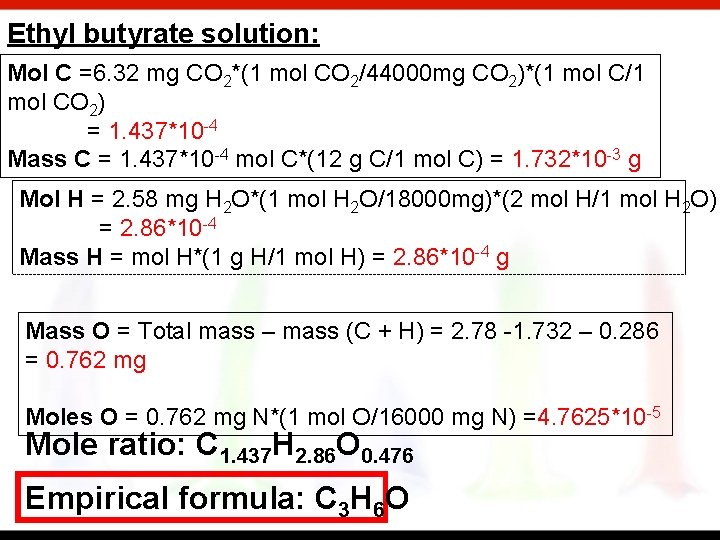
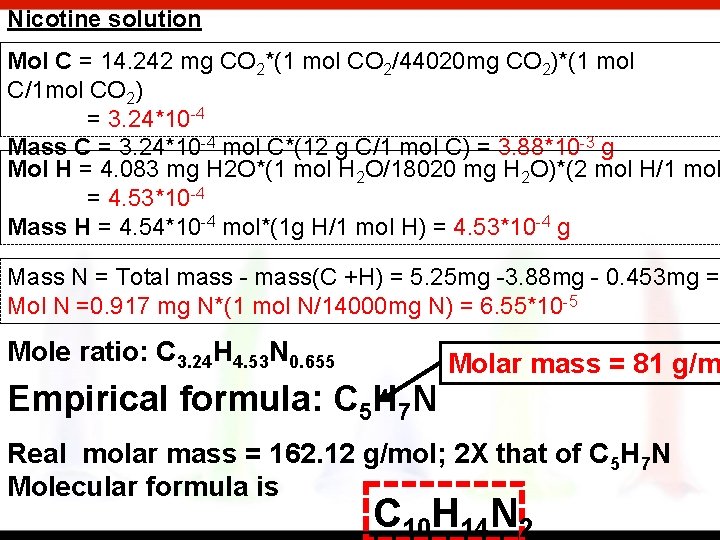
The characteristic odor of pineapple is due to ethyl butyrate, a compound containing carbon, hydrogen and oxygen. Combustion of 2. 78 mg of ethyl butyrate produces 6. 32 mg of CO 2 and 2. 58 mg of H 2 O. What is the empirical formula of the compound. Nicotine, a component of tobacco, is composed of C, H and N. A 5. 250 mg sample of nicotine was combusted, producing 14. 242 mg of CO 2 and 4. 083 mg of H 2 O. What is the empirical formula of nicotine? If the molar mass of nicotine is 162. 12 g/mol, what is its chemical formula

Ethyl butyrate solution: Mol C =6. 32 mg CO 2*(1 mol CO 2/44000 mg CO 2)*(1 mol C/1 mol CO 2) = 1. 437*10 -4 Mass C = 1. 437*10 -4 mol C*(12 g C/1 mol C) = 1. 732*10 -3 g Mol H = 2. 58 mg H 2 O*(1 mol H 2 O/18000 mg)*(2 mol H/1 mol H 2 O) = 2. 86*10 -4 Mass H = mol H*(1 g H/1 mol H) = 2. 86*10 -4 g Mass O = Total mass – mass (C + H) = 2. 78 -1. 732 – 0. 286 = 0. 762 mg Moles O = 0. 762 mg N*(1 mol O/16000 mg N) =4. 7625*10 -5 Mole ratio: C 1. 437 H 2. 86 O 0. 476 Empirical formula: C 3 H 6 O

Nicotine solution Mol C = 14. 242 mg CO 2*(1 mol CO 2/44020 mg CO 2)*(1 mol C/1 mol CO 2) = 3. 24*10 -4 Mass C = 3. 24*10 -4 mol C*(12 g C/1 mol C) = 3. 88*10 -3 g Mol H = 4. 083 mg H 2 O*(1 mol H 2 O/18020 mg H 2 O)*(2 mol H/1 mol = 4. 53*10 -4 Mass H = 4. 54*10 -4 mol*(1 g H/1 mol H) = 4. 53*10 -4 g Mass N = Total mass - mass(C +H) = 5. 25 mg -3. 88 mg - 0. 453 mg = Mol N =0. 917 mg N*(1 mol N/14000 mg N) = 6. 55*10 -5 Mole ratio: C 3. 24 H 4. 53 N 0. 655 Empirical formula: C 5 H 7 N Molar mass = 81 g/m Real molar mass = 162. 12 g/mol; 2 X that of C 5 H 7 N Molecular formula is C 10 H 14 N 2

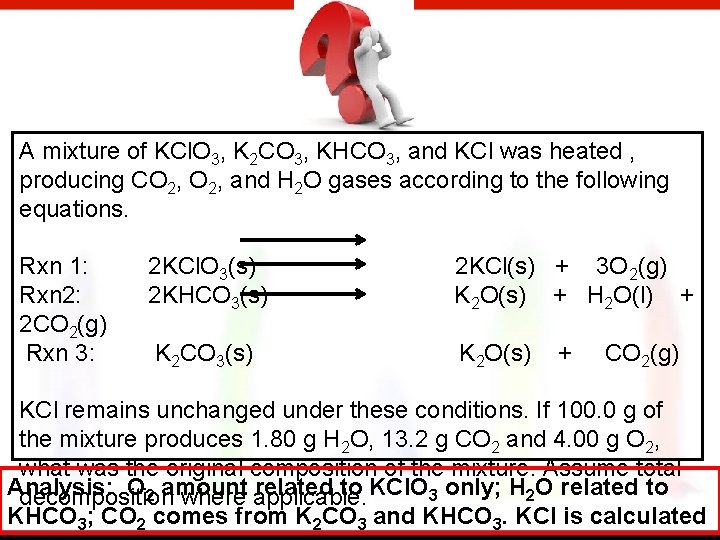
A mixture of KCl. O 3, K 2 CO 3, KHCO 3, and KCl was heated , producing CO 2, and H 2 O gases according to the following equations. Rxn 1: Rxn 2: 2 CO 2(g) Rxn 3: 2 KCl. O 3(s) 2 KHCO 3(s) 2 KCl(s) + 3 O 2(g) K 2 O(s) + H 2 O(l) + K 2 CO 3(s) K 2 O(s) + CO 2(g) KCl remains unchanged under these conditions. If 100. 0 g of the mixture produces 1. 80 g H 2 O, 13. 2 g CO 2 and 4. 00 g O 2, what was the original composition of the mixture. Assume total Analysis: O 2 amount related to KCl. O 3 only; H 2 O related to decomposition where applicable. KHCO 3; CO 2 comes from K 2 CO 3 and KHCO 3. KCl is calculated

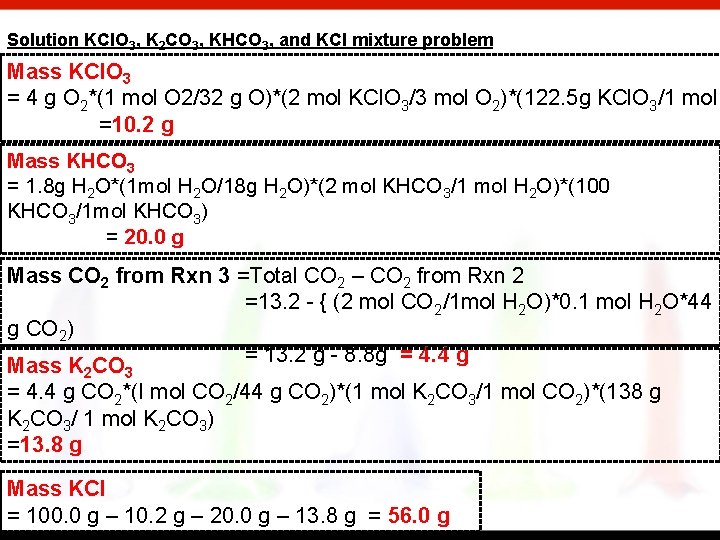
Solution KCl. O 3, K 2 CO 3, KHCO 3, and KCl mixture problem Mass KCl. O 3 = 4 g O 2*(1 mol O 2/32 g O)*(2 mol KCl. O 3/3 mol O 2)*(122. 5 g KCl. O 3/1 mol =10. 2 g Mass KHCO 3 = 1. 8 g H 2 O*(1 mol H 2 O/18 g H 2 O)*(2 mol KHCO 3/1 mol H 2 O)*(100 KHCO 3/1 mol KHCO 3) = 20. 0 g Mass CO 2 from Rxn 3 =Total CO 2 – CO 2 from Rxn 2 =13. 2 - { (2 mol CO 2/1 mol H 2 O)*0. 1 mol H 2 O*44 g CO 2) = 13. 2 g - 8. 8 g = 4. 4 g Mass K CO 2 3 = 4. 4 g CO 2*(I mol CO 2/44 g CO 2)*(1 mol K 2 CO 3/1 mol CO 2)*(138 g K 2 CO 3/ 1 mol K 2 CO 3) =13. 8 g Mass KCl = 100. 0 g – 10. 2 g – 20. 0 g – 13. 8 g = 56. 0 g

Stoichiometry The calculation of the quantities of reactants and products involved in a chemical reaction. Interpreting a Chemical Equation The coefficients of the balanced chemical equation may be interpreted in terms of either (1) numbers of molecules (or ions or formula units) or (2) numbers of moles, depending on your needs. Copyright © Cengage Learning. All rights reserved. 3 | 61

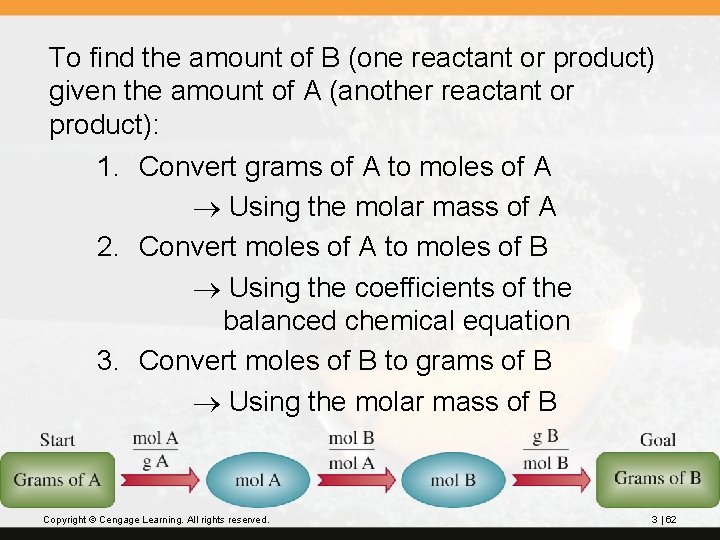
To find the amount of B (one reactant or product) given the amount of A (another reactant or product): 1. Convert grams of A to moles of A Using the molar mass of A 2. Convert moles of A to moles of B Using the coefficients of the balanced chemical equation 3. Convert moles of B to grams of B Using the molar mass of B Copyright © Cengage Learning. All rights reserved. 3 | 62

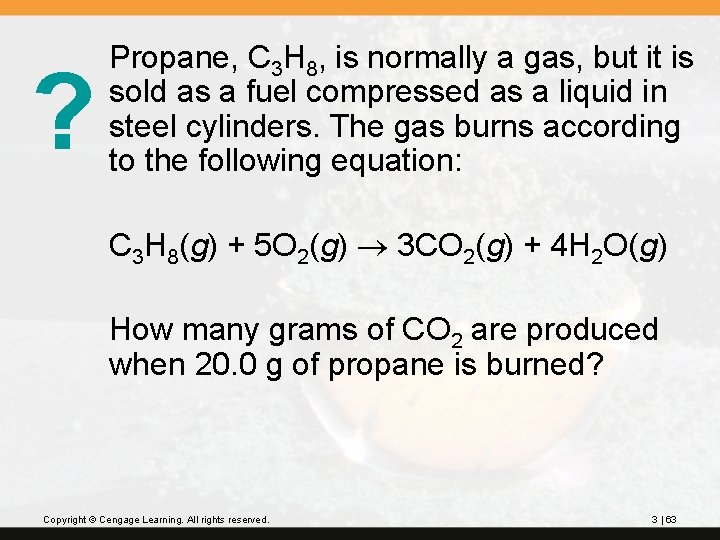
? Propane, C 3 H 8, is normally a gas, but it is sold as a fuel compressed as a liquid in steel cylinders. The gas burns according to the following equation: C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) How many grams of CO 2 are produced when 20. 0 g of propane is burned? Copyright © Cengage Learning. All rights reserved. 3 | 63

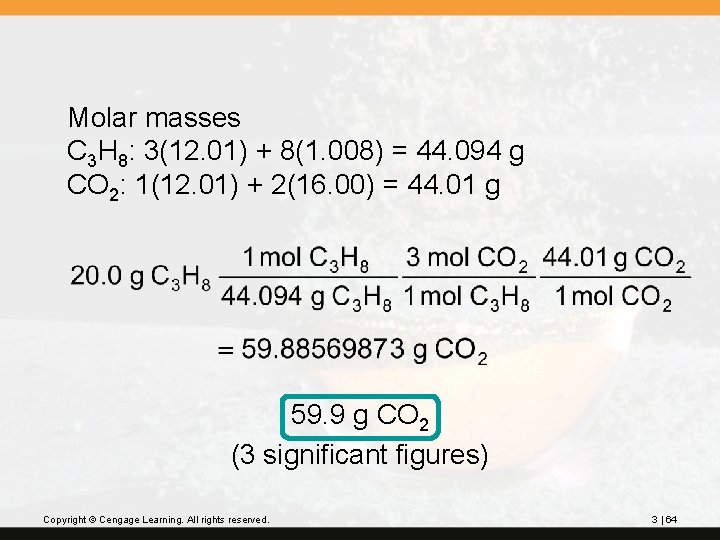
Molar masses C 3 H 8: 3(12. 01) + 8(1. 008) = 44. 094 g CO 2: 1(12. 01) + 2(16. 00) = 44. 01 g 59. 9 g CO 2 (3 significant figures) Copyright © Cengage Learning. All rights reserved. 3 | 64

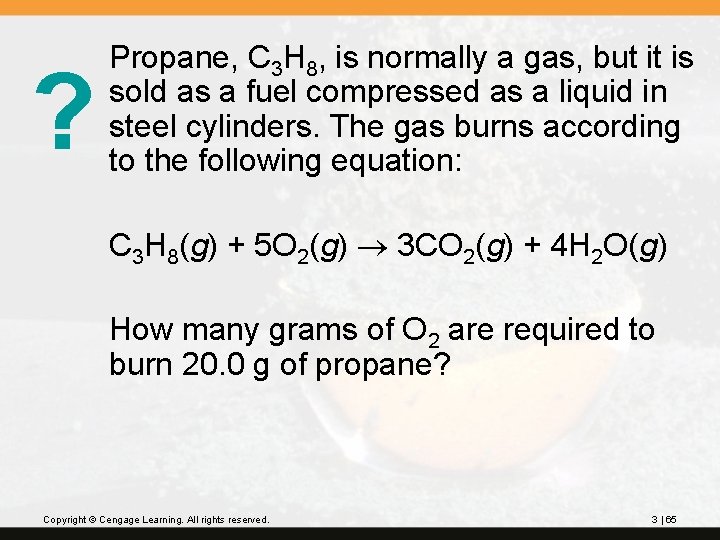
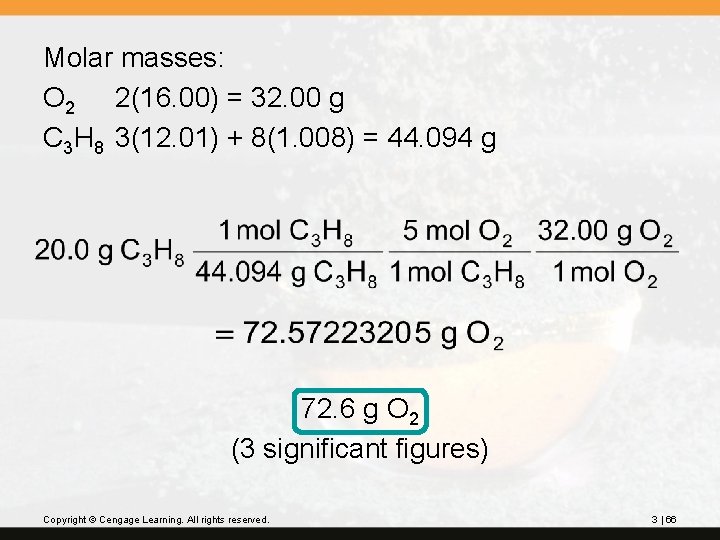
? Propane, C 3 H 8, is normally a gas, but it is sold as a fuel compressed as a liquid in steel cylinders. The gas burns according to the following equation: C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) How many grams of O 2 are required to burn 20. 0 g of propane? Copyright © Cengage Learning. All rights reserved. 3 | 65

Molar masses: O 2 2(16. 00) = 32. 00 g C 3 H 8 3(12. 01) + 8(1. 008) = 44. 094 g 72. 6 g O 2 (3 significant figures) Copyright © Cengage Learning. All rights reserved. 3 | 66

Limiting Reactant The reactant that is entirely consumed when a reaction goes to completion. Once one reactant has been completely consumed, the reaction stops. Any problem giving the starting amount for more than one reactant is a limiting reactant problem. Copyright © Cengage Learning. All rights reserved. 3 | 67

All amounts produced and reacted are determined by the limiting reactant. How can we determine the limiting reactant? 1. Use each given amount to calculate the amount of product produced. 2. The limiting reactant will produce the lesser or least amount of product. Copyright © Cengage Learning. All rights reserved. 3 | 68

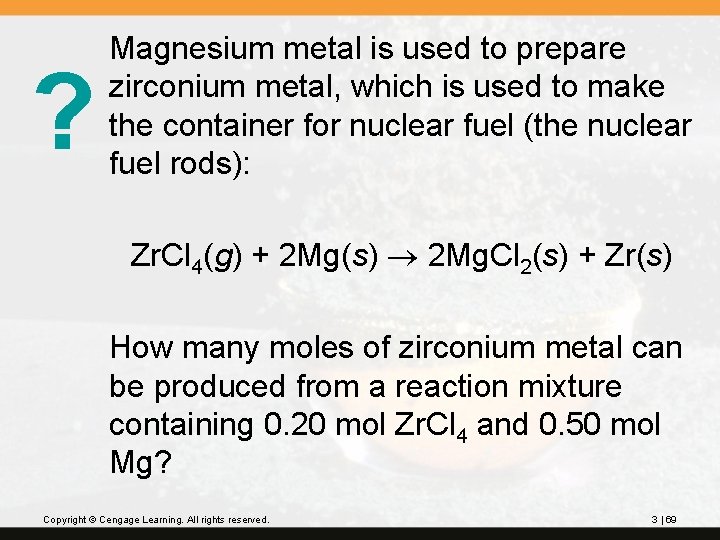
? Magnesium metal is used to prepare zirconium metal, which is used to make the container for nuclear fuel (the nuclear fuel rods): Zr. Cl 4(g) + 2 Mg(s) 2 Mg. Cl 2(s) + Zr(s) How many moles of zirconium metal can be produced from a reaction mixture containing 0. 20 mol Zr. Cl 4 and 0. 50 mol Mg? Copyright © Cengage Learning. All rights reserved. 3 | 69

Since Zr. Cl 4 gives the lesser amount of Zr, Zr. Cl 4 is the limiting reactant. 0. 20 mol Zr will be produced. Copyright © Cengage Learning. All rights reserved. 3 | 70

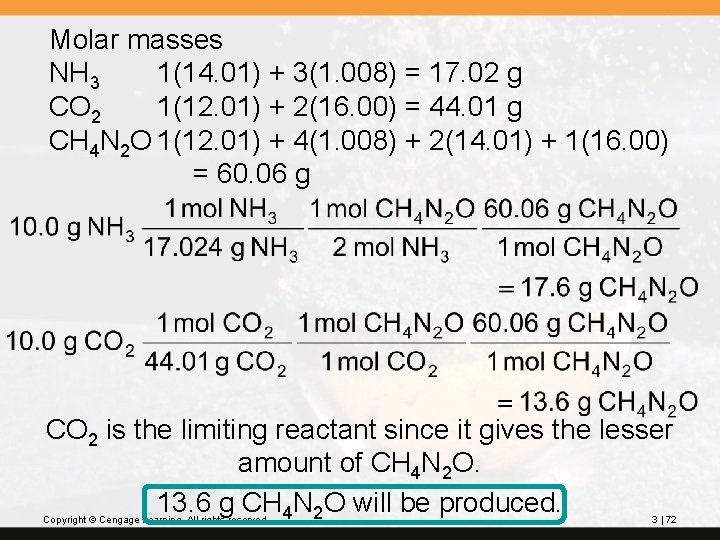
? Urea, CH 4 N 2 O, is used as a nitrogen fertilizer. It is manufactured from ammonia and carbon dioxide at high pressure and high temperature: 2 NH 3 + CO 2(g) CH 4 N 2 O + H 2 O In a laboratory experiment, 10. 0 g NH 3 and 10. 0 g CO 2 were added to a reaction vessel. What is the maximum quantity (in grams) of urea that can be obtained? How many grams of the excess reactant are left at the end of the reactions? Copyright © Cengage Learning. All rights reserved. 3 | 71

Molar masses NH 3 1(14. 01) + 3(1. 008) = 17. 02 g CO 2 1(12. 01) + 2(16. 00) = 44. 01 g CH 4 N 2 O 1(12. 01) + 4(1. 008) + 2(14. 01) + 1(16. 00) = 60. 06 g CO 2 is the limiting reactant since it gives the lesser amount of CH 4 N 2 O. 13. 6 g CH 4 N 2 O will be produced. Copyright © Cengage Learning. All rights reserved. 3 | 72

To find the excess NH 3, we need to find how much NH 3 reacted. We use the limiting reactant as our starting point. Now subtract the amount reacted from the at starting amount: 10. 0 − 7. 73 reacted 2. 27 g remains 2. 3 g NH 3 is left unreacted. (1 decimal place) Copyright © Cengage Learning. All rights reserved. 3 | 73

Theoretical/Expected Yield Theorerical yield is the maximum amount of product that can be obtained by a reaction from given amounts of reactants. This is a calculated amount based on a balanced chemical equation. Copyright © Cengage Learning. All rights reserved. 3 | 74

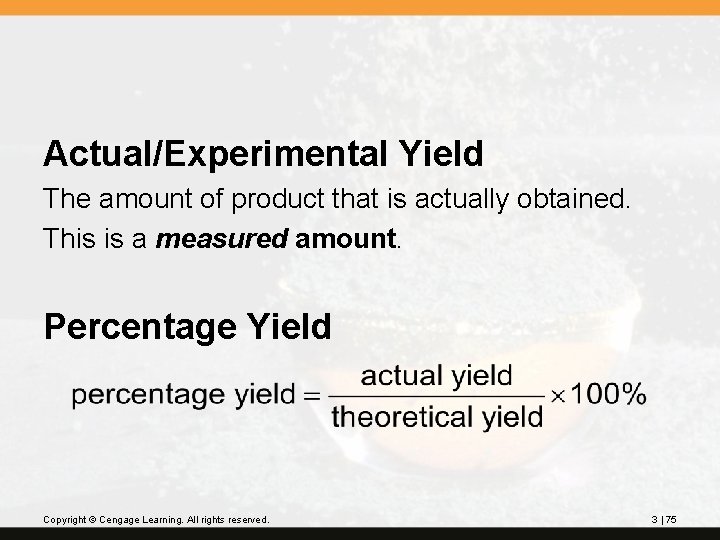
Actual/Experimental Yield The amount of product that is actually obtained. This is a measured amount. Percentage Yield Copyright © Cengage Learning. All rights reserved. 3 | 75

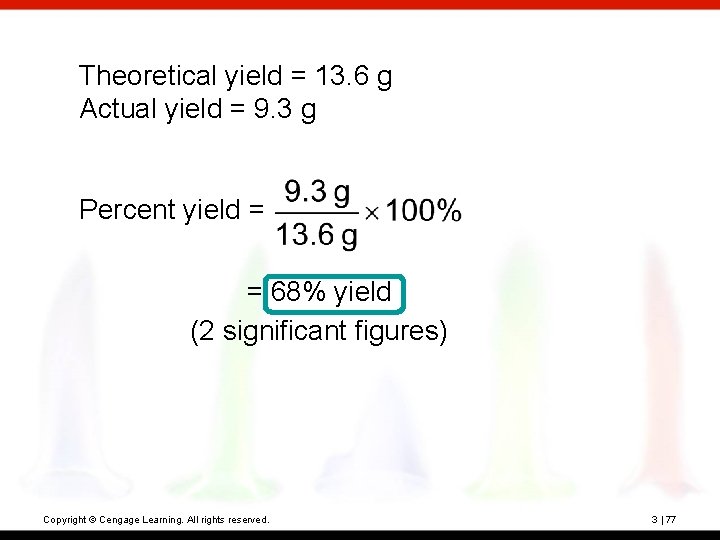
? 2 NH 3 + CO 2(g) CH 4 N 2 O + H 2 O When 10. 0 g NH 3 and 10. 0 g CO 2 are added to a reaction vessel, the limiting reactant is CO 2. The theoretical yield is 13. 6 of urea. When this reaction was carried out, 9. 3 g of urea was obtained. What is the percent yield? Copyright © Cengage Learning. All rights reserved. 3 | 76

Theoretical yield = 13. 6 g Actual yield = 9. 3 g Percent yield = = 68% yield (2 significant figures) Copyright © Cengage Learning. All rights reserved. 3 | 77

Silver chloride can be made according to the following chemical equation Na. Cl(aq) + Ag. NO 3(aq) → Na. NO 3(aq) + Ag. Cl(s) When solutions containing 0. 351 g Ag. NO 3 and 0. 250 g Na. Cl were mixed, the amount of Ag. Cl precipitated was 0. 250 g. Calculate the percent yield of Ag. Cl.