Chapter 3 Biochemistry 1 3 1 Organic Molecules

Chapter 3: Biochemistry 1
3. 1 Organic Molecules • Organic molecules contain carbon and hydrogen atoms. • Four classes of organic molecules (biomolecules) exist in living organisms: § Carbohydrates § Lipids § Proteins § Nucleic Acids 2
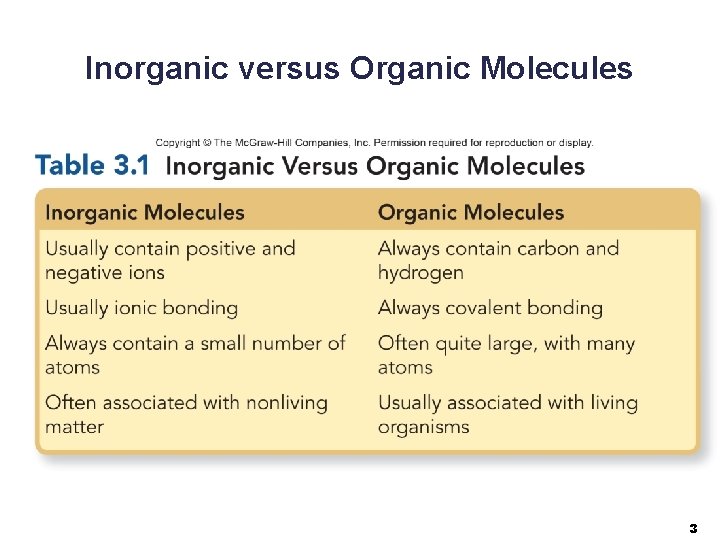
Inorganic versus Organic Molecules 3
The Carbon Skeleton and Functional Groups • The carbon chain of an organic molecule is called its skeleton or backbone. • Functional groups are clusters of specific atoms bonded to the carbon skeleton with characteristic structures and functions. § Determine the chemical reactivity and polarity of organic molecules 4
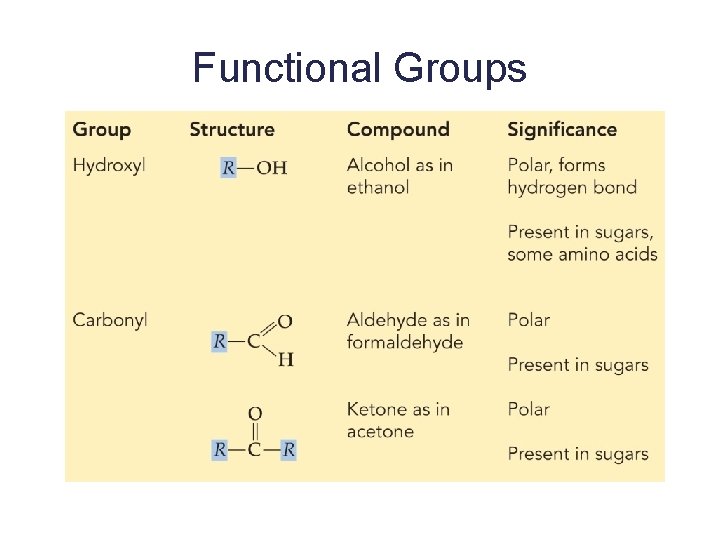
Functional Groups
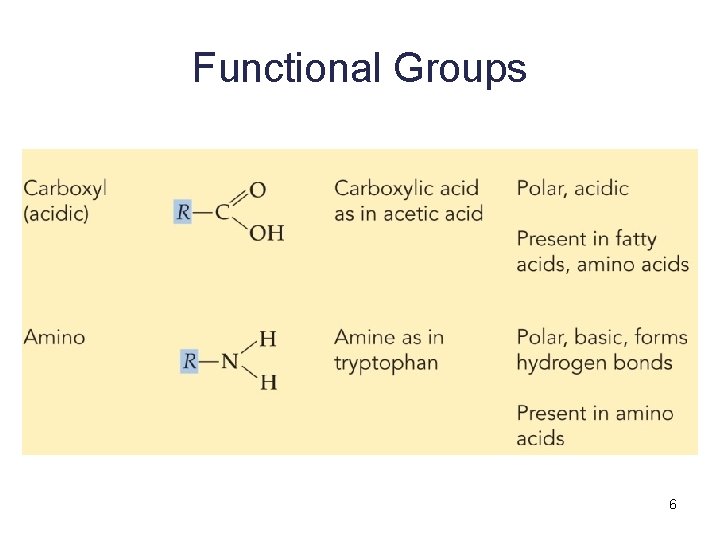
Functional Groups 6
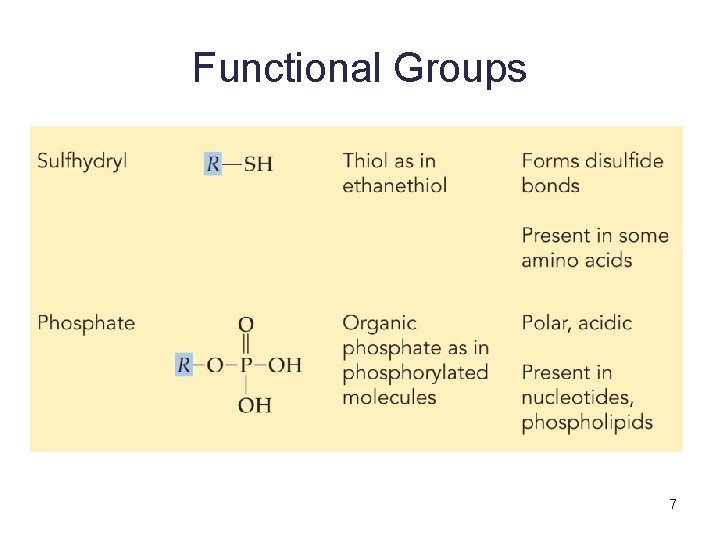
Functional Groups 7
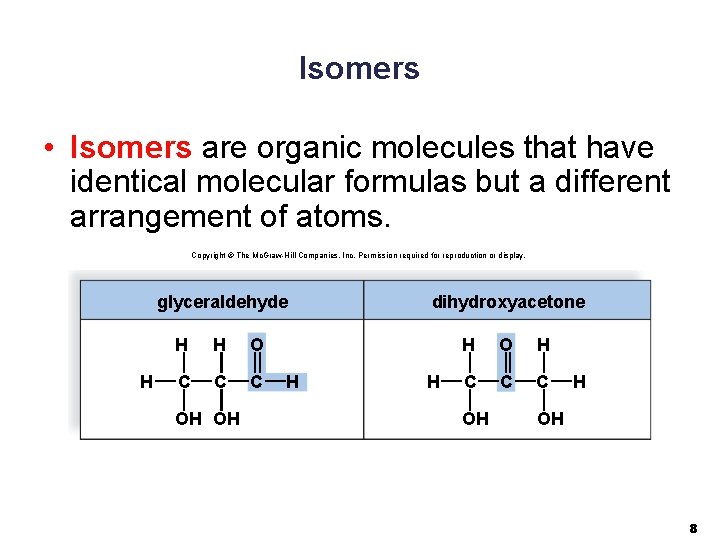
Isomers • Isomers are organic molecules that have identical molecular formulas but a different arrangement of atoms. Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. glyceraldehyde H H H O C C C OH OH H dihydroxyacetone H H O H C C C OH H OH 8
Biomolecules • Carbohydrates, lipids, proteins, and nucleic acids are called biomolecules. § Usually consist of many repeating units • Each repeating unit is called a monomer. • A molecule composed of monomers is called a polymer (many parts). – Example: amino acids (monomer) are joined together to form a protein (polymer) 9
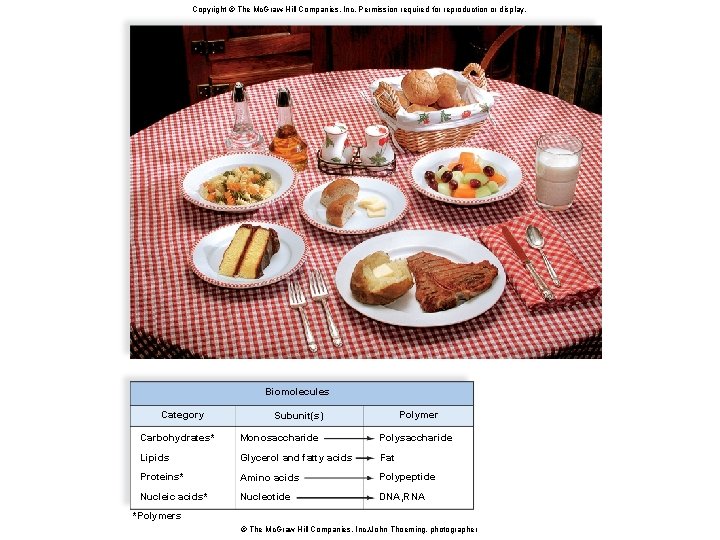
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Fig. 3. 3 Biomolecules Category Polymer Subunit(s) Carbohydrates* Monosaccharide Polysaccharide Lipids Glycerol and fatty acids Fat Proteins* Amino acids Polypeptide Nucleic acids* Nucleotide DNA, RNA *Polymers © The Mc. Graw Hill Companies, Inc. /John Thoeming, photographer
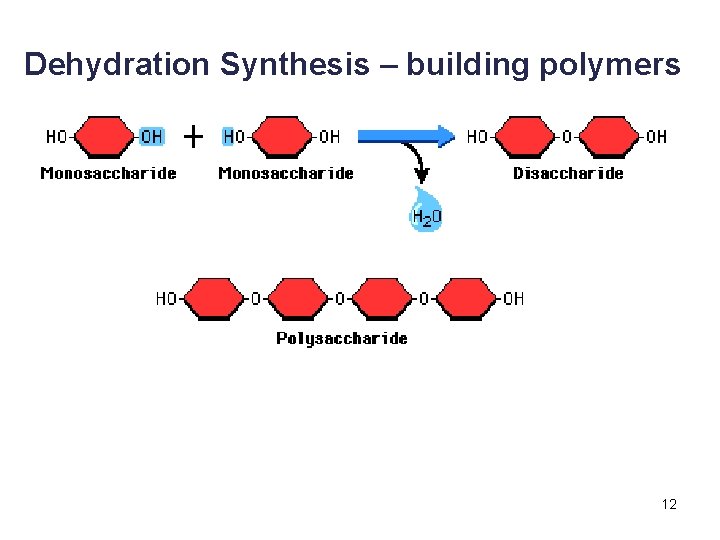
Synthesis and Degradation • A dehydration reaction is a chemical reaction in which subunits are joined together by the formation of a covalent bond and water is produced during the reaction. § Used to connect monomers together to make polymers § Example: formation of starch (polymer) from glucose subunits (monomer) • A hydrolysis reaction is a chemical reaction in which a water molecule is added to break a covalent bond. § Used to breakdown polymers into monomers § Example: digestion of starch into glucose monomers 11
Dehydration Synthesis – building polymers 12
Dehydration Synthesis – building polymers 13
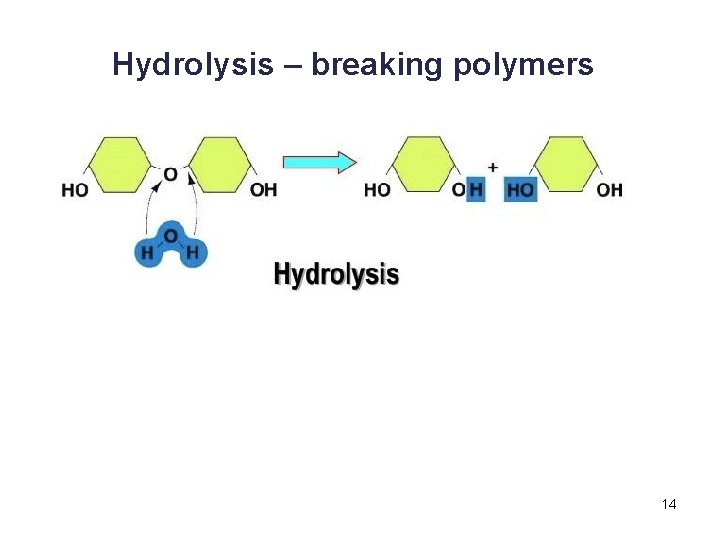
Hydrolysis – breaking polymers 14
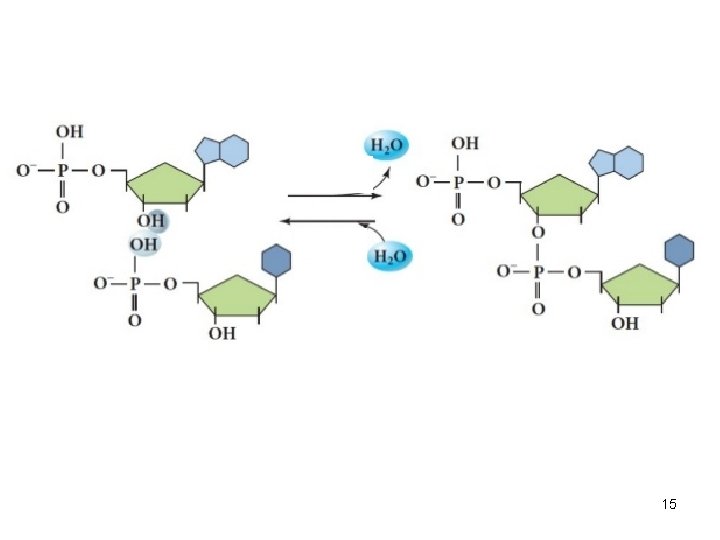
15
Synthesis and Degradation • Enzymes are required for cells to carry out dehydration synthesis and hydrolysis reactions. § An enzyme is a molecule that speeds up a chemical reaction. • Enzymes are not consumed in the reaction. • Enzymes are not changed by the reaction. 16

3. 2 Carbohydrates • Functions: § Energy source § Provide building material (structural role) • Contain carbon, hydrogen and oxygen in a 1: 2: 1 ratio • Varieties: monosaccharides, disaccharides, and polysaccharides

Monosaccharides • A monosaccharide is a single sugar molecule. • Also called simple sugars • Have a backbone of 3 to 7 carbon atoms • Examples: § Glucose (blood), fructose (fruit) and galactose • Hexoses - six carbon atoms § Ribose and deoxyribose (in nucleotides) • Pentoses – five carbon atoms 18
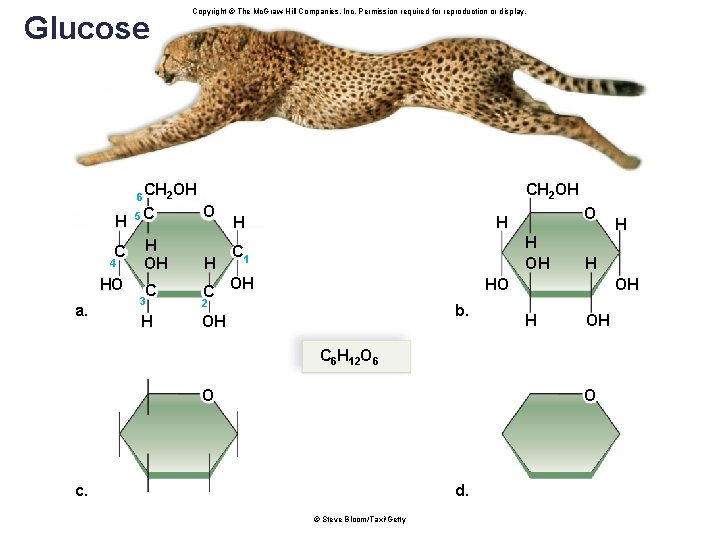
Glucose 6 H C 4 HO a. Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Fig. 3. 6 CH 2 OH 5 C O H OH H C C 3 2 H OH H OH C 1 OH HO b. H OH C 6 H 12 O 6 O O c. d. © Steve Bloom/Taxi/Getty H

Disaccharides • A disaccharide contains two monosaccharides joined together by dehydration synthesis. • Examples: § Lactose (milk sugar) is composed of galactose and glucose. § Sucrose (table sugar) is composed of glucose and fructose. § Maltose is composed of two glucose molecules. 20
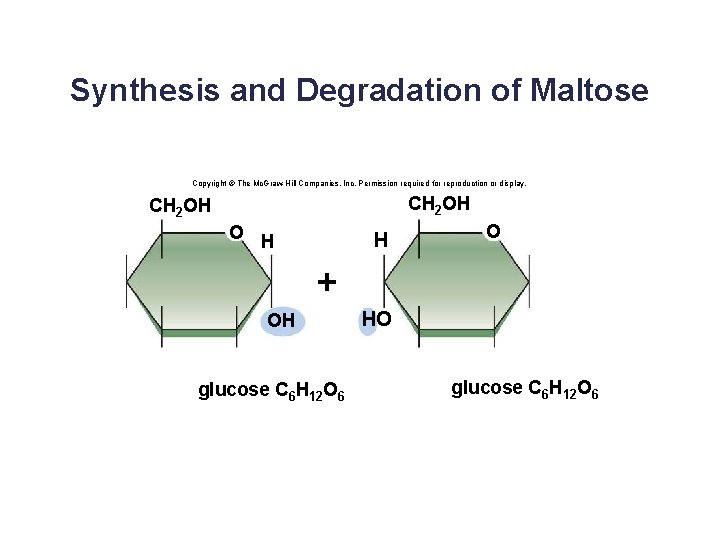
Synthesis and Degradation of Maltose Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. CH 2 OH O H H O + OH glucose C 6 H 12 O 6 HO glucose C 6 H 12 O 6
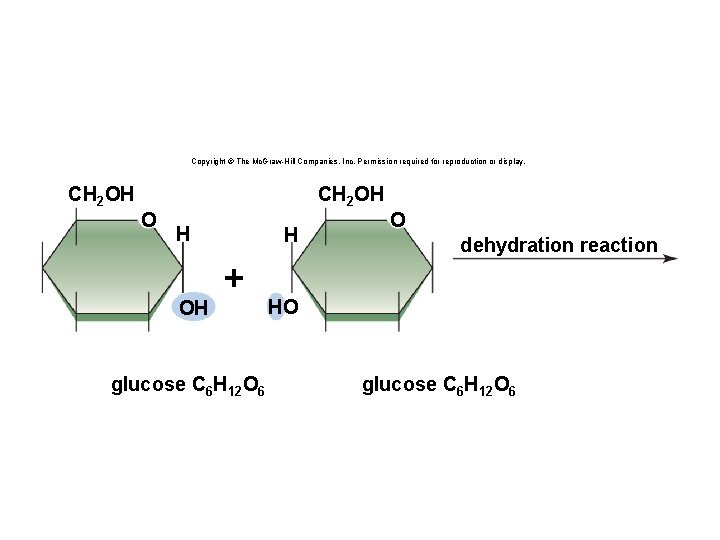
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. CH 2 OH O H OH H + glucose C 6 H 12 O 6 O dehydration reaction HO glucose C 6 H 12 O 6
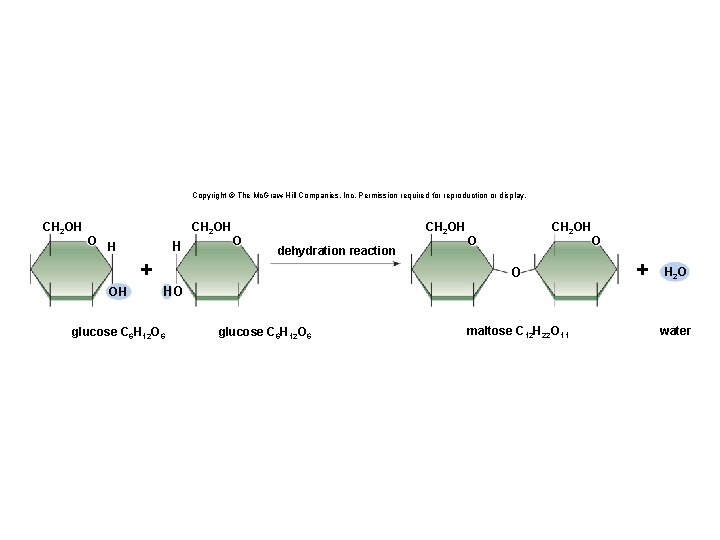
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. CH 2 OH O CH 2 OH H H O CH 2 OH dehydration reaction + OH CH 2 OH O O O + H 2 O HO glucose C 6 H 12 O 6 maltose C 12 H 22 O 11 water
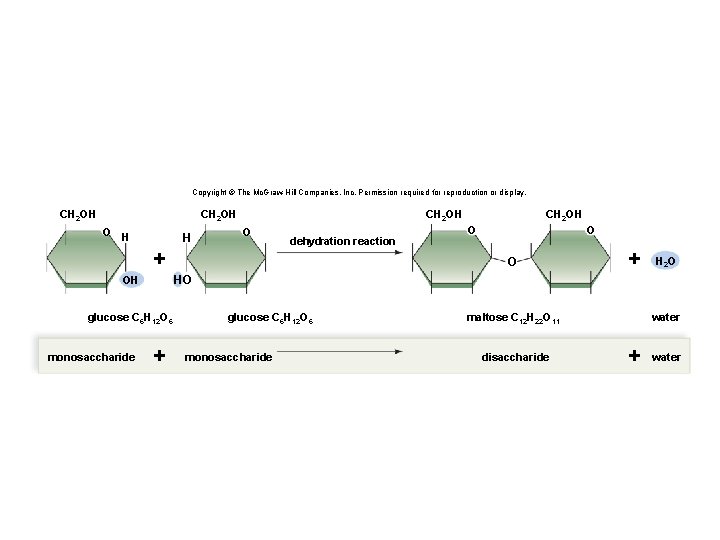
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. CH 2 OH O H H CH 2 OH O dehydration reaction + O O O + H 2 O HO OH glucose C 6 H 12 O 6 monosaccharide CH 2 OH + glucose C 6 H 12 O 6 monosaccharide maltose C 12 H 22 O 11 disaccharide water + water
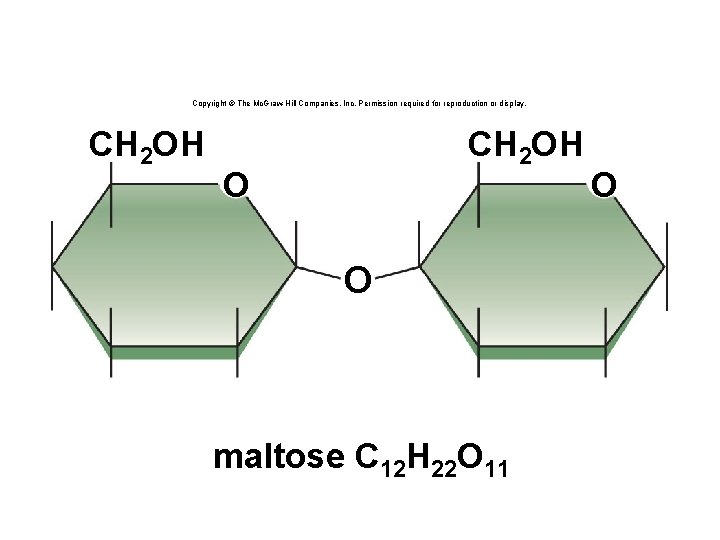
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. CH 2 OH O O maltose C 12 H 22 O 11 O
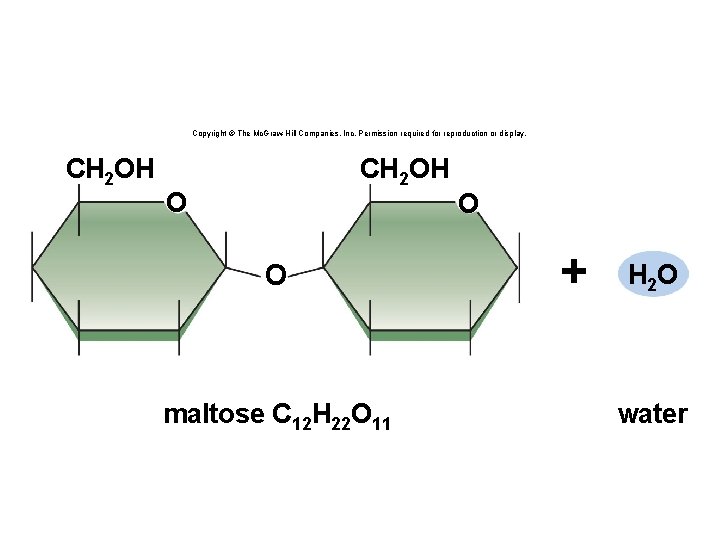
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. CH 2 OH O O maltose C 12 H 22 O 11 O + H 2 O water
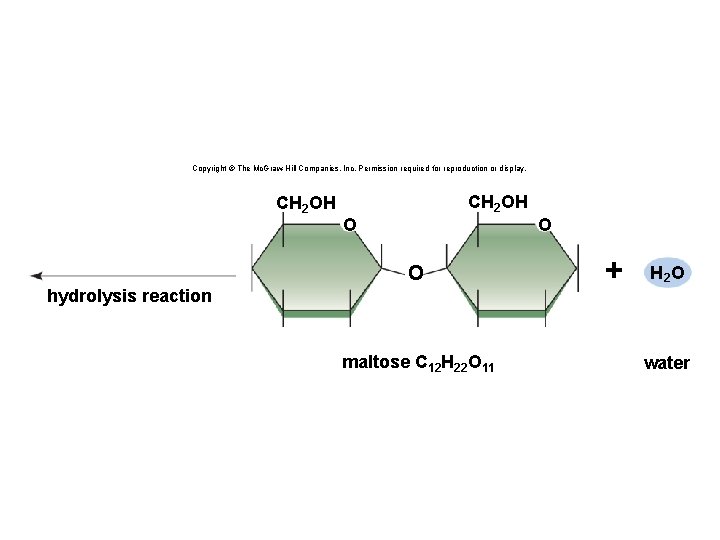
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. CH 2 OH O O hydrolysis reaction maltose C 12 H 22 O 11 O + H 2 O water
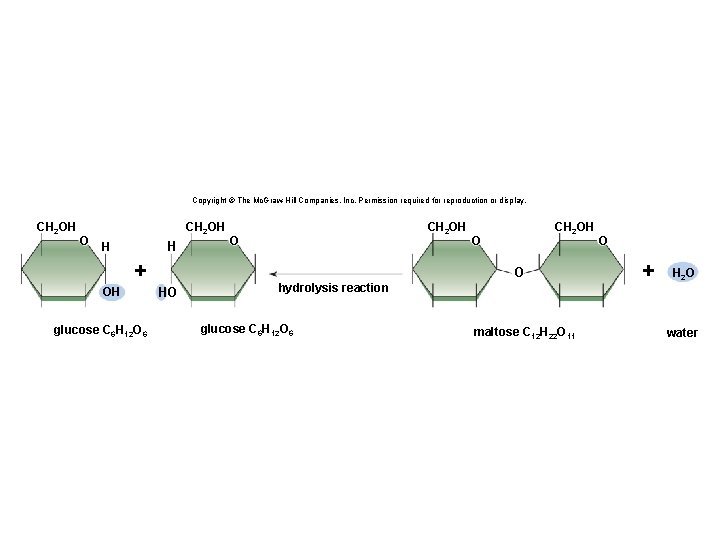
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. CH 2 OH O CH 2 OH H H + OH glucose C 6 H 12 O 6 CH 2 OH O O HO hydrolysis reaction glucose C 6 H 12 O 6 maltose C 12 H 22 O 11 O + H 2 O water
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. CH 2 OH O CH 2 OH H H + glucose C 6 H 12 O 6 monosaccharide + CH 2 OH O O hydrolysis reaction HO OH CH 2 OH O glucose C 6 H 12 O 6 monosaccharide O + maltose C 12 H 22 O 11 disaccharide H 2 O water + water
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. dehydration reaction hydrolysis reaction glucose C 6 H 12 O 6 monosaccharide + glucose C 6 H 12 O 6 monosaccharide maltose C 12 H 22 O 11 disaccharide water + water
Polysaccharides • A polysaccharide is a polymer of monosaccharides. • Examples: § Starch provides energy storage in plants. § Glycogen provides energy storage in animals. § Cellulose is found in the cell walls of plants. § Chitin is found in the cell walls of fungi and exoskeleton of some animals. § Peptidoglycan is found in the cell walls of bacteria.
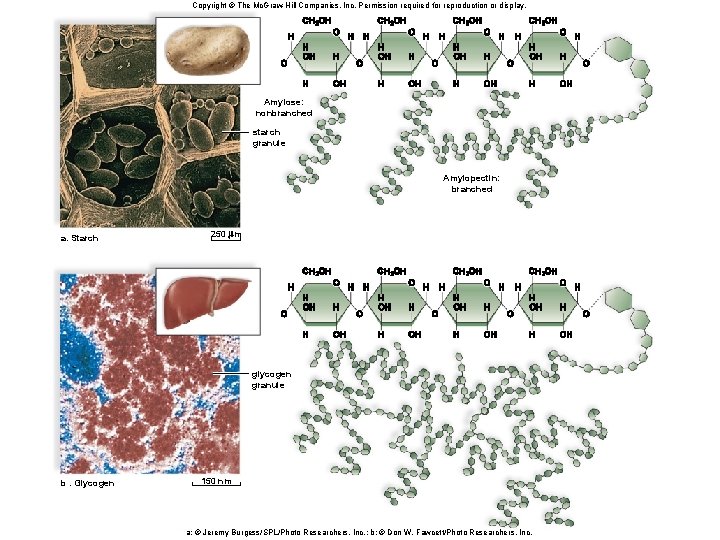
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Amylose: nonbranched starch granule Amylopectin: branched a. Starch 250 m glycogen granule b. Glycogen 150 nm a: © Jeremy Burgess/SPL/Photo Researchers, Inc. ; b: © Don W. Fawcett/Photo Researchers, Inc.
3. 3 Lipids • Lipids are varied in structure. • Large nonpolar molecules that are insoluble in water • Functions: § Long-term energy storage § Structural components § Cell communication and regulation § Protection • Varieties: fats, oils, phospholipids, steroids, waxes
Triglycerides: Long-Term Energy Storage § Also called fats and oils § Functions: long-term energy storage and insulation § Consist of one glycerol molecule linked to three fatty acids by dehydration synthesis
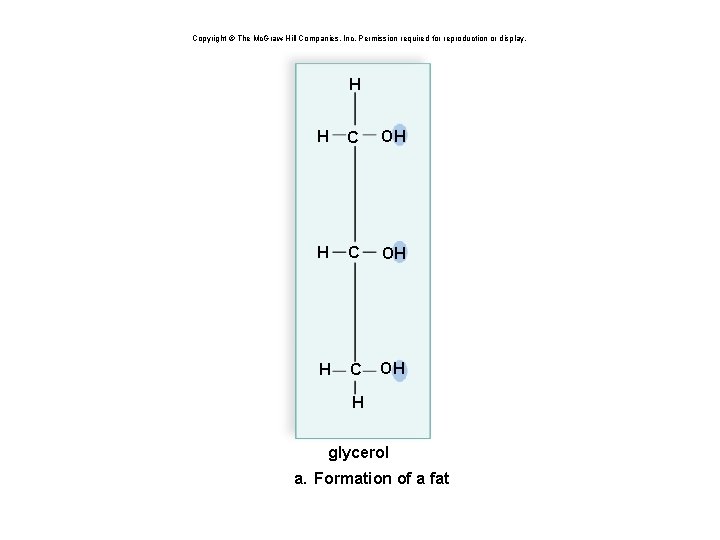
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. H H C OH H glycerol a. Formation of a fat
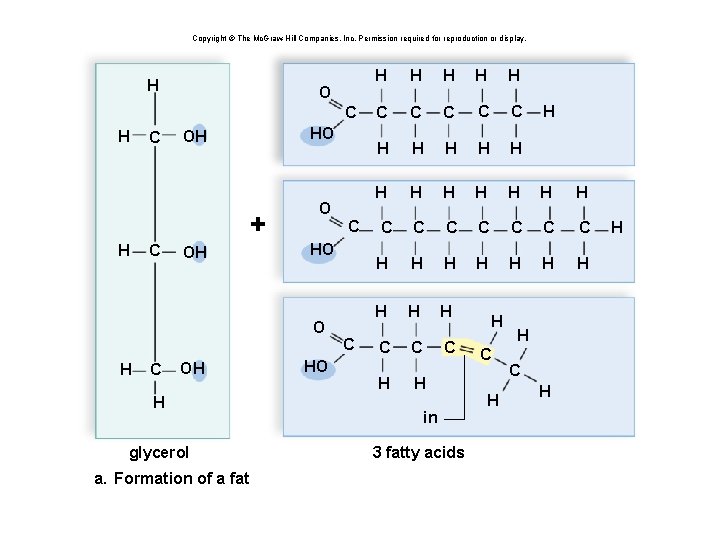
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. H O C HO OH + H C OH O HO O H C OH H glycerol a. Formation of a fat C HO C H H H C C C H H H C C C C H H H H H C C C H H in 3 fatty acids H C H H
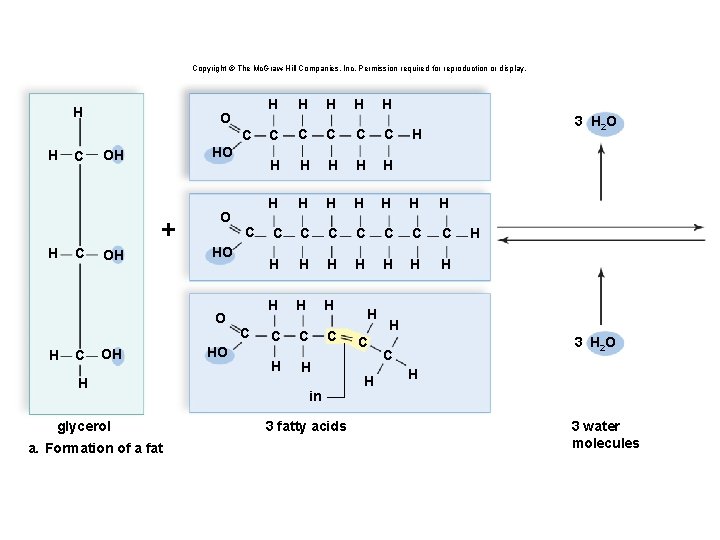
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. H O C HO OH + H C OH O HO O H C OH H glycerol a. Formation of a fat C HO C H H H C C C H H H C C C C H H H H H C C C H H in 3 fatty acids H C H 3 H 2 O H H H 3 H 2 O C H 3 water molecules
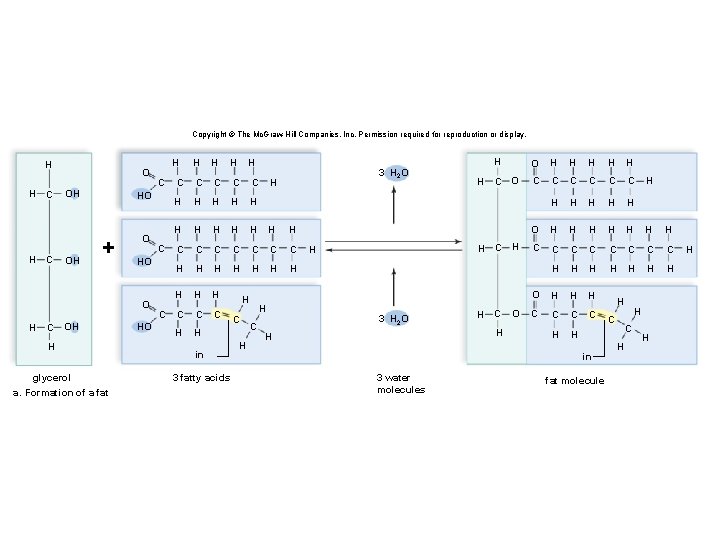
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. H H H C C O OH OH C C HO + O OH H glycerol a. Formation of a fat C HO O H H HO C C H H 3 H 2 O C C H H H C C C C H H H H H C C C H H in 3 fatty acids H C H H O H H H C O C C C H H H O H H H H C C C C H H H H O H H H C C H H H C H 3 H 2 O H C H H H O in 3 water molecules fat molecule H C H H
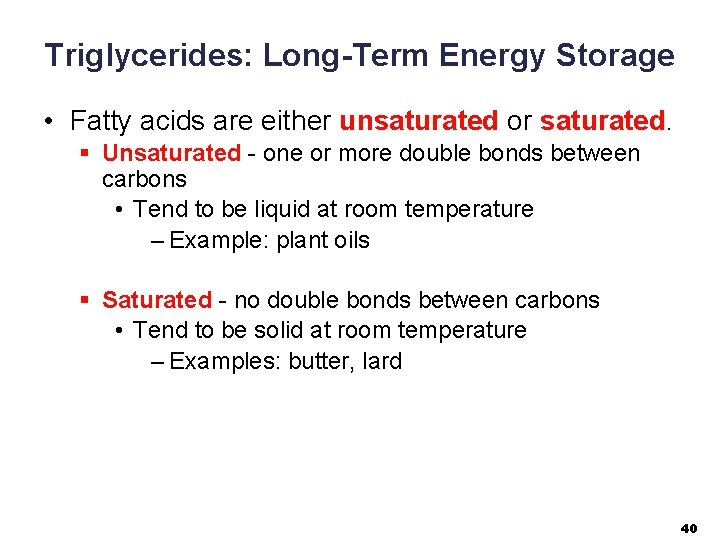
Triglycerides: Long-Term Energy Storage • Fatty acids are either unsaturated or saturated. § Unsaturated - one or more double bonds between carbons • Tend to be liquid at room temperature – Example: plant oils § Saturated - no double bonds between carbons • Tend to be solid at room temperature – Examples: butter, lard 40
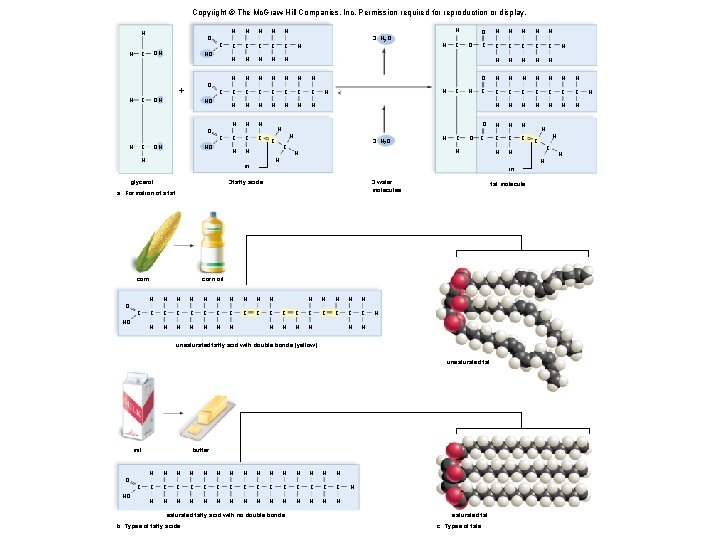
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. H O C H H OH C HO OH C O + C HO H H H C C C H H H H C H OH C HO C H H C C C H H H C H H in glycerol Fig. 3. 10 3 H 2 O O C HO C O H H H C C C H H H O H H H H C C C C H H H H O H H H C C O H H C C H O H H 3 water molecules corn oil H H H H C C C C C H H H H unsaturated fat H C HO butter H H H H C C C C H H H H saturated fatty acid with no double bonds b. Types of fatty acids H saturated fat c. Types of fats H H C H fat molecule unsaturated fatty acid with double bonds (yellow) O H C H 3 fatty acids mil H in a. Formation of a fat corn H H H O H 3 H 2 O H H H
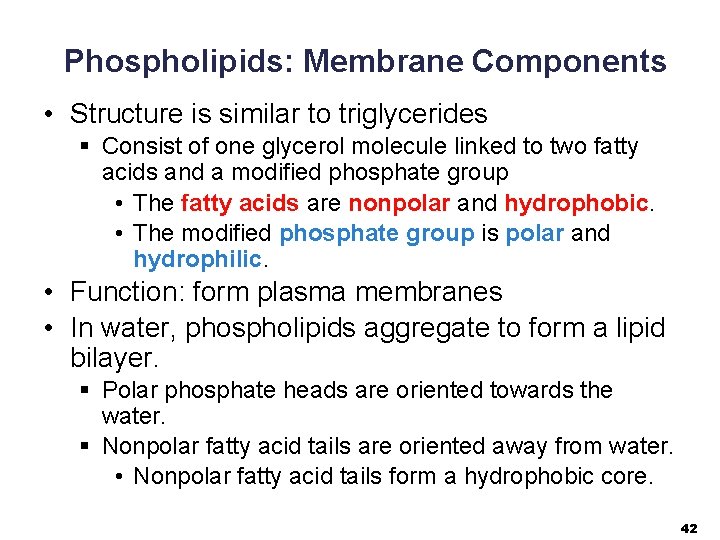
Phospholipids: Membrane Components • Structure is similar to triglycerides § Consist of one glycerol molecule linked to two fatty acids and a modified phosphate group • The fatty acids are nonpolar and hydrophobic. • The modified phosphate group is polar and hydrophilic. • Function: form plasma membranes • In water, phospholipids aggregate to form a lipid bilayer. § Polar phosphate heads are oriented towards the water. § Nonpolar fatty acid tails are oriented away from water. • Nonpolar fatty acid tails form a hydrophobic core. 42
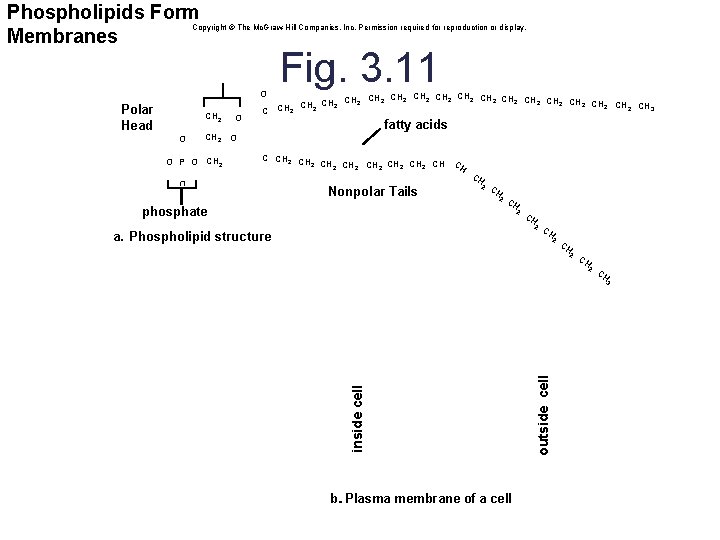
Phospholipids Form Membranes Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. glycerol O Polar Head 1 O CH 2 2 CH 2 3 R O P O CH 2 O Fig. 3. 11 CH 2 CH 2 CH 2 CH 2 2 CH 3 CH 2 C fatty acids O C CH 2 CH 2 CH C H O Nonpolar Tails phosphate CH 2 a. Phospholipid structure CH 2 CH b. . Plasma membrane of a cell outside cell inside cell 3

Steroids: Four Fused Carbon Rings • Composed of four fused carbon rings § Various functional groups attached to the carbon skeleton • Functions: component of animal cell membrane, regulation • Examples: cholesterol, testosterone, estrogen • Cholesterol is the precursor molecule for several other steroids. 44
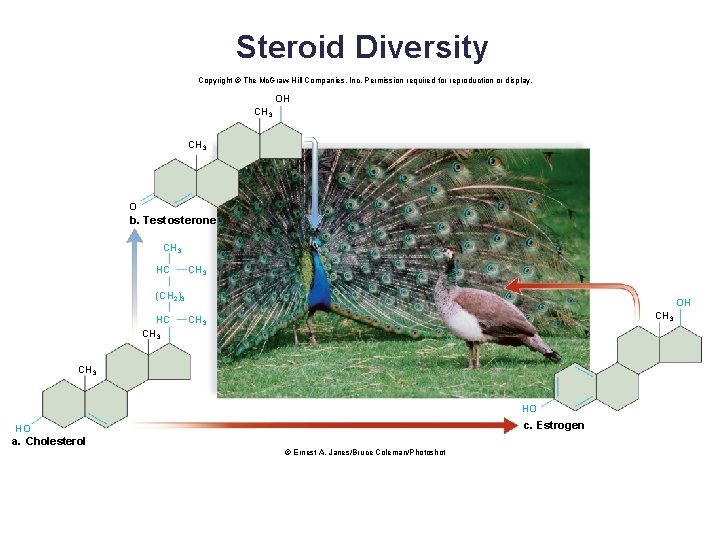
Steroid Diversity Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. OH CH 3 O b. Testosterone CH 3 HC CH 3 (CH 2)3 HC CH 3 OH CH 3 HO c. Estrogen HO a. Cholesterol © Ernest A. Janes/Bruce Coleman/Photoshot

Waxes • Long-chain fatty acid bonded to a long-chain alcohol • Solid at room temperature • Waterproof • Resistant to degradation • Function: protection • Examples: earwax, plant cuticle, beeswax 46

Waxes Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. a. b. a: © Das Fotoarchiv/Peter Arnold, Inc. ; b: © Martha Cooper/Peter Arnold, Inc. 47
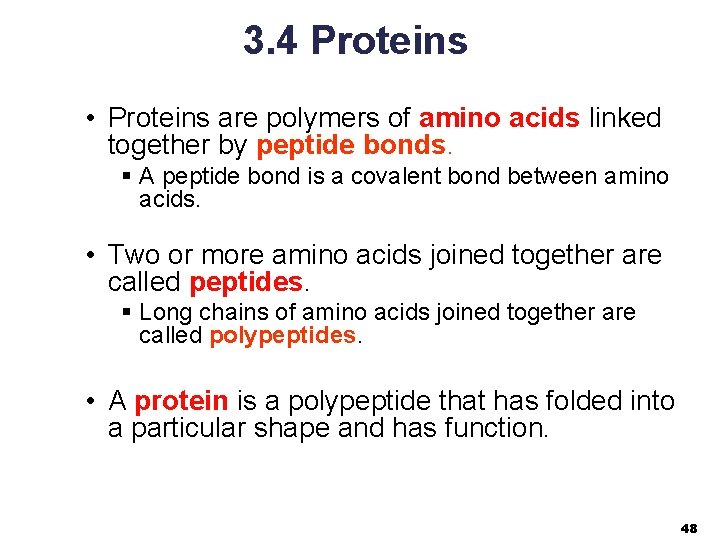
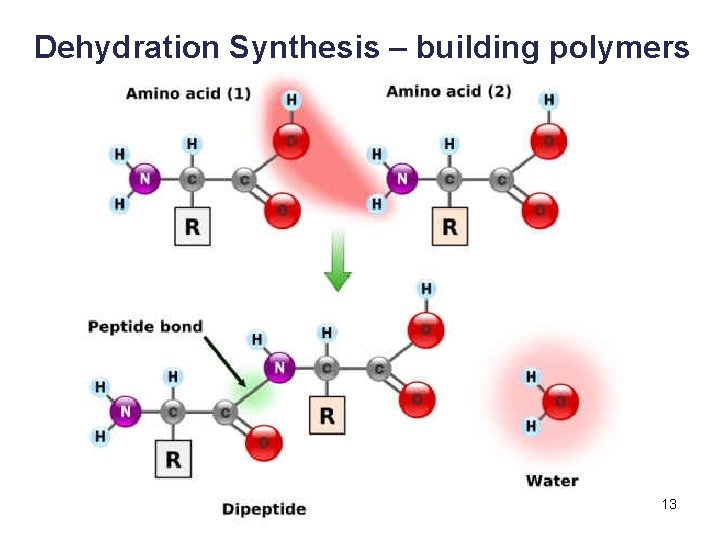
3. 4 Proteins • Proteins are polymers of amino acids linked together by peptide bonds. § A peptide bond is a covalent bond between amino acids. • Two or more amino acids joined together are called peptides. § Long chains of amino acids joined together are called polypeptides. • A protein is a polypeptide that has folded into a particular shape and has function. 48
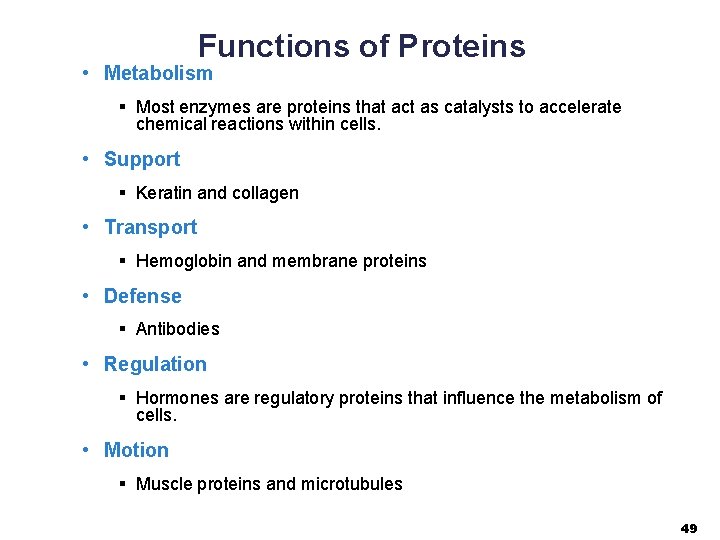
Functions of Proteins • Metabolism § Most enzymes are proteins that act as catalysts to accelerate chemical reactions within cells. • Support § Keratin and collagen • Transport § Hemoglobin and membrane proteins • Defense § Antibodies • Regulation § Hormones are regulatory proteins that influence the metabolism of cells. • Motion § Muscle proteins and microtubules 49
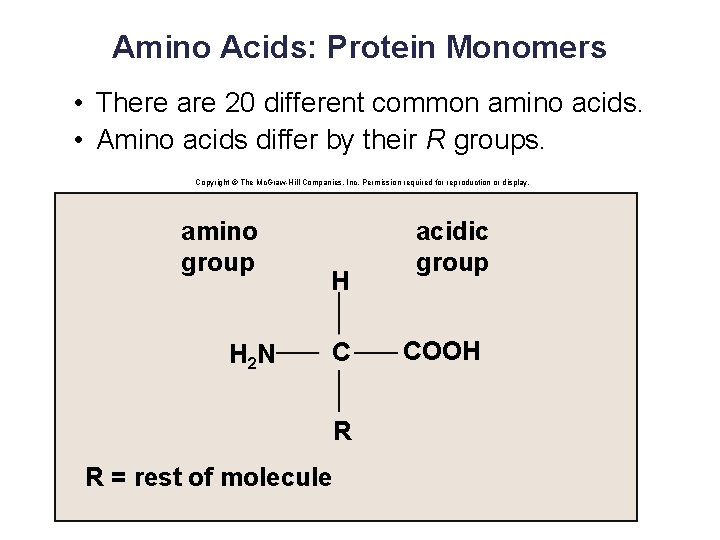
Amino Acids: Protein Monomers • There are 20 different common amino acids. • Amino acids differ by their R groups. Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. amino group H 2 N H C R R = rest of molecule acidic group COOH
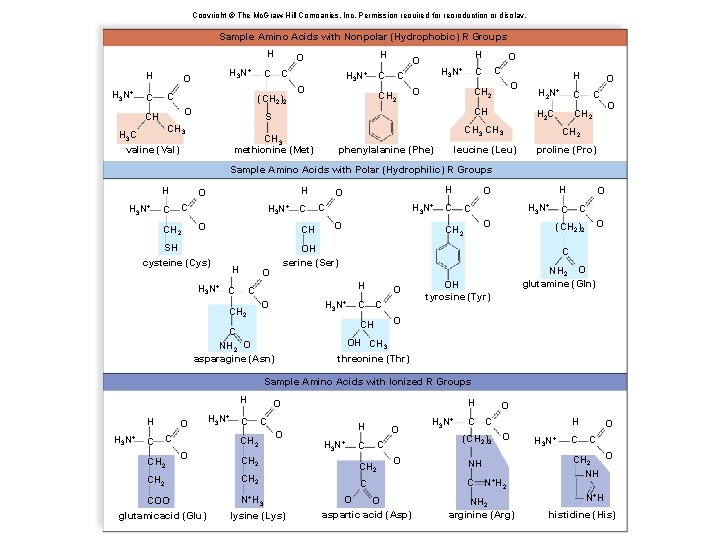
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Sample Amino Acids with Nonpolar (Hydrophobic) R Groups H H H 3 N+ O C C (CH 2)2 O CH H O H 3 N+ O H O C H 3 N+ C CH 2 O O CH 2 CH S CH 3 H 3 C valine (Val) O C C H 2 C CH 2 CH 3 methionine (Met) phenylalanine (Phe) leucine (Leu) O H 2 N+ C O CH 2 proline (Pro) Sample Amino Acids with Polar (Hydrophilic) R Groups H H 3 N+ C H O C CH 2 H 3 N+ C O CH SH cysteine (Cys) H H 3 N+ C O CH 2 H O C C H 3 N+ O C C O (CH 2)2 NH 2 O glutamine (Gln) OH tyrosine (Tyr) O CH C NH 2 O asparagine (Asn) O C C CH 2 H O OH serine (Ser) O H 3 N+ C H O OH CH 3 threonine (Thr) Sample Amino Acids with Ionized R Groups H H H 3 N+ C O C CH 2 H 3 N+ C C CH 2 O O H H 3 N+ CH 2 COO- N+H 3 glutamicacid (Glu) H O lysine (Lys) C CH 2 O C O O aspartic acid (Asp) H 3 N+ C O C (CH 2)3 H O NH C N+H 2 NH 2 arginine (Arg) H 3 N+ C O C CH 2 O NH N+H histidine (His)
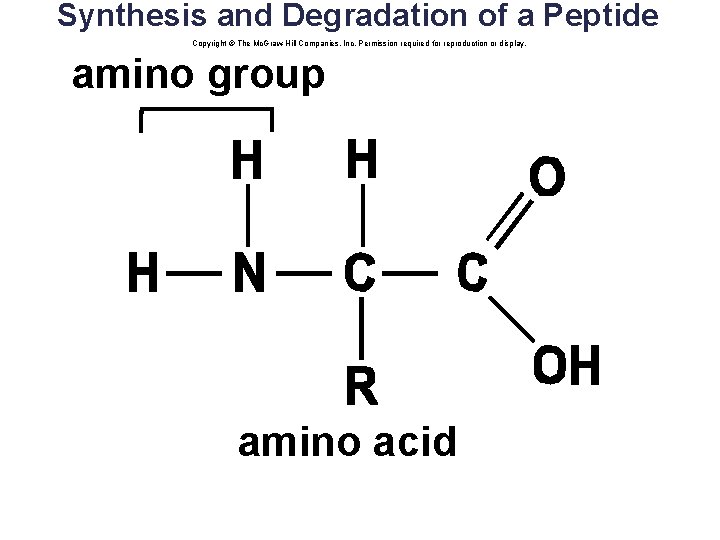
Synthesis and Degradation of a Peptide Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. amino group amino acid
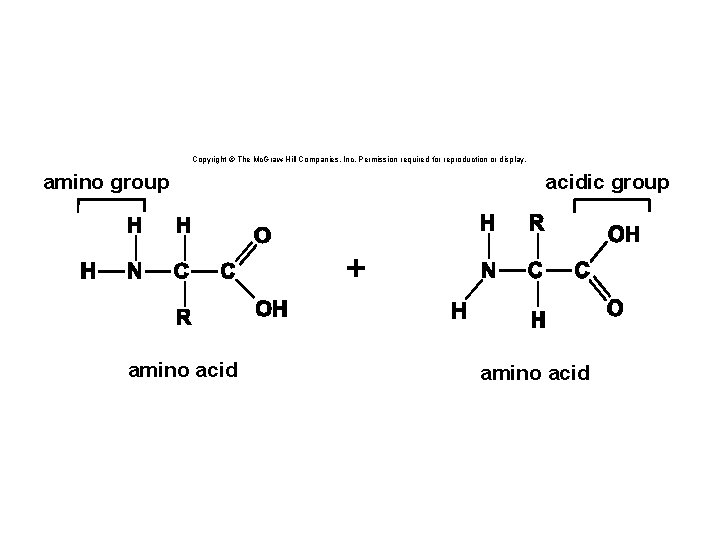
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. amino group acidic group + amino acid
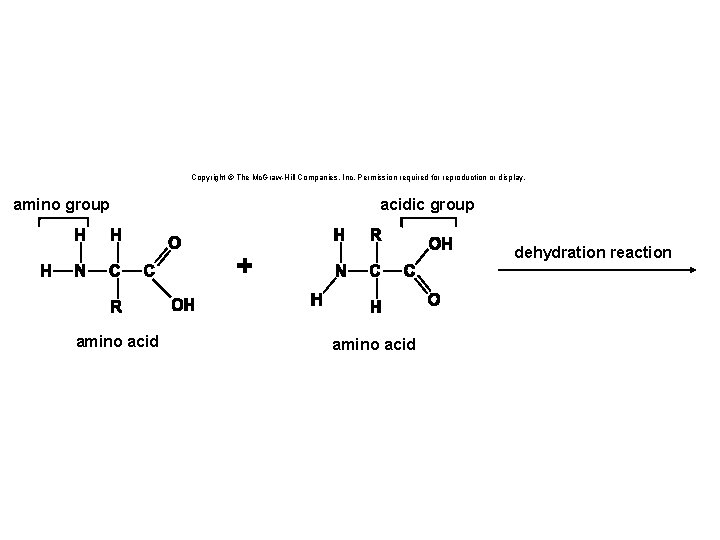
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. amino group acidic group dehydration reaction amino acid
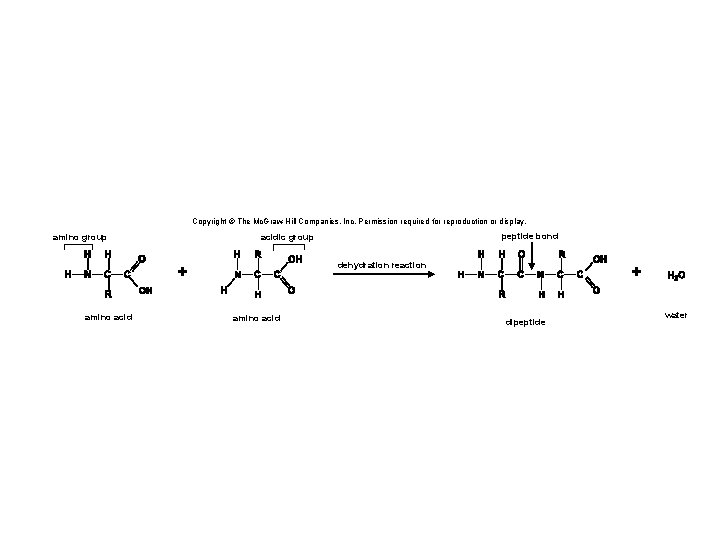
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. amino group peptide bond acidic group dehydration reaction amino acid dipeptide water
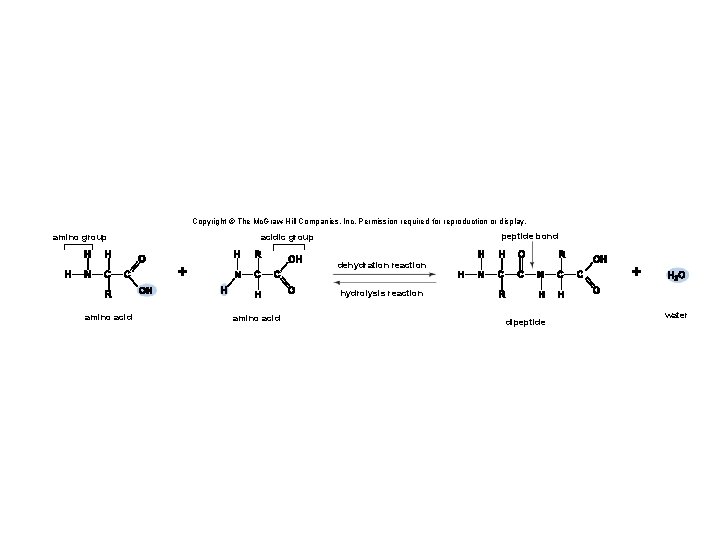
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. amino group peptide bond acidic group dehydration reaction hydrolysis reaction amino acid dipeptide water
Levels of Protein Structure • Proteins cannot function properly unless they fold into their proper shape. § When a protein loses it proper shape, it said to be denatured. • Exposure of proteins to certain chemicals, a change in p. H, or high temperature can disrupt protein structure. • Proteins can have up to four levels of structure: § § Primary Secondary Tertiary Quaternary 57
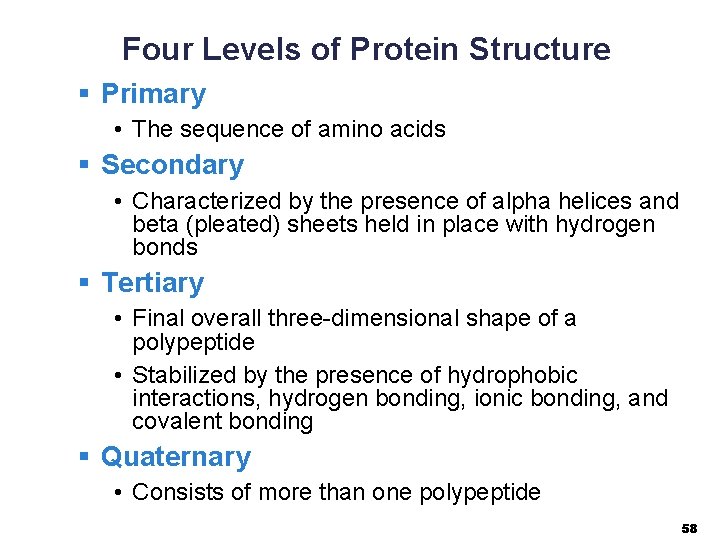
Four Levels of Protein Structure § Primary • The sequence of amino acids § Secondary • Characterized by the presence of alpha helices and beta (pleated) sheets held in place with hydrogen bonds § Tertiary • Final overall three-dimensional shape of a polypeptide • Stabilized by the presence of hydrophobic interactions, hydrogen bonding, ionic bonding, and covalent bonding § Quaternary • Consists of more than one polypeptide 58
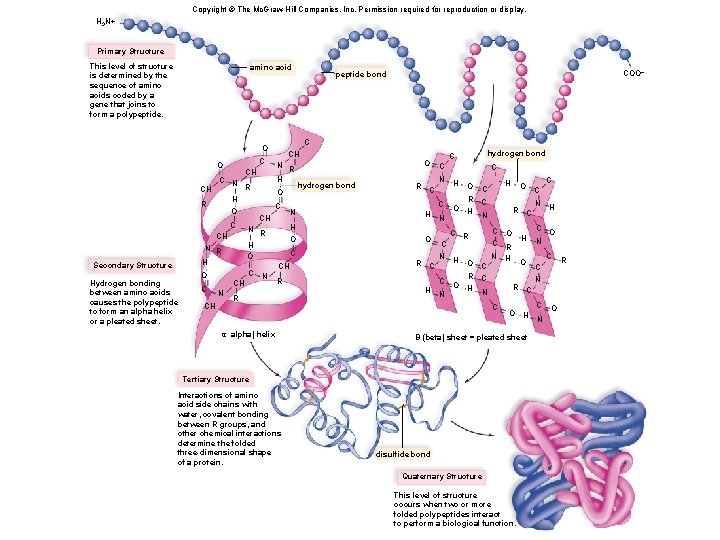
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. H 3 N+ Primary Structure This level of structure is determined by the sequence of amino acids coded by a gene that joins to form a polypeptide. amino acid C O C CH R O H O O C CH CH N C H O O CH R R hydrogen bond C C C N H O C H C N R C N CH N R H C hydrogen bond O N R O R H N R H C Hydrogen bonding between amino acids causes the polypeptide to form an alpha helix or a pleated sheet. N CH CH Secondary Structure CH C O COO– peptide bond N O H C R C H N C R C N H R C O C R C C O H H N N R C C O C R N H H O R C C α alpha) helix O O Interactions of amino acid side chains with water, covalent bonding between R groups, and other chemical interactions determine the folded three-dimensional shape of a protein. disulfide bond Quaternary Structure This level of structure occurs when two or more folded polypeptides interact to perform a biological function. N H C O N C C N C H Β (beta) sheet = pleated sheet Tertiary Structure C C N O R
Protein-Folding Diseases • Chaperone proteins help proteins fold into their normal shape. § Defects in chaperone proteins may play a role in several human diseases such as Alzheimer disease and cystic fibrosis. • Prions are misfolded proteins that have been implicated in a group of fatal brain diseases known as TSEs. § Mad cow disease is one example of a TSE disease. 60
3. 5 Nucleic Acids • Nucleic acids are polymers of nucleotides. • Two varieties of nucleic acids: § DNA (deoxyribonucleic acid) • Genetic material that stores information for its own replication and for the sequence of amino acids in proteins. § RNA (ribonucleic acid) • Perform a wide range of functions within cells which include protein synthesis and regulation of gene expression 61
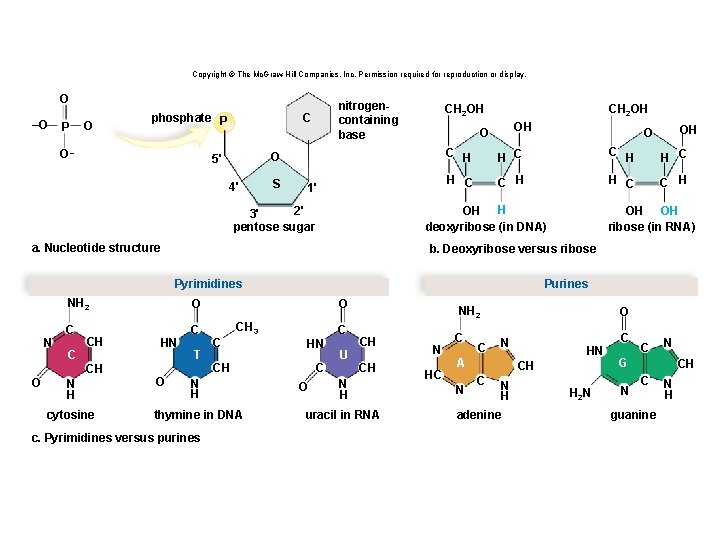
Structure of a Nucleotide • Each nucleotide is composed of three parts: § A phosphate group § A pentose sugar § A nitrogen-containing (nitrogenous) base • There are five types of nucleotides found in nucleic acids. § DNA contains adenine, guanine, cytosine, and thymine. § RNA contains adenine, guanine, cytosine, and uracil. • Nucleotides are joined together by a series of dehydration synthesis reactions to form a linear molecule called a strand. 62
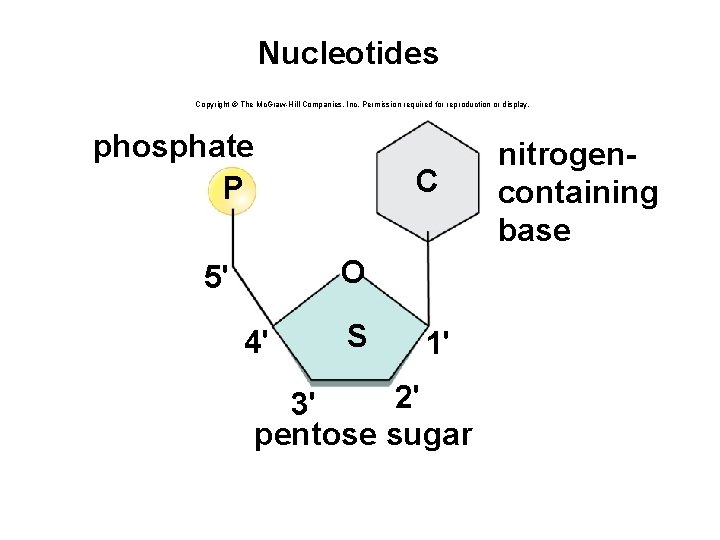
Nucleotides Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. phosphate P C O 5' 4' S 1' 2' 3' pentose sugar nitrogencontaining base
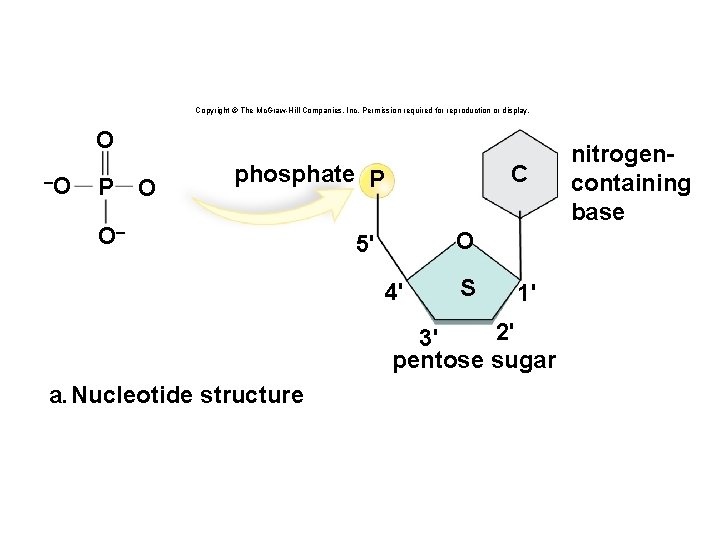
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. O –O P O C phosphate P O– O 5' 4' S 1' 2' 3' pentose sugar a. Nucleotide structure nitrogencontaining base
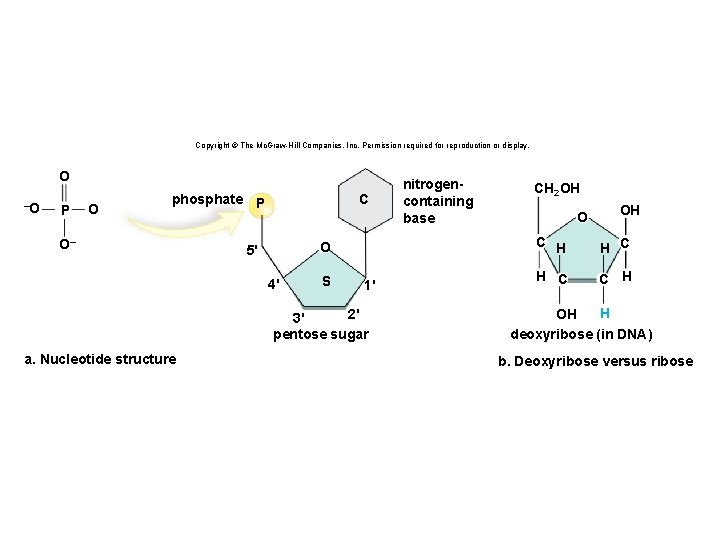
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. O –O P O C phosphate P O– O 5' 4' S 1' 2' 3' pentose sugar a. Nucleotide structure nitrogencontaining base CH 2 OH O OH C H C C H H OH deoxyribose (in DNA) b. Deoxyribose versus ribose
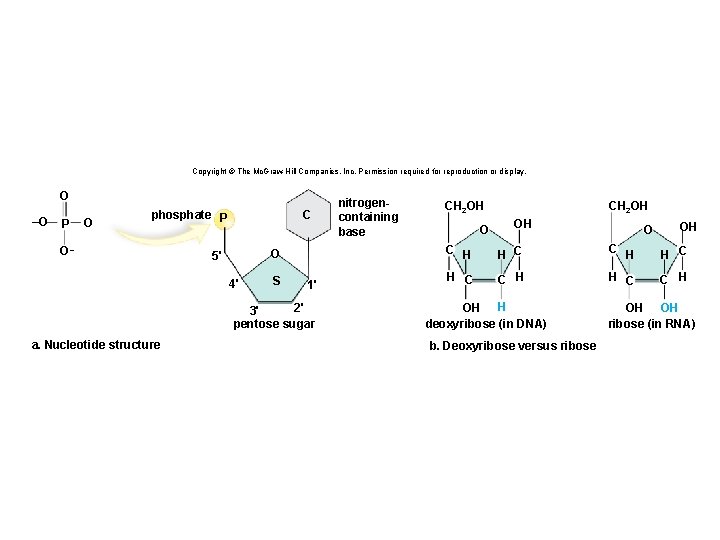
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. O –O P O C phosphate P O– O 5' 4' S 1' 2' 3' pentose sugar a. Nucleotide structure nitrogencontaining base CH 2 OH OH O OH C H C C H OH H deoxyribose (in DNA) b. Deoxyribose versus ribose OH OH ribose (in RNA)
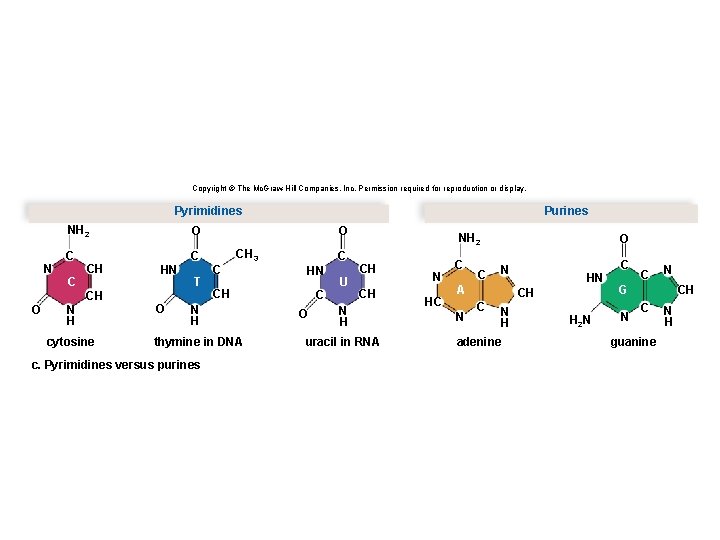
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. Pyrimidines N NH 2 O C C CH O N H cytosine HN O T O CH 3 C C HN CH N H thymine in DNA c. Pyrimidines versus purines Purines C O U NH 2 CH CH N H uracil in RNA N HC C C O N A N HN CH C N H adenine H 2 N C C N G N CH C guanine N H
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. O –O P O O– nitrogencontaining base C phosphate P CH 2 OH 4' S OH O O 5' CH 2 OH 1' C H H C H C C H OH H deoxyribose (in DNA) 2' 3' pentose sugar a. Nucleotide structure NH 2 C CH O N H cytosine Purines O HN O OH OH ribose (in RNA) b. Deoxyribose versus ribose Pyrimidines N OH O C T O CH 3 C HN CH N H thymine in DNA c. Pyrimidines versus purines C C O U NH 2 CH CH N H uracil in RNA N HC C C O N A N HN CH C N H adenine H 2 N C C N G N CH C guanine N H
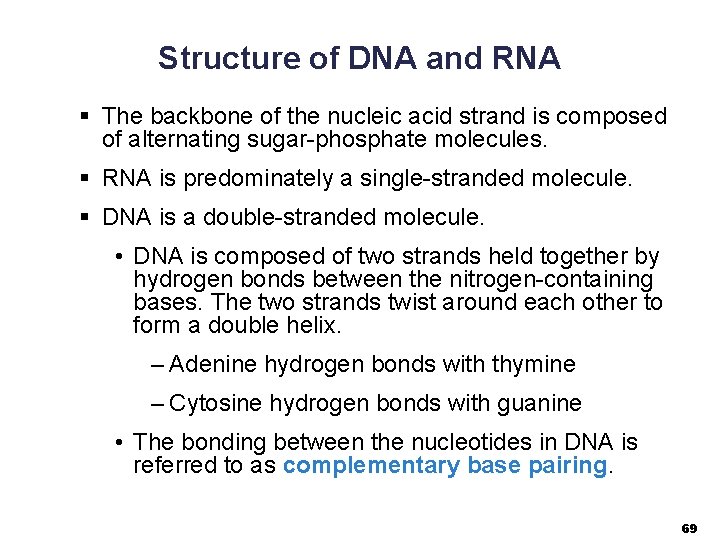
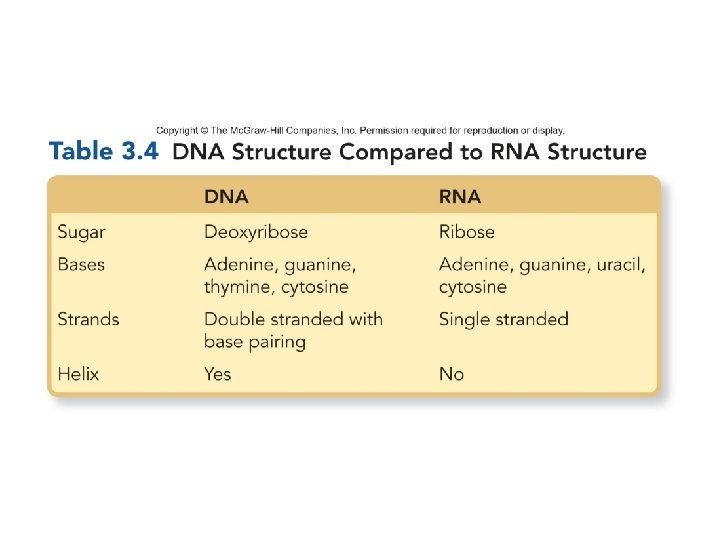
Structure of DNA and RNA § The backbone of the nucleic acid strand is composed of alternating sugar-phosphate molecules. § RNA is predominately a single-stranded molecule. § DNA is a double-stranded molecule. • DNA is composed of two strands held together by hydrogen bonds between the nitrogen-containing bases. The two strands twist around each other to form a double helix. – Adenine hydrogen bonds with thymine – Cytosine hydrogen bonds with guanine • The bonding between the nucleotides in DNA is referred to as complementary base pairing. 69
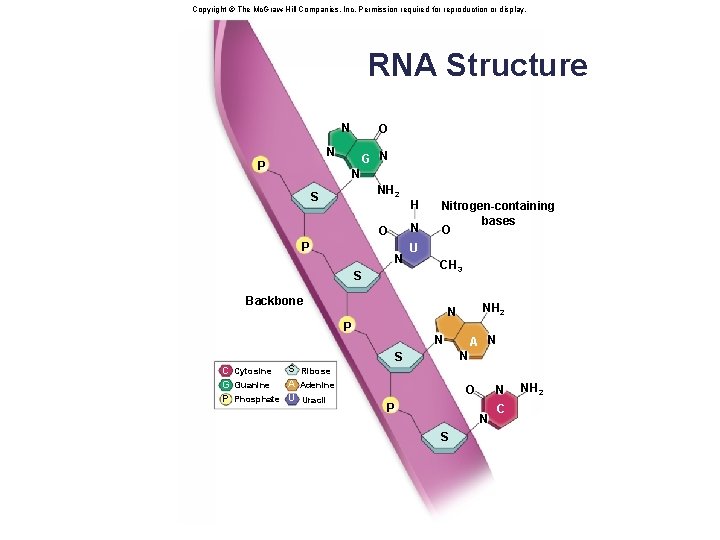
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. RNA Structure Fig. 3. 19 N O N P G N N NH 2 S N O P H N S Nitrogen-containing bases O U CH 3 Backbone P N A N N S S Ribose C Cytosine A Guanine G Adenine P Phosphate U Uracil NH 2 N N O P N S C NH 2
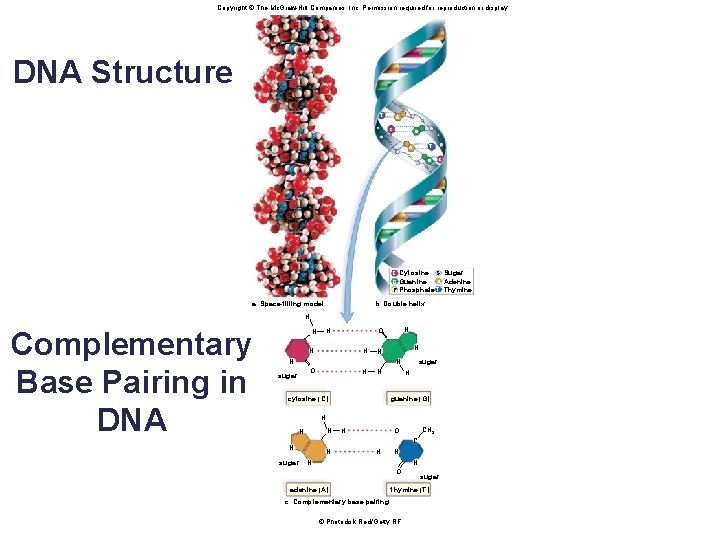
Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. DNA Structure T A C G T A G C C CCytosine S Sugar Guanine A AAdenine GG P Phosphate T TThymine b. Double helix a. Space-filling model ― H ― N O sugar N ― ― ― N H cytosine (C) sugar N ― ― ― N N N O H H guanine (G) H N N CH 3 O H C H N N ― ― suga r ― N N O adenine (A) ― ― ― Complementary Base Pairing in DNA N N sugar thymine (T) c. Complementary base pairing © Photodisk Red/Getty RF
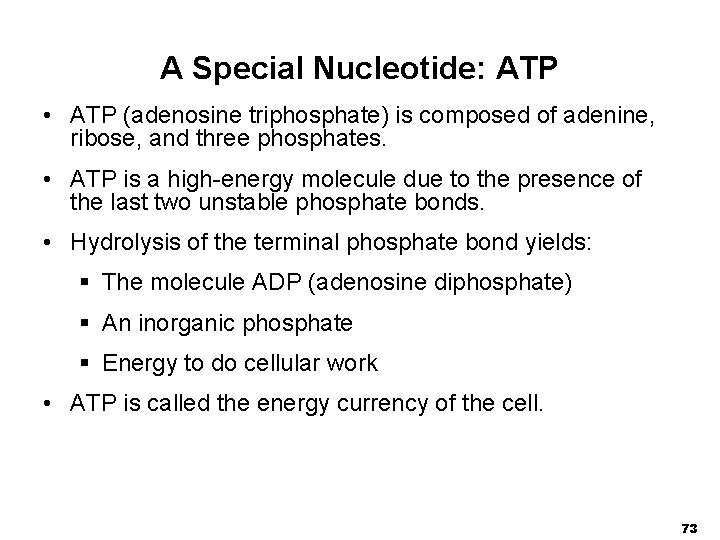
A Special Nucleotide: ATP • ATP (adenosine triphosphate) is composed of adenine, ribose, and three phosphates. • ATP is a high-energy molecule due to the presence of the last two unstable phosphate bonds. • Hydrolysis of the terminal phosphate bond yields: § The molecule ADP (adenosine diphosphate) § An inorganic phosphate § Energy to do cellular work • ATP is called the energy currency of the cell. 73
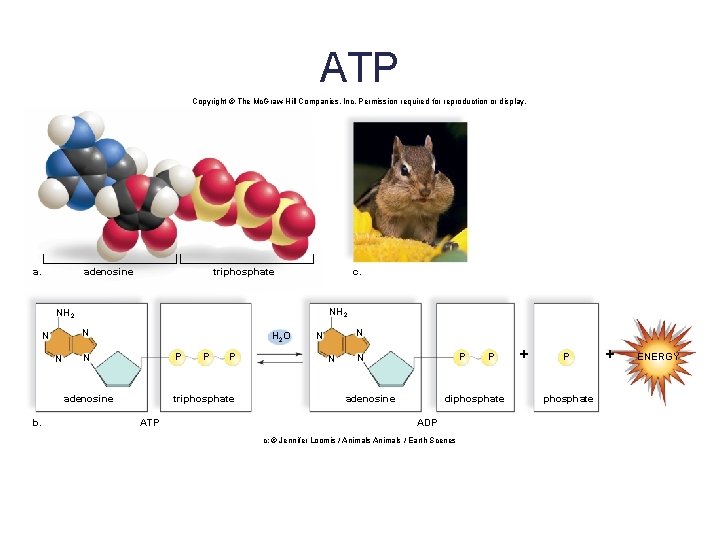
ATP Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. adenosine triphosphate c. NH 2 N N N H 2 O P N adenosine b. P P triphosphate ATP N N N P N adenosine P diphosphate ADP c: © Jennifer Loomis / Animals / Earth Scenes + P phosphate + ENERGY
- Slides: 74