CHAPTER 3 BACTERIAL IDENTIFICATION METHODS CONTENT Purification of

CHAPTER 3 BACTERIAL IDENTIFICATION METHODS

CONTENT Purification of cultures Morphological and pure culture studies Biochemical tests
PURIFICATION OF CULTURES Reason to purify cultures. To characterize an individual species. To study the morphology and physiology of individual bacterial species To study their biochemical behavior and response. To purify, pure cultures techniques can be used. Method: Streak plate method Pour plate method Spread plate method

IMPORTANT PROCEDURE!! Need to have a control procedure to avoid contamination. Specimen collection Preparation of media Microbiological tecniques Staining and reagents Equipment used.

SPECIMEN COLLECTION Applied the sterile techniques Use correct media for transportation and stock. The transport media used to preserve and ensure the viability of bacteria during the transportation period Important! Label your specimen. Crucial for cerebrospinal fluid, blood culture and fecal specimens, etc.

USING STERILE TECHNIQUES 6 Bacteria are everywhere Media used for bacteria growth welcoming for many bacteria We only want specific ones to grow Sterile techniques Sterile remain sterile as long as doesn’t touch anything that isn’t sterile Also avoid prolonged exposure to air

7 ASEPTIC TECHNIQUE: These are various techniques that are used to minimize the introduction of microorganisms into media especially during transfer processes, such as : pouring of media into Petri dishes inoculation of cultures These techniques include: cleaning the bench top work areas with disinfectant solution washing hands before starting work other specific techniques that will be demonstrated in the lab.

STERILE TECHNIQUES: WHAT CAN YOU DO IN THE LAB? 8 Wash your hands Keep your bench clean Wear gloves Flame loop, neck of tube Keep cap facing down Work quickly and efficiently Limit talking when opening cultures
PREPARATION OF MEDIA The media should be packed well to prevent from leakage and breaks, protected from moisture and sunlight and excessive heat The expiry date should be noted and the instruction of storage should be followed The mix bacterial colonies should be sub cultured until the culture are purified the bacterial colony characteristic should only derive from a single colony
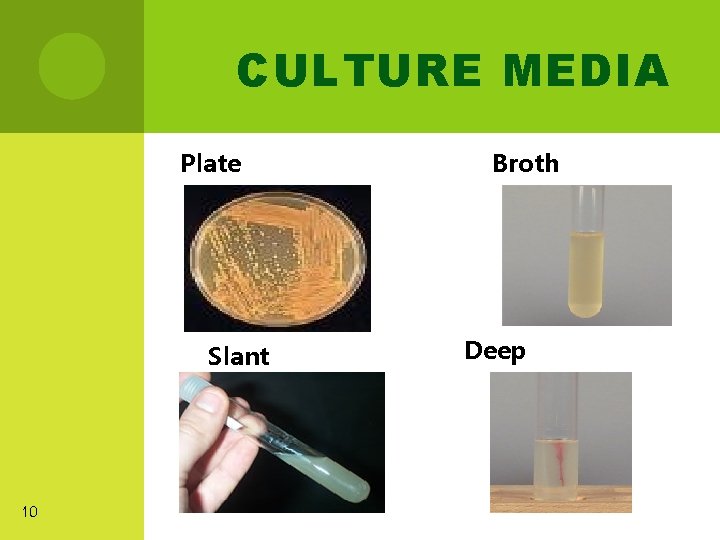
CULTURE MEDIA Plate Slant 10 Broth Deep

MORPHOLOGICAL AND PURE CULTURE STUDIES

Morphological studies: - Sizes, shapes, cell arrangement, cell wall, surface adherents or appendages, flagella, pili, endospores, ribosomes. - Macroscopic examintation Techniques used in the study: - Microscopic examintion - Staining techniques

MORPHOLOGICAL AND PURE CULTURE STUDIES
ISOLATION OF PURE BACTERIAL CULTURES Divide into 3 groups: Selective media Differental media Enrichment media

SELECTIVE MEDIA Prepared by the addition of specific subtances to a culture medium that will permit growth of one bacteria while inhibiting the growth of others. Contain antimicrobial agents such as crytal violet, bile salts, sodium azide, antibiotic and e. t. c. Salmonella-Shigella Agar- media contain bile salts (inhibits many coliform bacteria). Produce colorless colonies (unable to ferment lactose) Mannitol Salt Agar -Isolation of Staphylococci. Bismuth Sulfite Agar-Isolation for Salmonella typhi. Reduces the sulfite to sulfide results in black colonies and with metallic sheen.
DIFFERENTIAL MEDIA The incorporation of certain chemicals into a medium may result in diagnostically useful growth or visible change in the medium after incubation. Eosin Methylene Blue(EMB)- Differentiate between lactse and non-lactose fermenters. Mac Conkey Agar-contain crystal violet and bile salts. Use for selection of Enterobacteriaceae and related gram negative rods. Hektoen Enteric Agar-High concenration of bile salts. Inhibit Gram positive bacteria and retards the growth of many coliform strains.
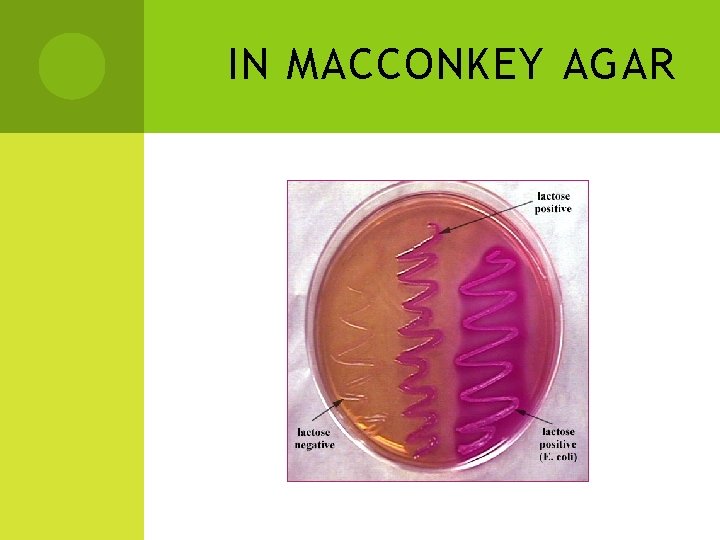
IN MACCONKEY AGAR

IN MACCONKEY AGAR
ENRICHMENT MEDIA These are routinely employed in a laboratory e. g. nutrient broth, nutrient agar, infusion broth, blood agar. They support the growth of fastidious bacteria.

IN NUTRIENT AGAR
PURE COLONY
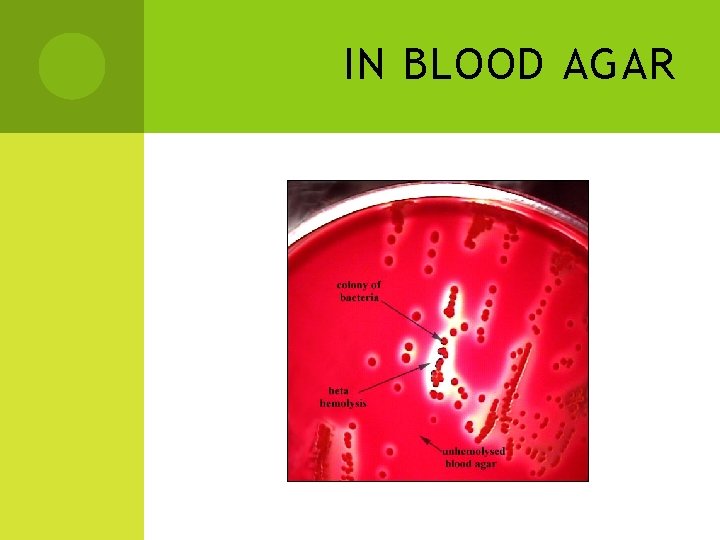
IN BLOOD AGAR
HEMOLYSIS Destruction of erythrocytes nd hemoglobin in medium. Can be divided into 3 categories: alpha hemolysis, beta hemolysis and gamma hemolysis Alpha hemolysis-greenish to brownish discolouration around the colonies. (Streptococous gordonii, Streptococcus pneumoniae) Beta hemolysis-complete lysis of blood cell resulting in clearing effect around the growth of colony. (S. aureus) Gamma hemolysis-no change in the medium. (Enterococcus faecalis)
BIOCHEMICAL TESTS Catalase test Oxidase test Coagulase test Sugar fermentation test MRVP test Indole test Citrate test Motility test H 2 S test Litmus milk test
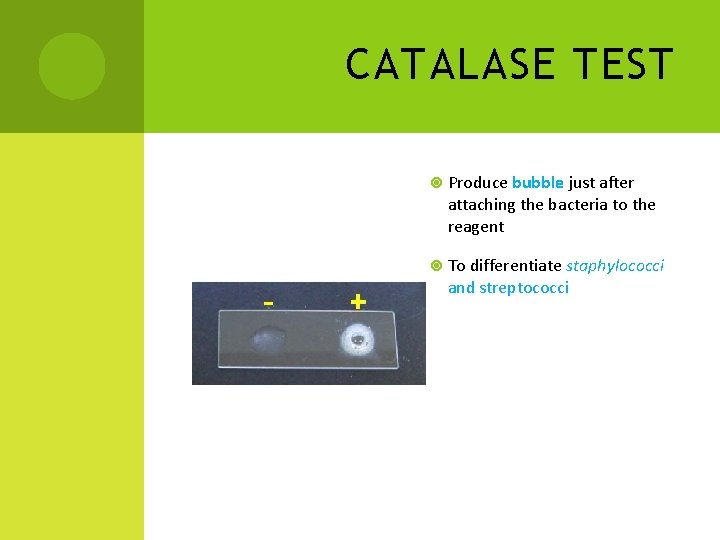
CATALASE TEST Produce bubble just after attaching the bacteria to the reagent To differentiate staphylococci and streptococci

OXIDASE TEST Have 2 methods: Filter paper/Sterile swab To help identify Vibrio, Neisseria, Pasteurella and Pseudomonas sp. Oxidase enzymes oxydize phenylenediamine. Deep purple colour on reagent paper

OXIDASE TEST

COAGULASE TEST To identify S. aureus The enzyme coagulase clots plasma Tube : fibrin clot Slide: clumping of bacterial cells

SUGAR FERMENTATION TEST Glucose test Maltose test Sucrose test Lactose test Some will appear with gas production

VOGES-PROSKAUER TEST To differentiate enterobacteria Organism ferments glucose with acetoin production. Acetoin is oxidised to diacetyl which reacts with creatine. Brick red colour develop slowly Eg: E. coli (-) Klebsiella sp. (+)
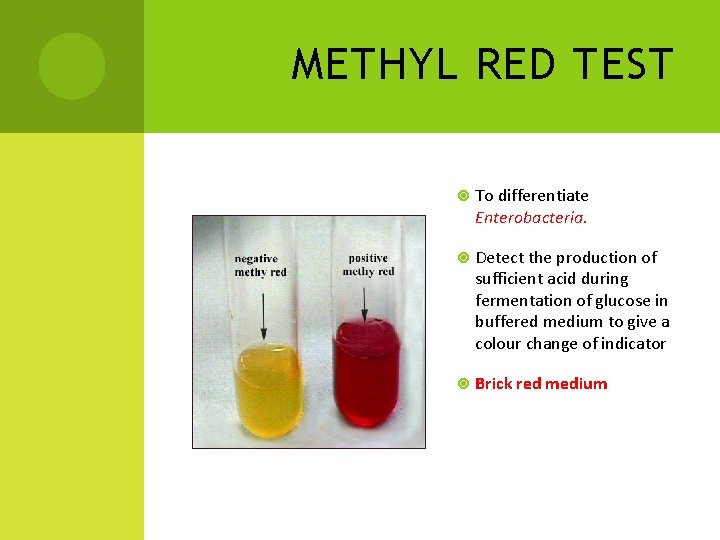
METHYL RED TEST To differentiate Enterobacteria. Detect the production of sufficient acid during fermentation of glucose in buffered medium to give a colour change of indicator Brick red medium
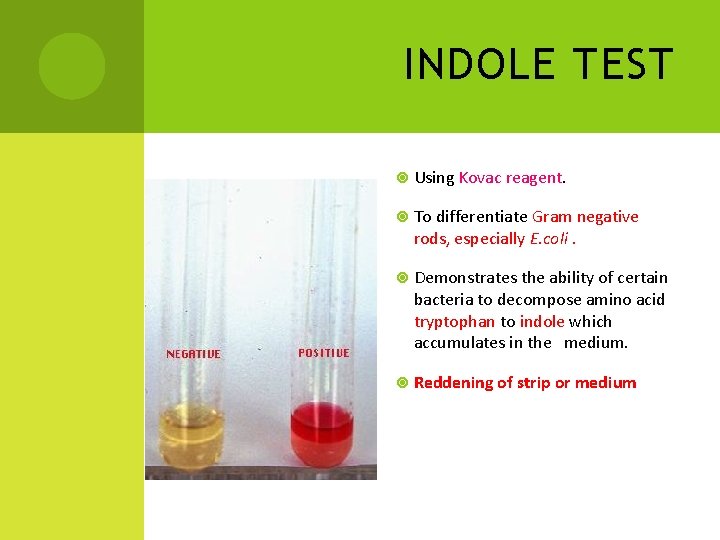
INDOLE TEST Using Kovac reagent. To differentiate Gram negative rods, especially E. coli. Demonstrates the ability of certain bacteria to decompose amino acid tryptophan to indole which accumulates in the medium. Reddening of strip or medium

INDOLE TEST USING OTHER REAGENT

CITRATE TEST Test the ability of organism to utilise citrate as a sole carbon source and ammonium salt for nitrogen. Result in alkalinization in the medium with colour change indicator. Use Koser’s liquid citrate medium. Differentiate Enterobacteria from other bacteria. Positive result : Blue and turbid medium

MOTILITY TEST

LITMUS TEST Medium consisting of LACTOSE, CASEIN and the p. H indicator azolitmin. It is used to differentiate members within the genus Clostridium. It differentiates Enterobacteriaceae from other Gram-negative bacilli based on enterics' ability to reduce litmus. The skim milk provides nutients for growth. The protein is casein and the lactose is for fermentation. Azolitmin is purple between p. H of 4. 6 and 8. 2. It turns pink when p. H reaches 4. 5 and blue at a p. H of 8. 3. Because of this, litmus milk can give quite unreliable results. Thus, you would be advised to use litmus milk as a confirmatory test but not a definitive test (except as a last resort).


TRIPLE SUGAR ION Triple Sugar Iron medium is a differential medium that can distinguish between a number of Gram-negative enteric bacteria based on their physiological ability (or lack thereof) to: a. metabolize lactose and/or sucrose b. conduct fermentation to produce acid c. produce gas during fermentation d. generate H 2 S.

TERMS FOR TODAY Culture collection centre. ATCC American type culture Collection Centre NCTCC National Collection of Type Culture NCIM Natonal Collection of Industrial and Marine Bacterial NCDO National Collection of Dairy Organism

THE END
- Slides: 41