Chapter 3 Atoms and Elements 3 6 Isotopes
- Slides: 17

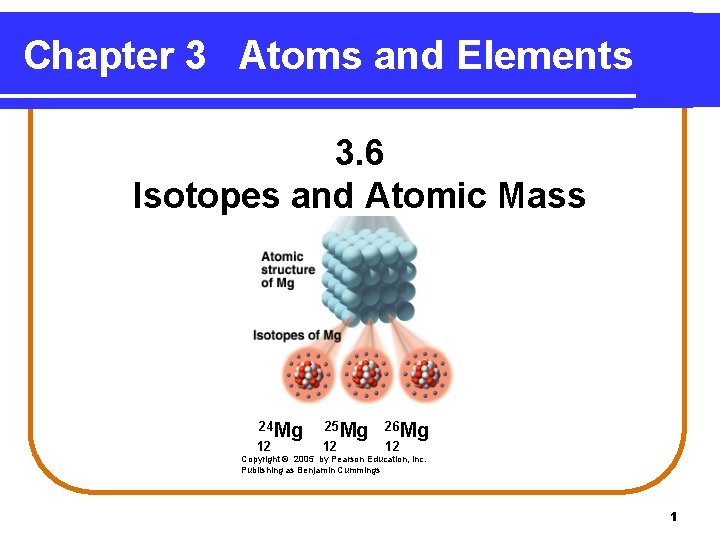
Chapter 3 Atoms and Elements 3. 6 Isotopes and Atomic Mass 24 Mg 12 25 Mg 12 26 Mg 12 Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

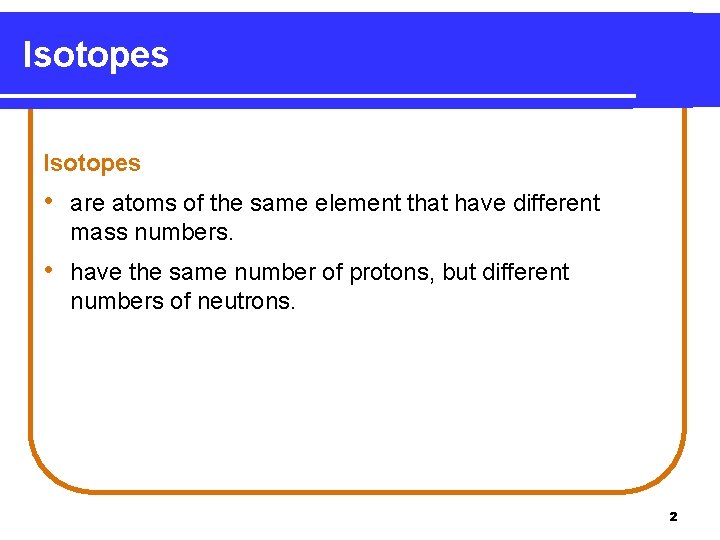
Isotopes • are atoms of the same element that have different mass numbers. • have the same number of protons, but different numbers of neutrons. 2

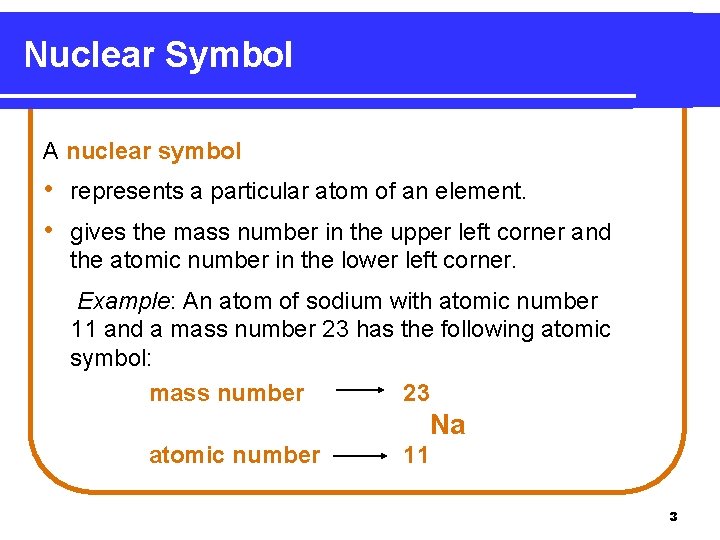
Nuclear Symbol A nuclear symbol • represents a particular atom of an element. • gives the mass number in the upper left corner and the atomic number in the lower left corner. Example: An atom of sodium with atomic number 11 and a mass number 23 has the following atomic symbol: mass number 23 Na atomic number 11 3

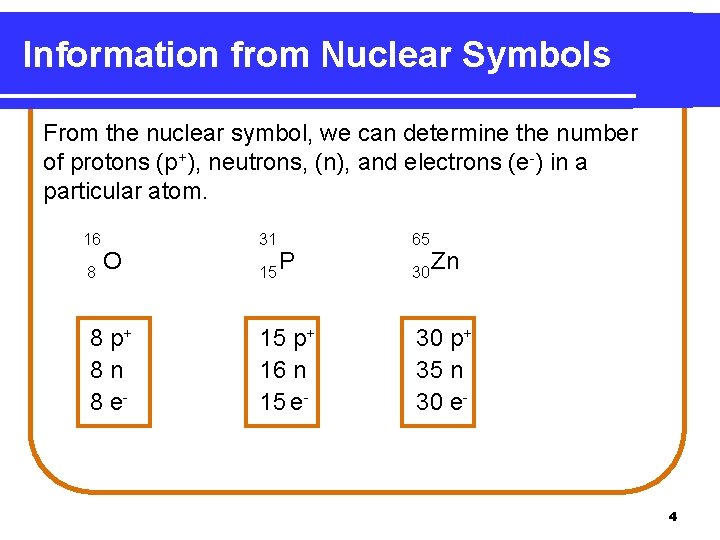
Information from Nuclear Symbols From the nuclear symbol, we can determine the number of protons (p+), neutrons, (n), and electrons (e-) in a particular atom. 16 8 O 8 p+ 8 n 8 e- 31 15 P 15 p+ 16 n 15 e- 65 30 Zn 30 p+ 35 n 30 e- 4

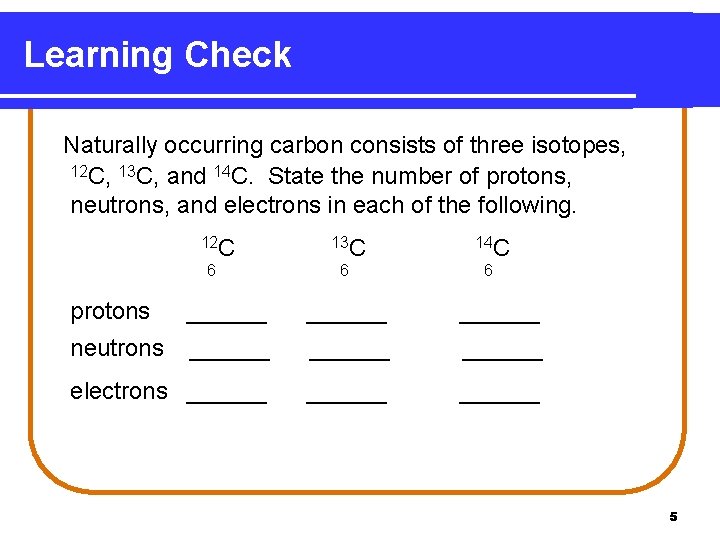
Learning Check Naturally occurring carbon consists of three isotopes, 12 C, 13 C, and 14 C. State the number of protons, neutrons, and electrons in each of the following. 12 C 6 13 C 14 C 6 6 protons ______ neutrons ______ electrons ______ 5

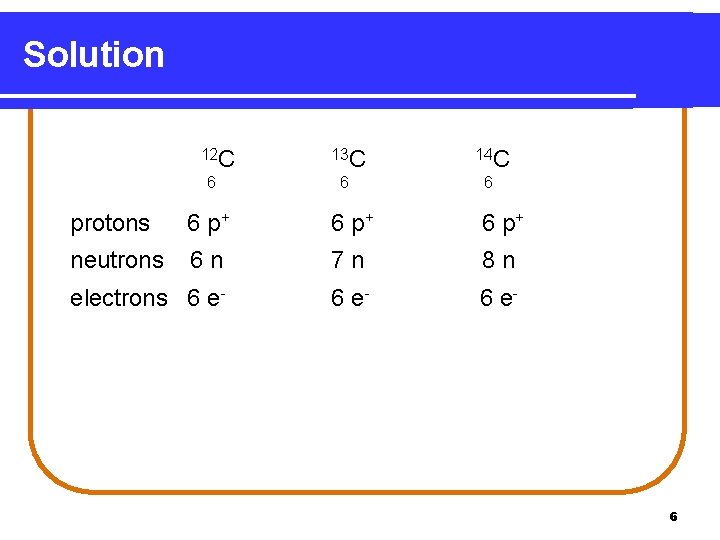
Solution 12 C 6 13 C 14 C 6 6 protons 6 p+ neutrons 6 n 7 n 8 n electrons 6 e- 6

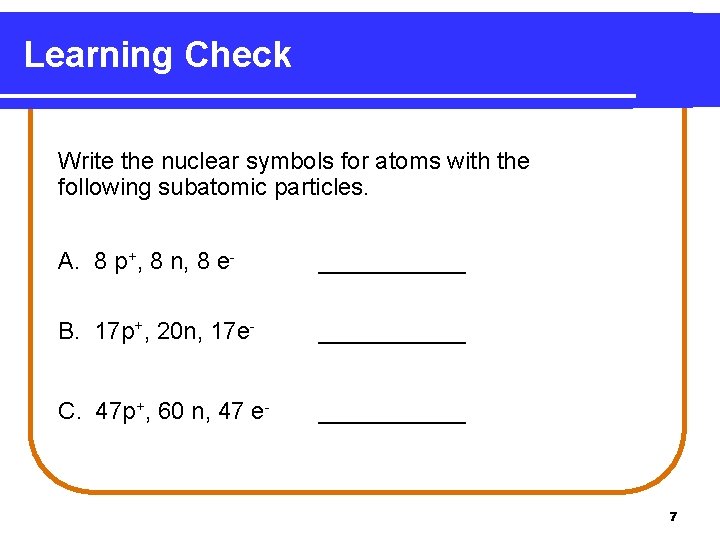
Learning Check Write the nuclear symbols for atoms with the following subatomic particles. A. 8 p+, 8 n, 8 e- ______ B. 17 p+, 20 n, 17 e- ______ C. 47 p+, 60 n, 47 e- ______ 7

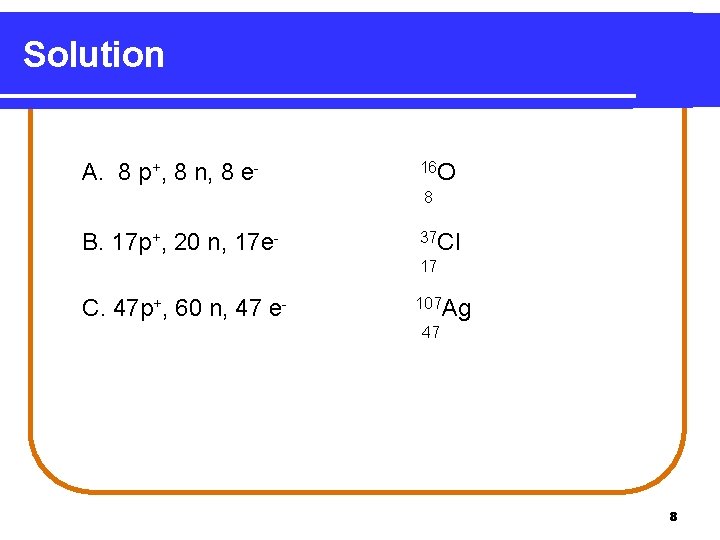
Solution A. 8 p+, 8 n, 8 e- 16 O 8 B. 17 p+, 20 n, 17 e- 37 Cl 17 C. 47 p+, 60 n, 47 e- 107 Ag 47 8

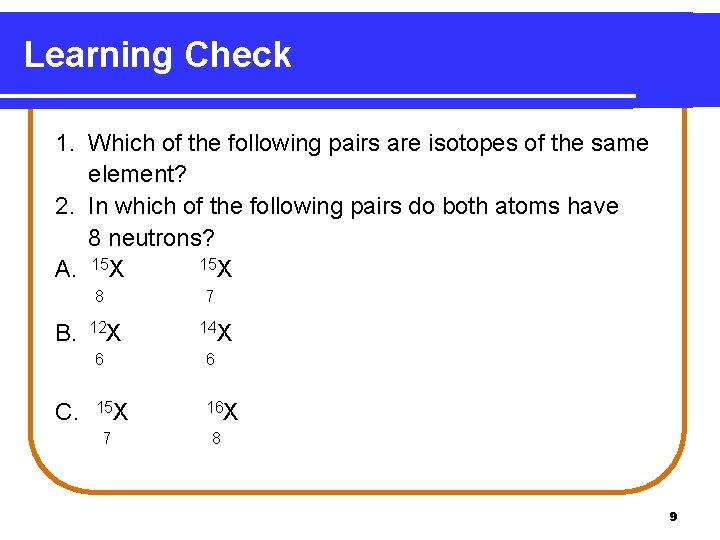
Learning Check 1. Which of the following pairs are isotopes of the same element? 2. In which of the following pairs do both atoms have 8 neutrons? 15 X A. 15 X 8 B. C. 12 X 7 14 X 6 6 15 X 16 X 7 8 9

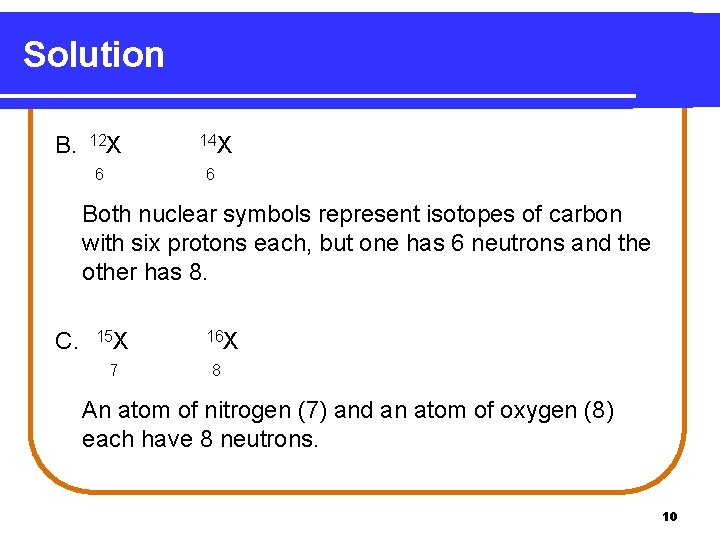
Solution B. 12 X 6 14 X 6 Both nuclear symbols represent isotopes of carbon with six protons each, but one has 6 neutrons and the other has 8. C. 15 X 7 16 X 8 An atom of nitrogen (7) and an atom of oxygen (8) each have 8 neutrons. 10

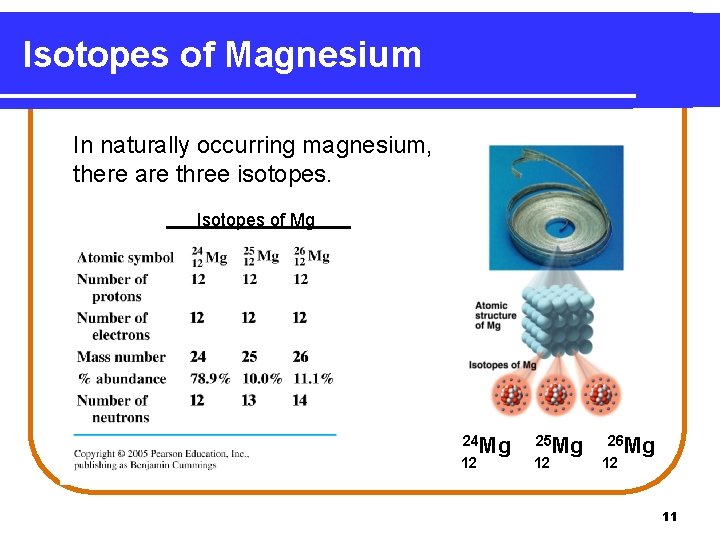
Isotopes of Magnesium In naturally occurring magnesium, there are three isotopes. Isotopes of Mg 24 Mg 12 25 Mg 12 26 Mg 12 11

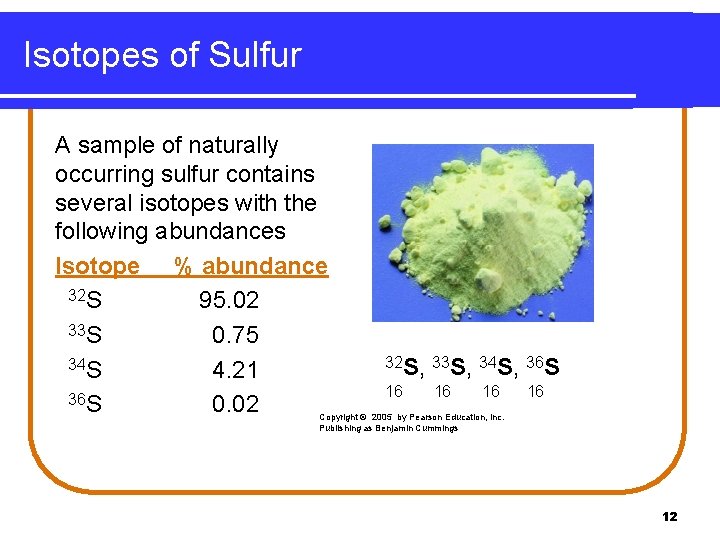
Isotopes of Sulfur A sample of naturally occurring sulfur contains several isotopes with the following abundances Isotope % abundance 32 S 95. 02 33 S 0. 75 34 S 4. 21 36 S 0. 02 32 S, 33 S, 34 S, 36 S 16 16 Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 12

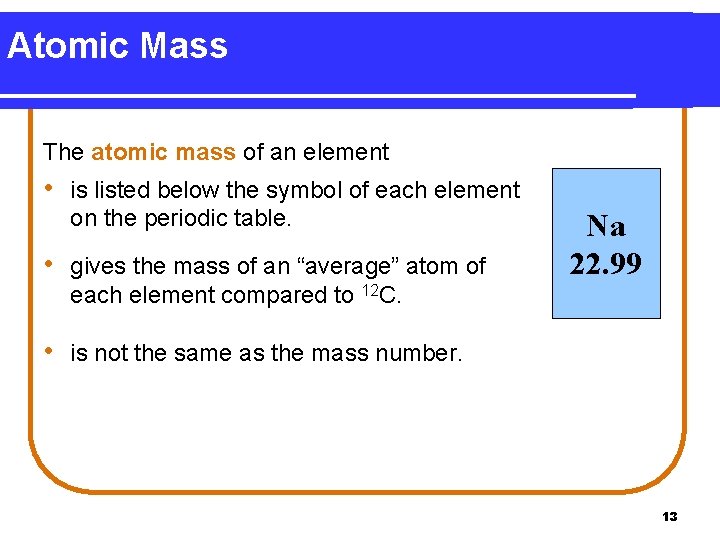
Atomic Mass The atomic mass of an element • is listed below the symbol of each element on the periodic table. • gives the mass of an “average” atom of Na 22. 99 each element compared to 12 C. • is not the same as the mass number. 13

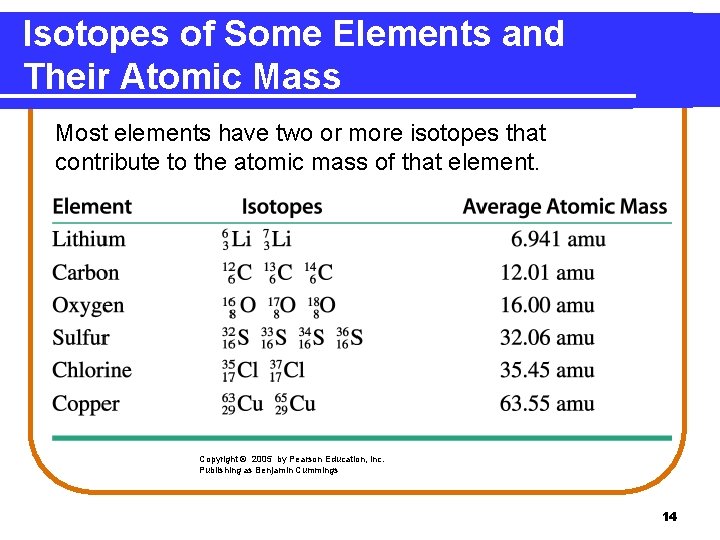
Isotopes of Some Elements and Their Atomic Mass Most elements have two or more isotopes that contribute to the atomic mass of that element. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 14

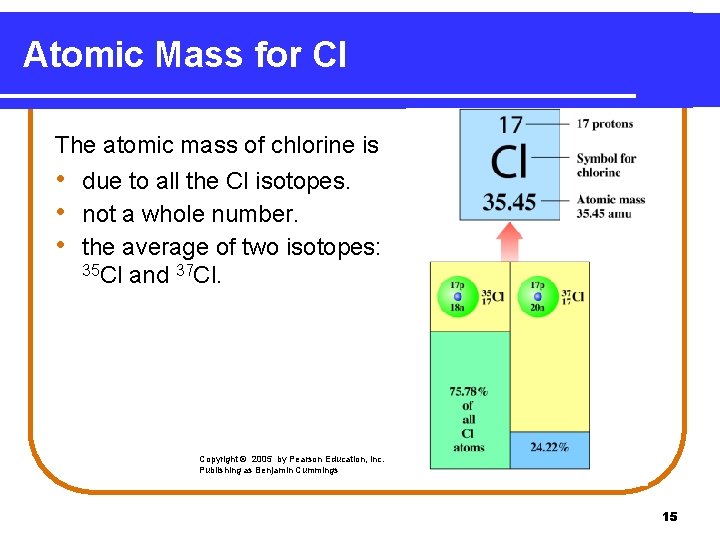
Atomic Mass for Cl The atomic mass of chlorine is • due to all the Cl isotopes. • not a whole number. • the average of two isotopes: 35 Cl and 37 Cl. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 15

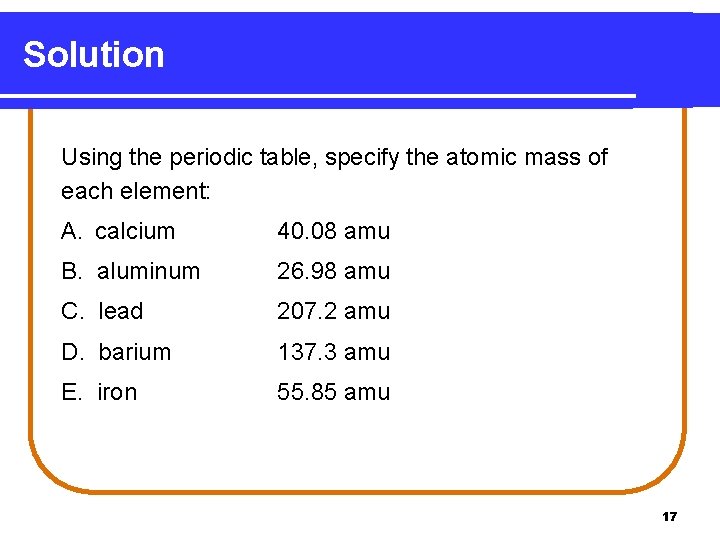
Learning Check Using the periodic table, specify the atomic mass of each element. A. calcium _____ B. aluminum _____ C. lead _____ D. barium _____ E. iron _____ 16

Solution Using the periodic table, specify the atomic mass of each element: A. calcium 40. 08 amu B. aluminum 26. 98 amu C. lead 207. 2 amu D. barium 137. 3 amu E. iron 55. 85 amu 17