Chapter 3 Atomic Theory of Matter The theory





- Slides: 31

Chapter 3

Atomic Theory of Matter The theory that atoms are the fundamental building blocks of matter reemerged in the early 19 th century, championed by John Dalton. © 2009, Prentice-Hall, Inc.

Dalton's Postulates Each element is composed of extremely small particles called atoms. © 2009, Prentice-Hall, Inc.

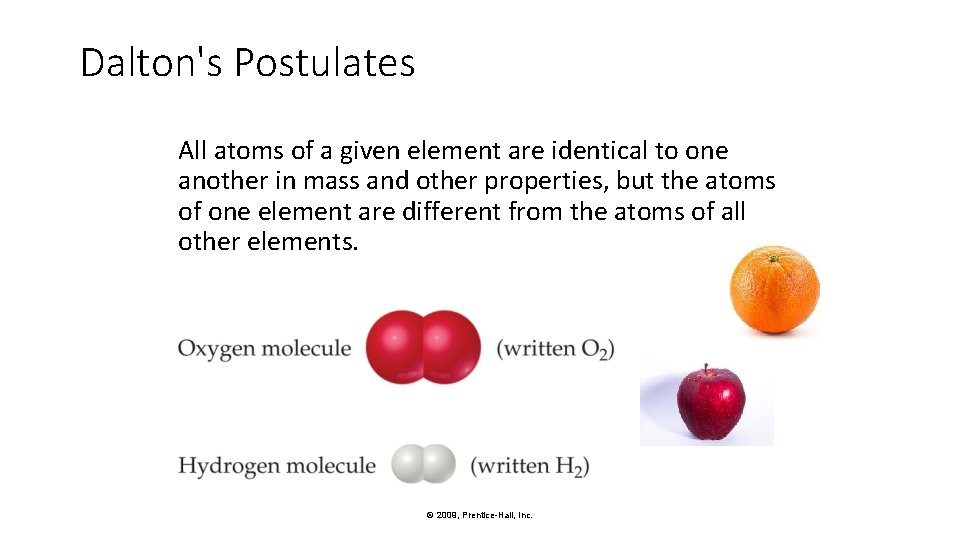
Dalton's Postulates All atoms of a given element are identical to one another in mass and other properties, but the atoms of one element are different from the atoms of all other elements. © 2009, Prentice-Hall, Inc.

Dalton's Postulates © 2009, Prentice-Hall, Inc.

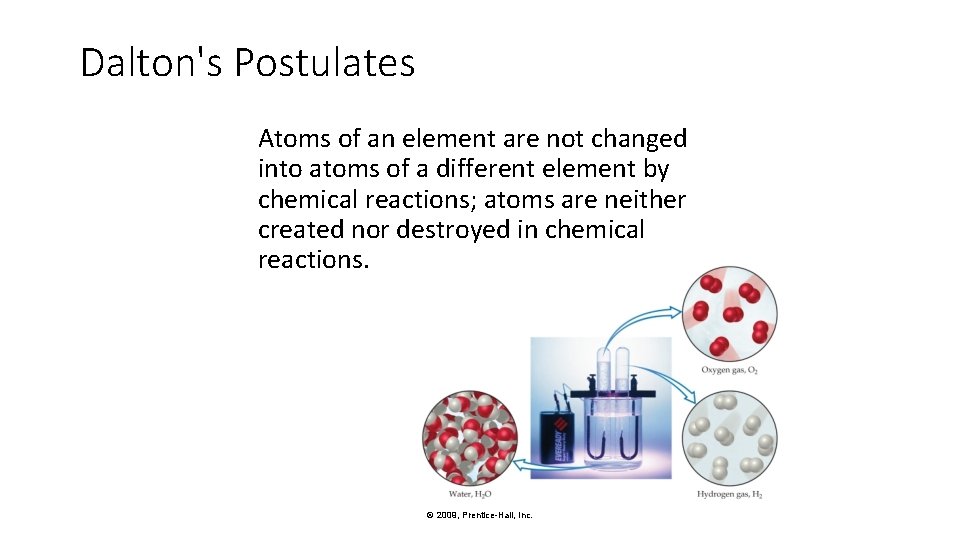
Dalton's Postulates Atoms of an element are not changed into atoms of a different element by chemical reactions; atoms are neither created nor destroyed in chemical reactions. © 2009, Prentice-Hall, Inc.

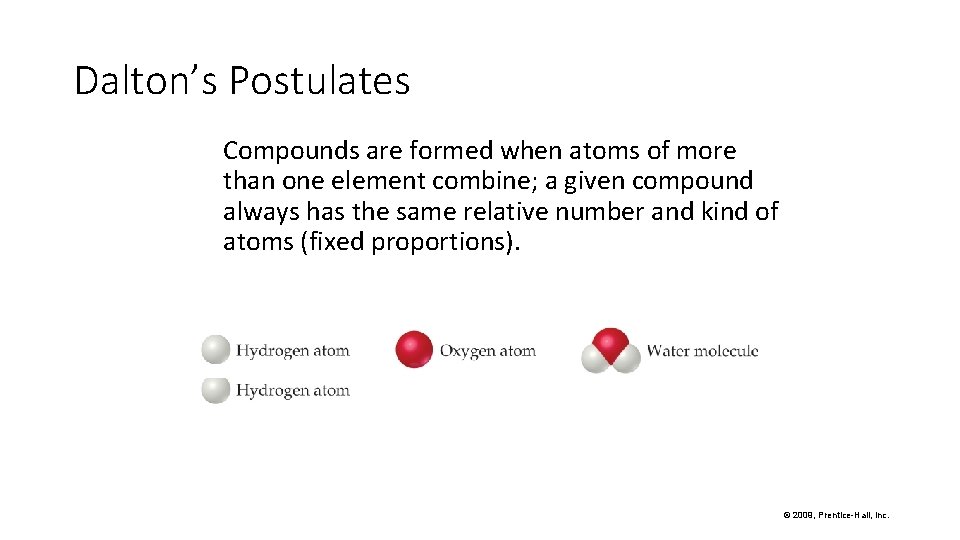
Dalton’s Postulates Compounds are formed when atoms of more than one element combine; a given compound always has the same relative number and kind of atoms (fixed proportions). © 2009, Prentice-Hall, Inc.

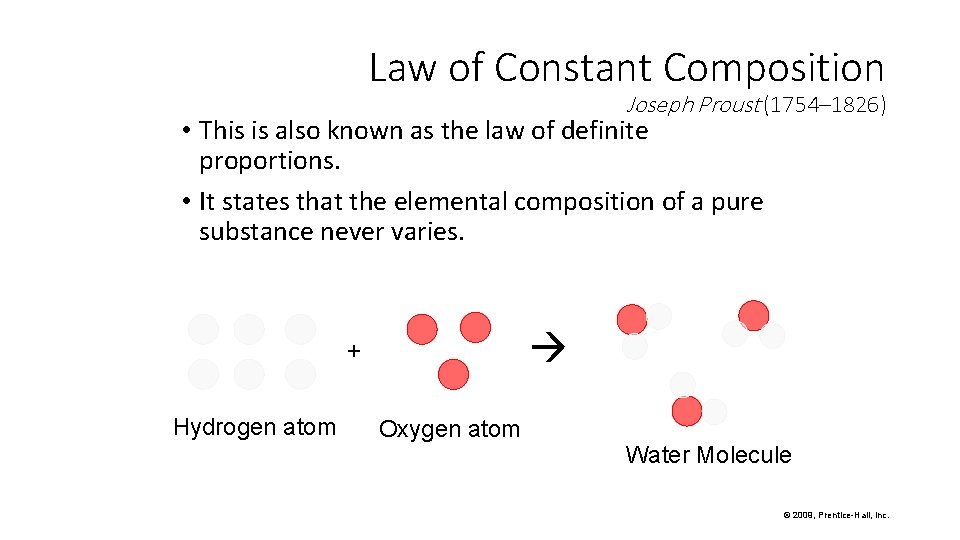
Law of Constant Composition Joseph Proust (1754– 1826) • This is also known as the law of definite proportions. • It states that the elemental composition of a pure substance never varies. + Hydrogen atom Oxygen atom Water Molecule © 2009, Prentice-Hall, Inc.

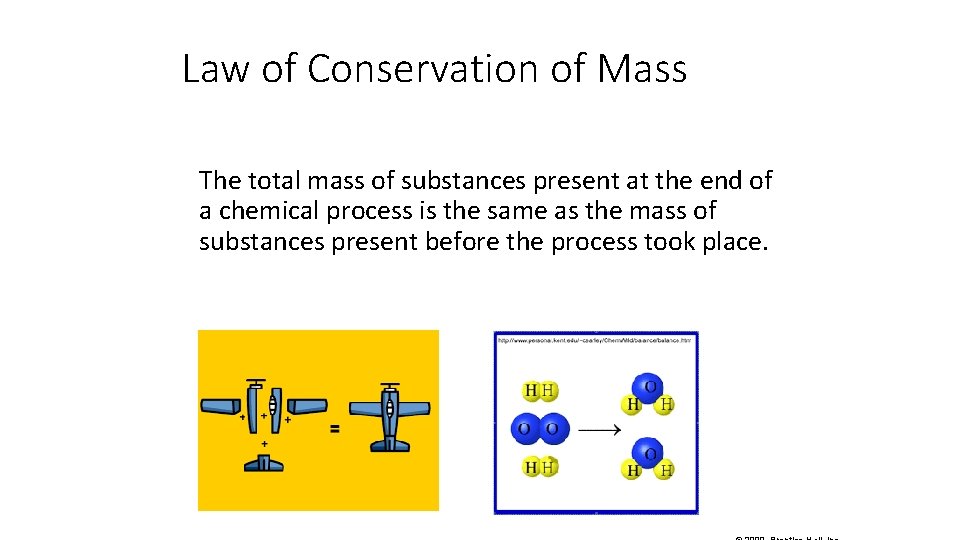
Law of Conservation of Mass The total mass of substances present at the end of a chemical process is the same as the mass of substances present before the process took place.

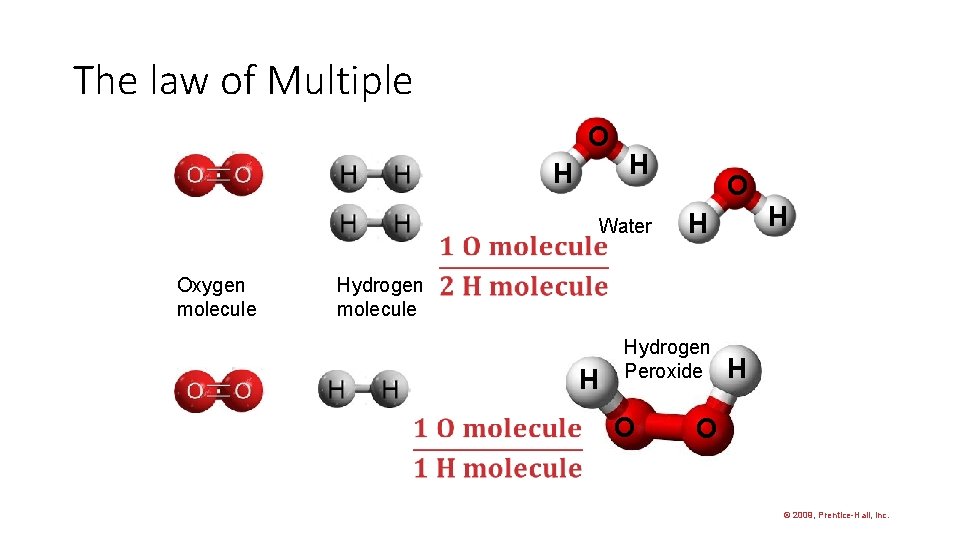
The law of Multiple O H Oxygen molecule H Water O H H Hydrogen molecule H Hydrogen Peroxide O H O © 2009, Prentice-Hall, Inc.

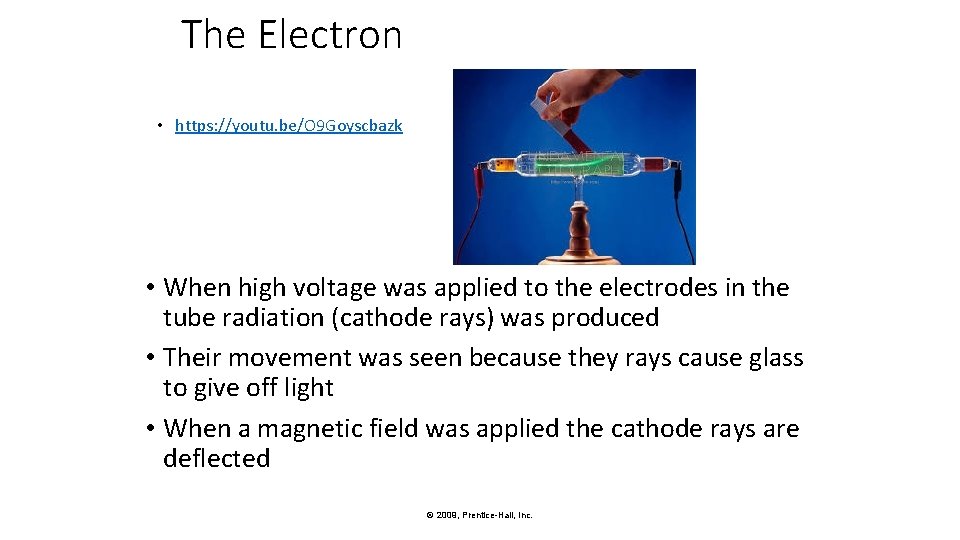
The Electron • https: //youtu. be/O 9 Goyscbazk • When high voltage was applied to the electrodes in the tube radiation (cathode rays) was produced • Their movement was seen because they rays cause glass to give off light • When a magnetic field was applied the cathode rays are deflected © 2009, Prentice-Hall, Inc.

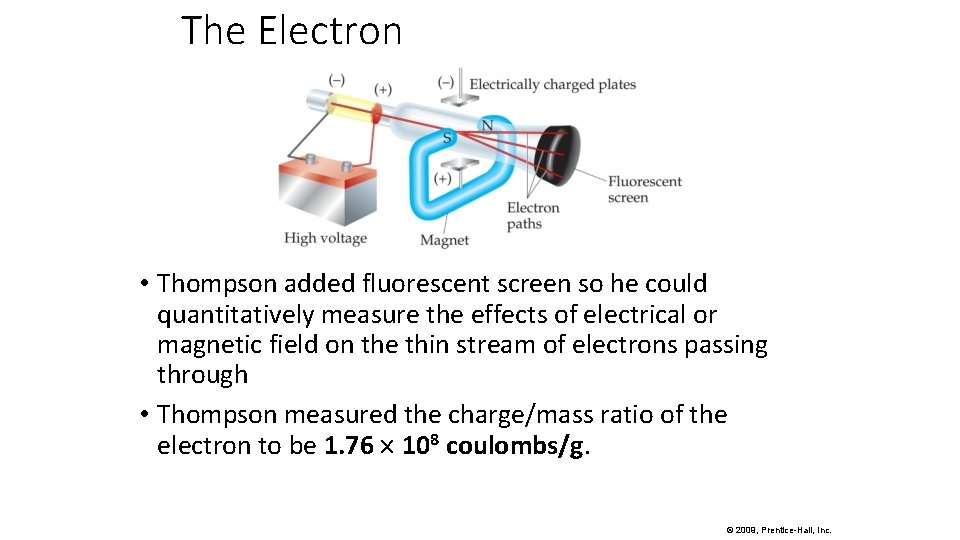
The Electron • Thompson added fluorescent screen so he could quantitatively measure the effects of electrical or magnetic field on the thin stream of electrons passing through • Thompson measured the charge/mass ratio of the electron to be 1. 76 108 coulombs/g. © 2009, Prentice-Hall, Inc.

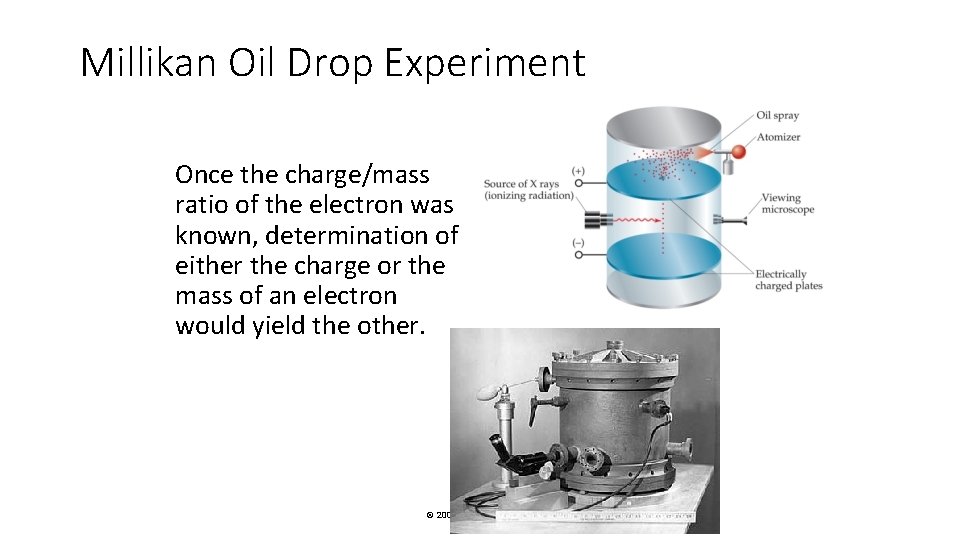
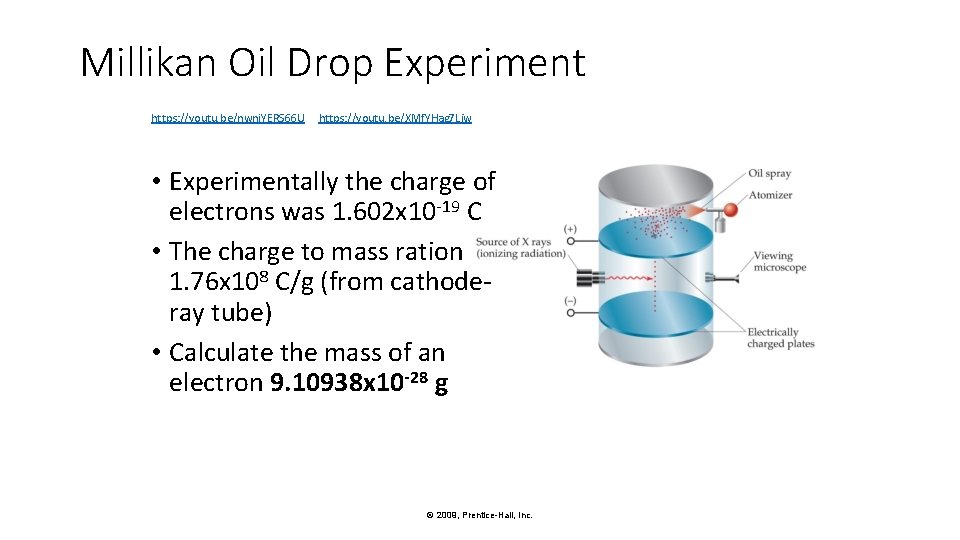
Millikan Oil Drop Experiment Once the charge/mass ratio of the electron was known, determination of either the charge or the mass of an electron would yield the other. © 2009, Prentice-Hall, Inc.

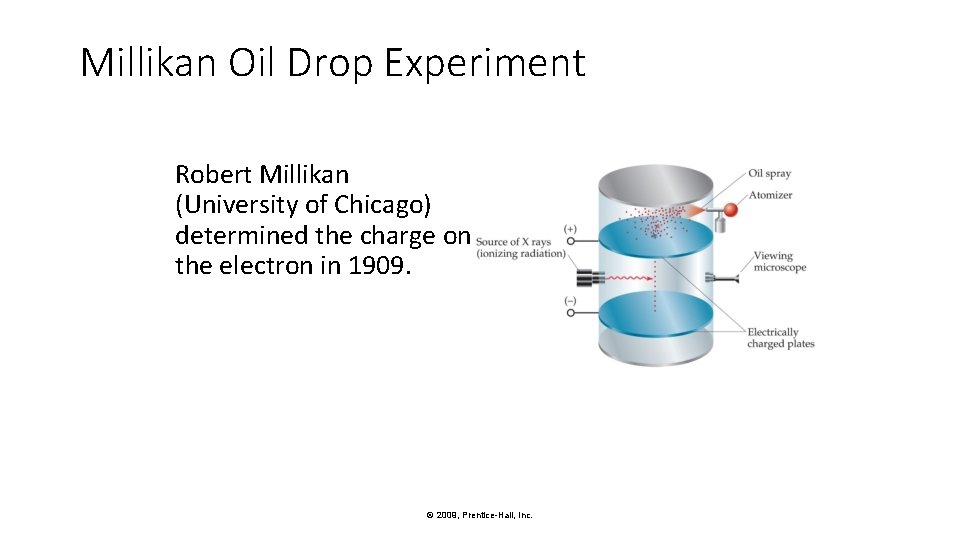
Millikan Oil Drop Experiment Robert Millikan (University of Chicago) determined the charge on the electron in 1909. © 2009, Prentice-Hall, Inc.

Millikan Oil Drop Experiment https: //youtu. be/nwnj. YERS 66 U https: //youtu. be/XMf. YHag 7 Liw • Experimentally the charge of electrons was 1. 602 x 10 -19 C • The charge to mass ration 1. 76 x 108 C/g (from cathoderay tube) • Calculate the mass of an electron 9. 10938 x 10 -28 g © 2009, Prentice-Hall, Inc.

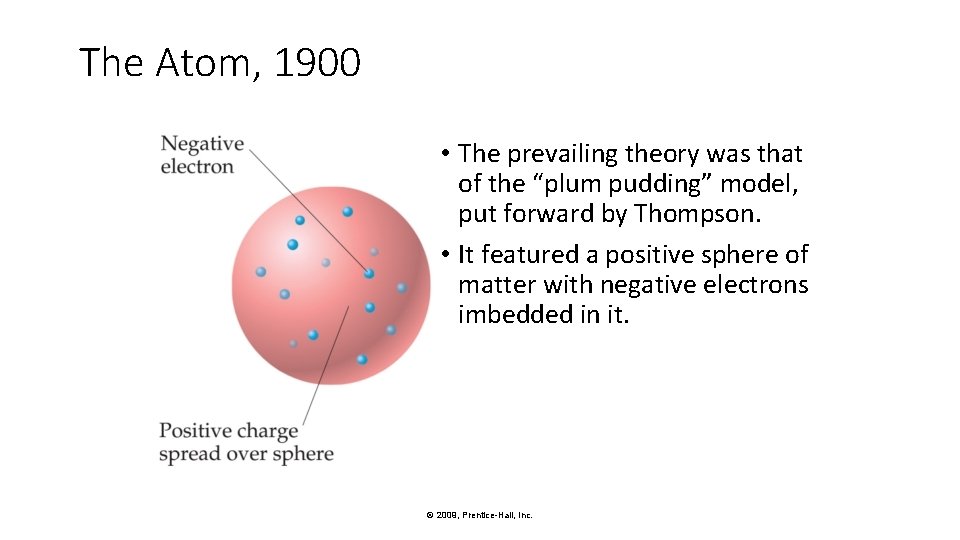
The Atom, 1900 • The prevailing theory was that of the “plum pudding” model, put forward by Thompson. • It featured a positive sphere of matter with negative electrons imbedded in it. © 2009, Prentice-Hall, Inc.

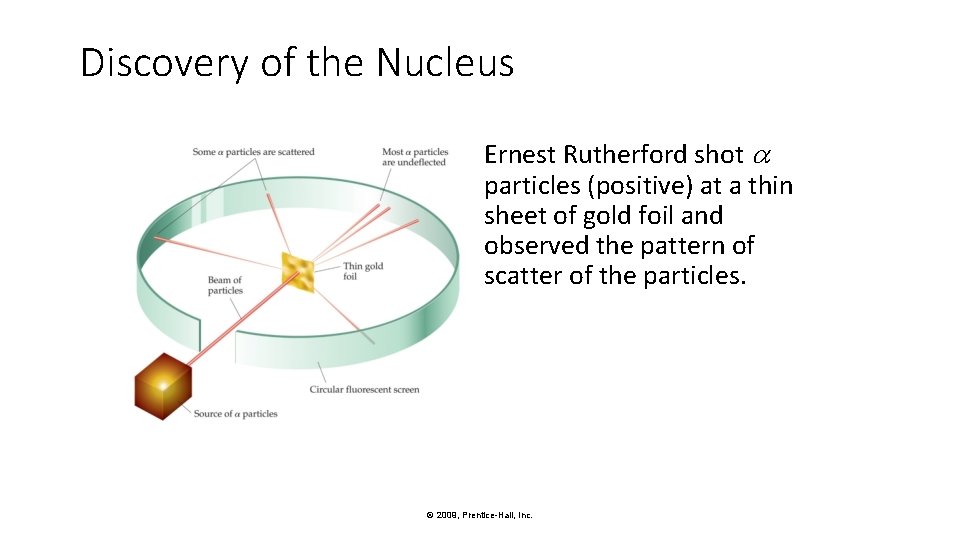
Discovery of the Nucleus Ernest Rutherford shot particles (positive) at a thin sheet of gold foil and observed the pattern of scatter of the particles. © 2009, Prentice-Hall, Inc.

Discovery of the Nucleus Why did the α particles behaved the way they did? © 2009, Prentice-Hall, Inc.

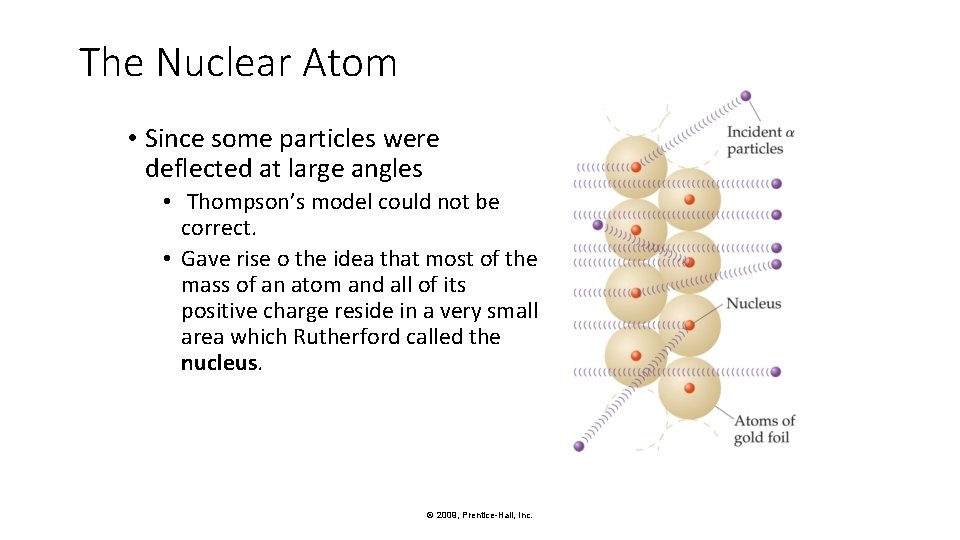
The Nuclear Atom • Since some particles were deflected at large angles • Thompson’s model could not be correct. • Gave rise o the idea that most of the mass of an atom and all of its positive charge reside in a very small area which Rutherford called the nucleus. © 2009, Prentice-Hall, Inc.

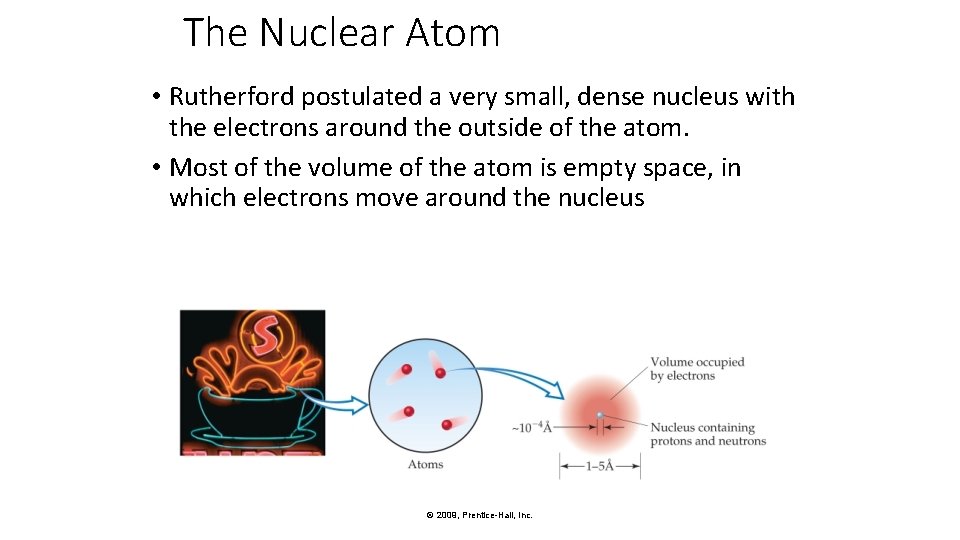
The Nuclear Atom • Rutherford postulated a very small, dense nucleus with the electrons around the outside of the atom. • Most of the volume of the atom is empty space, in which electrons move around the nucleus © 2009, Prentice-Hall, Inc.

Other Subatomic Particles • Protons were discovered by Rutherford in 1919. • Neutrons were discovered by James Chadwick in 1932. © 2009, Prentice-Hall, Inc.

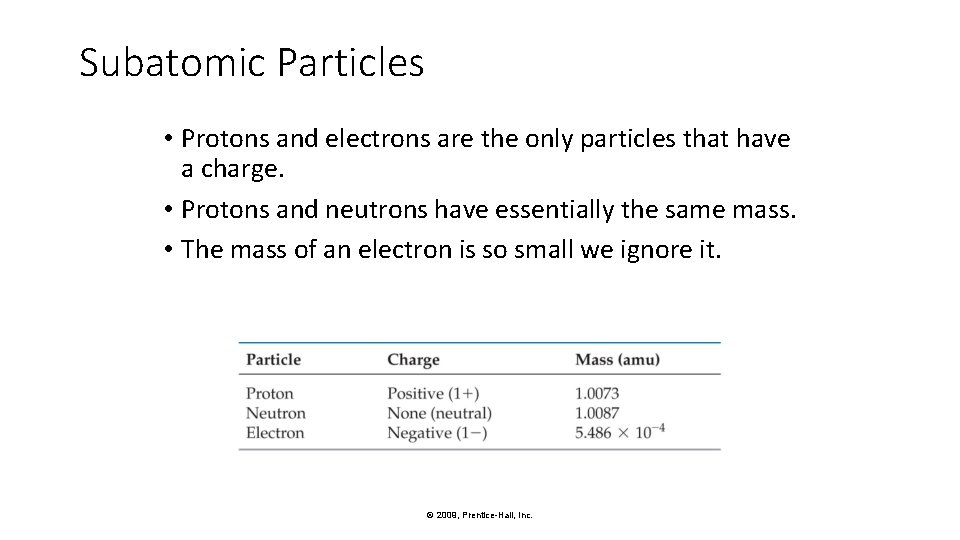
Subatomic Particles • Protons and electrons are the only particles that have a charge. • Protons and neutrons have essentially the same mass. • The mass of an electron is so small we ignore it. © 2009, Prentice-Hall, Inc.

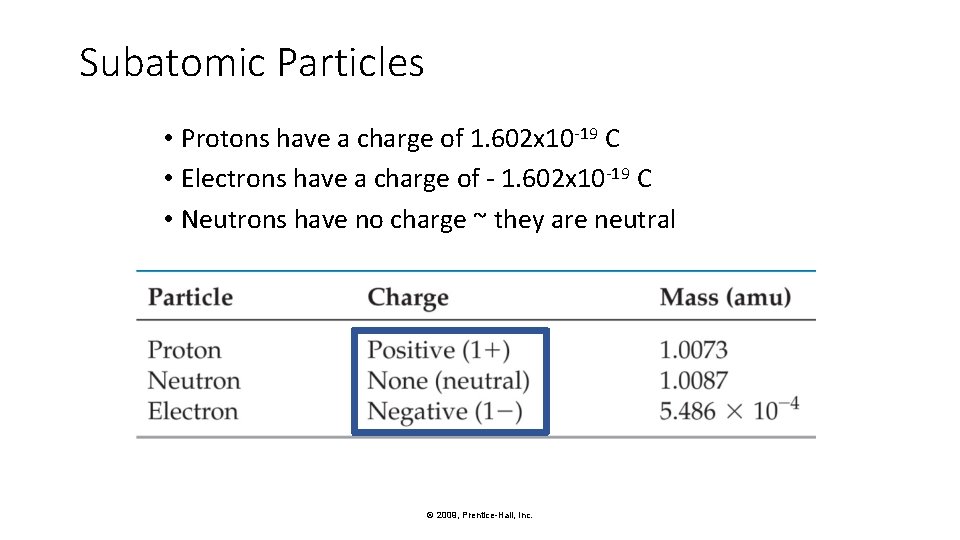
Subatomic Particles • Protons have a charge of 1. 602 x 10 -19 C • Electrons have a charge of - 1. 602 x 10 -19 C • Neutrons have no charge ~ they are neutral © 2009, Prentice-Hall, Inc.

Subatomic Particles • Protons and neutrons reside together in the nucleus • The vast majority of an atom’s volume is the space in which the electrons reside • The electrons are attracted to the protons in the nucleus by the electrostatic force between + and – charge © 2009, Prentice-Hall, Inc.

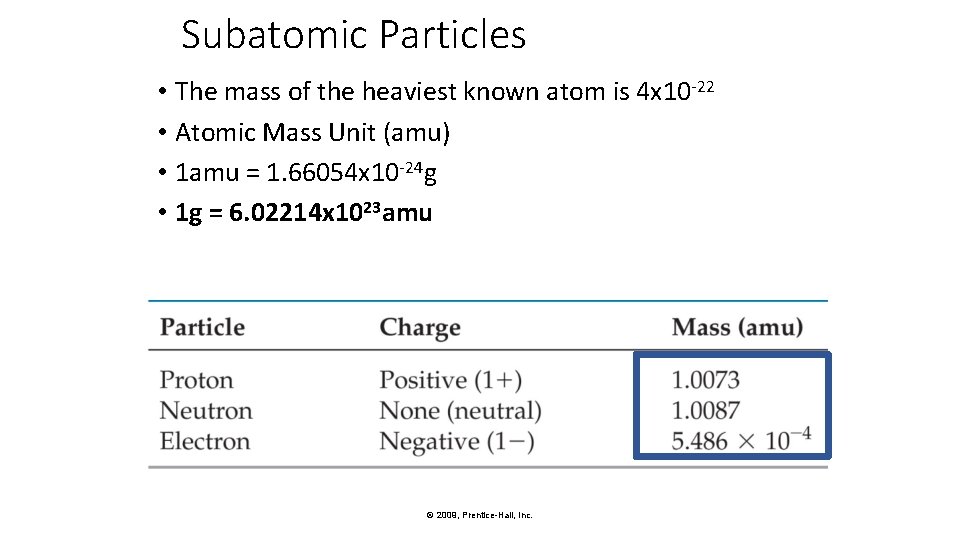
Subatomic Particles • The mass of the heaviest known atom is 4 x 10 -22 • Atomic Mass Unit (amu) • 1 amu = 1. 66054 x 10 -24 g • 1 g = 6. 02214 x 1023 amu © 2009, Prentice-Hall, Inc.

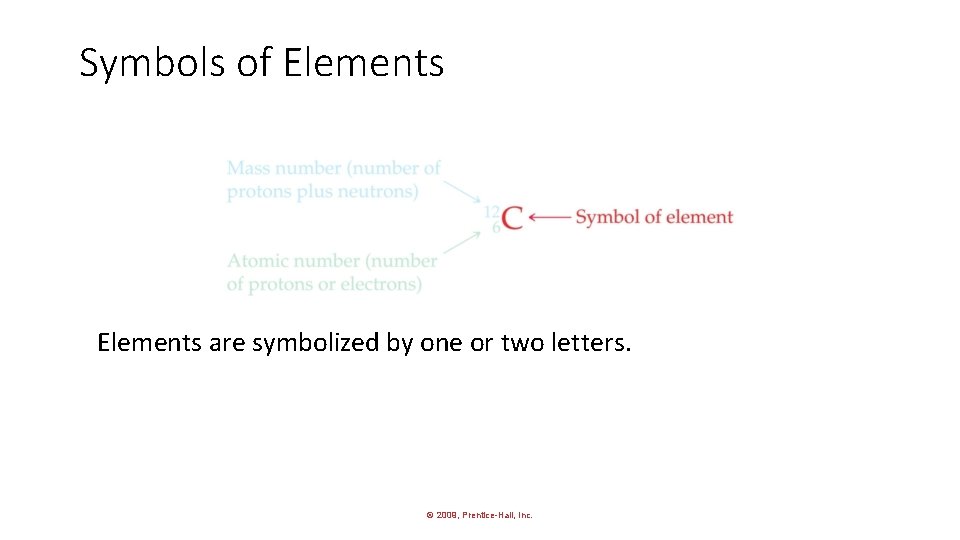
Symbols of Elements are symbolized by one or two letters. © 2009, Prentice-Hall, Inc.

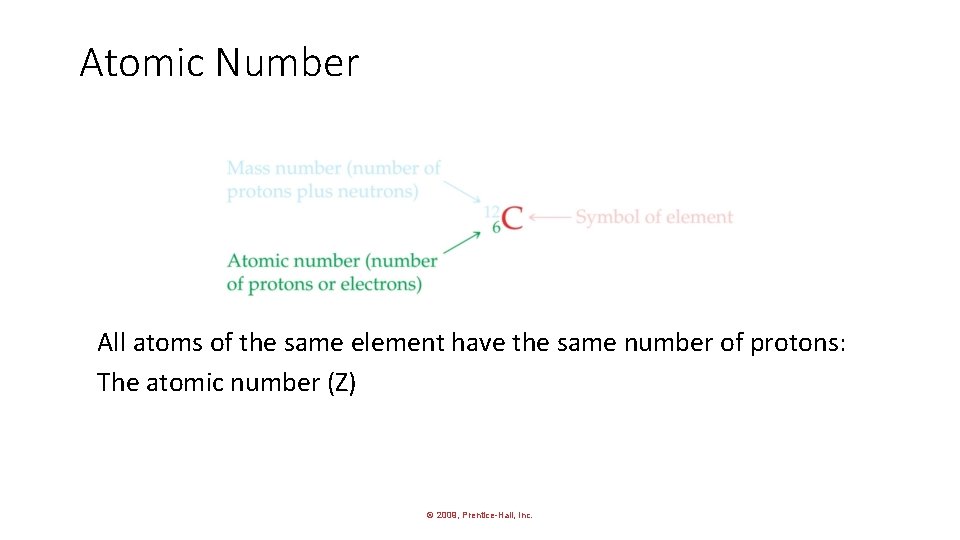
Atomic Number All atoms of the same element have the same number of protons: The atomic number (Z) © 2009, Prentice-Hall, Inc.

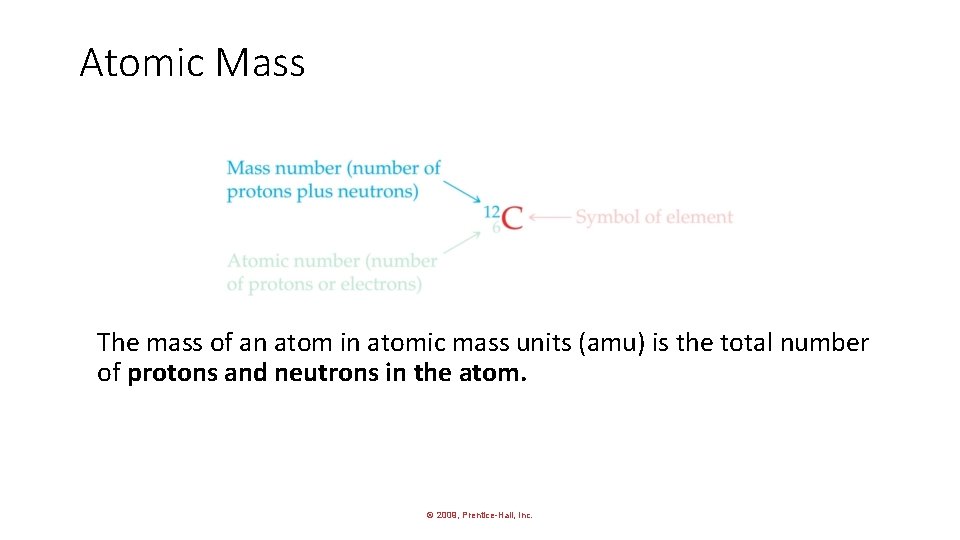
Atomic Mass The mass of an atom in atomic mass units (amu) is the total number of protons and neutrons in the atom. © 2009, Prentice-Hall, Inc.

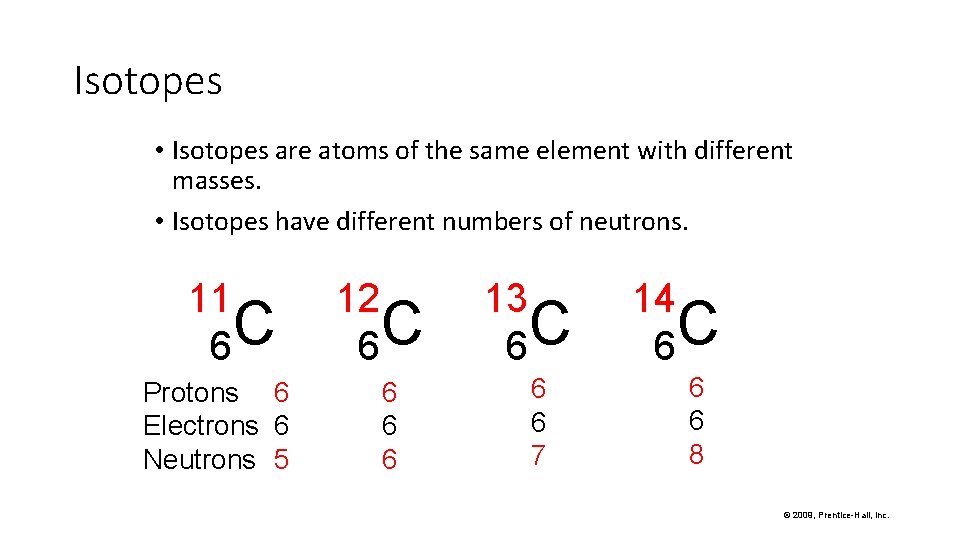
Isotopes • Isotopes are atoms of the same element with different masses. • Isotopes have different numbers of neutrons. 11 C 6 Protons 6 Electrons 6 Neutrons 5 12 C 6 6 13 C 6 6 6 7 14 C 6 6 6 8 © 2009, Prentice-Hall, Inc.

Isotopes 1. How many protons, neutrons, and electros are in a. 138 Ba atom b. an atom of phosphorous-31 a. 56 protons, 56 electrons, 82 neutrons (138 -56) b. 15 protons, 15 electrons, 16 neutrons (31 -15) © 2009, Prentice-Hall, Inc.

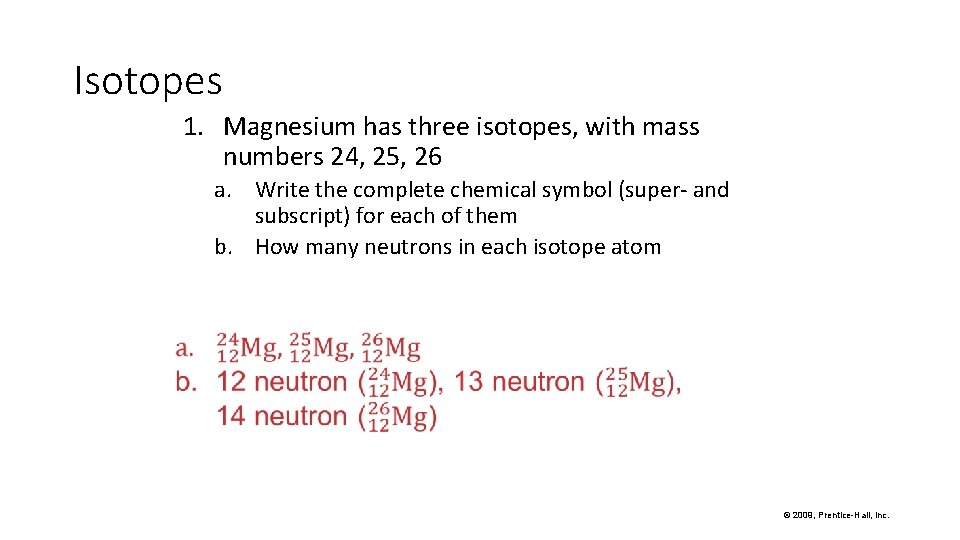
Isotopes 1. Magnesium has three isotopes, with mass numbers 24, 25, 26 a. Write the complete chemical symbol (super- and subscript) for each of them b. How many neutrons in each isotope atom © 2009, Prentice-Hall, Inc.