Chapter 3 Atomic Structure the Periodic Table The

Chapter 3 Atomic Structure & the Periodic Table The Development of Atomic Theory In 1803 John Dalton proposed the “Atomic Theory” 1. All matter is made up of atoms. 2. Atoms of a particular element are similar to one another. Atoms of different elements have different properties. 3. Atoms combine in simple whole-number ratios to form compounds. 4. Chemical change involves joining, separating, or rearranging atoms. Atoms are never created or destroyed in a chemical reaction.
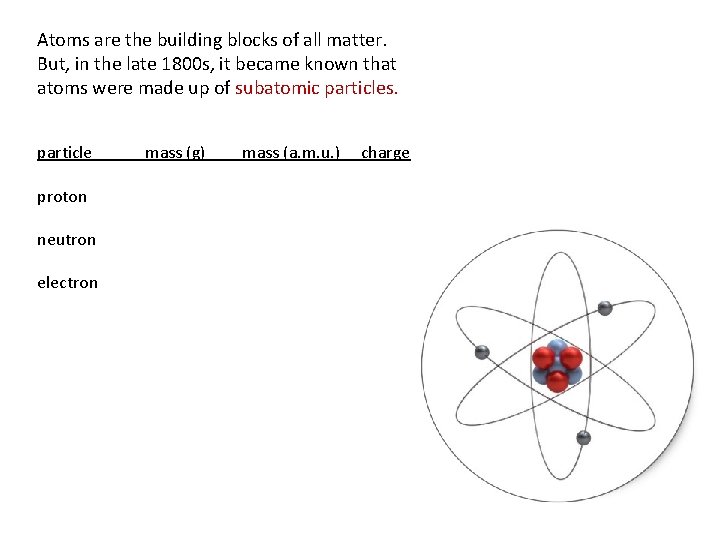
Atoms are the building blocks of all matter. But, in the late 1800 s, it became known that atoms were made up of subatomic particles. particle proton neutron electron mass (g) mass (a. m. u. ) charge
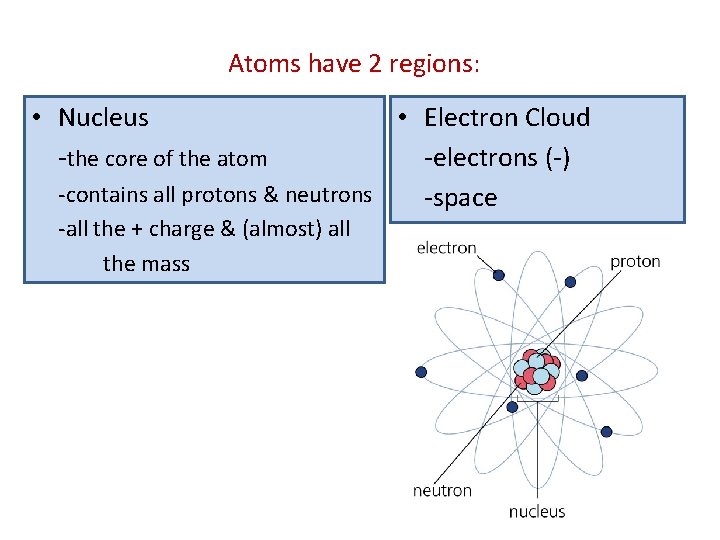
Atoms have 2 regions: • Nucleus -the core of the atom • Electron Cloud -electrons (-) -contains all protons & neutrons -space -all the + charge & (almost) all the mass
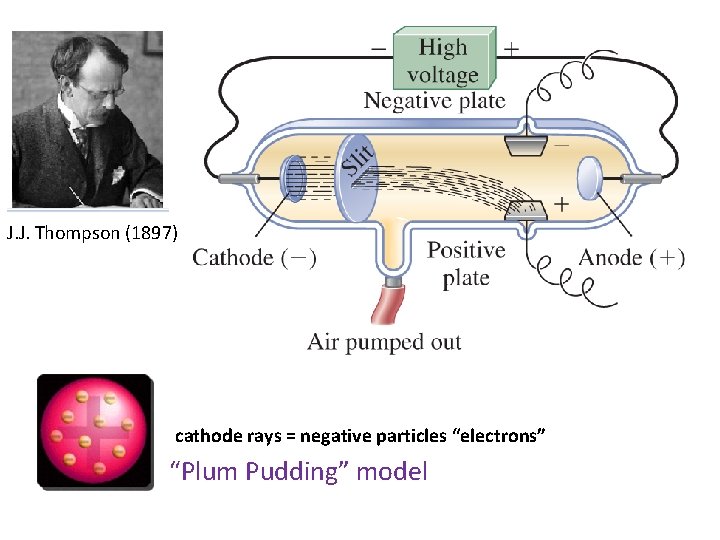
J. J. Thompson (1897) cathode rays = negative particles “electrons” “Plum Pudding” model
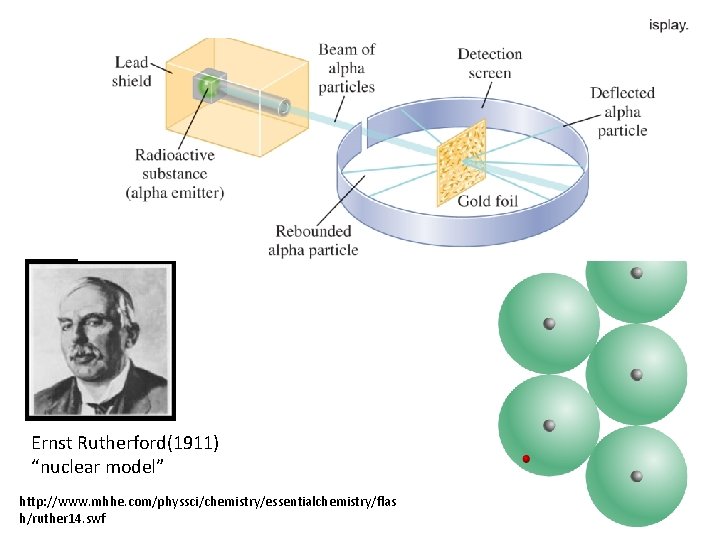
Ernst Rutherford(1911) “nuclear model” http: //www. mhhe. com/physsci/chemistry/essentialchemistry/flas h/ruther 14. swf
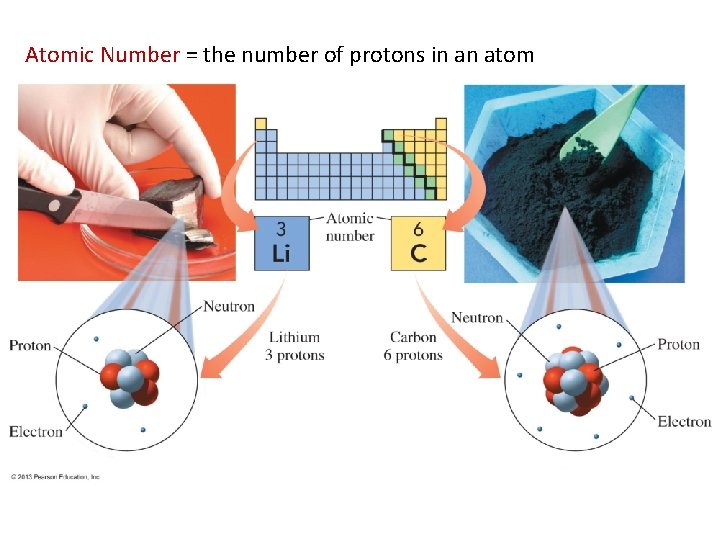
Atomic Number = the number of protons in an atom
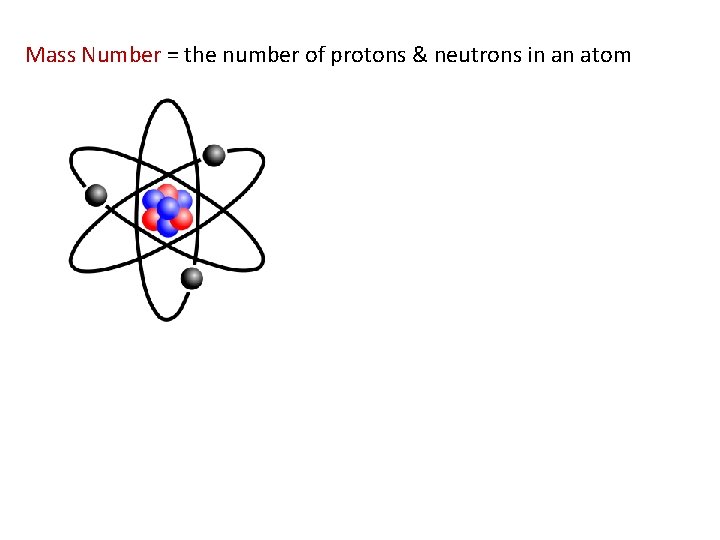
Mass Number = the number of protons & neutrons in an atom

Isotopic Notation – shows the symbol, Mass Number & Atomic Number of a particular Isotope of an Element Isotopes = Atoms of the same element that have different numbers of neutrons and therefore, different Mass Numbers
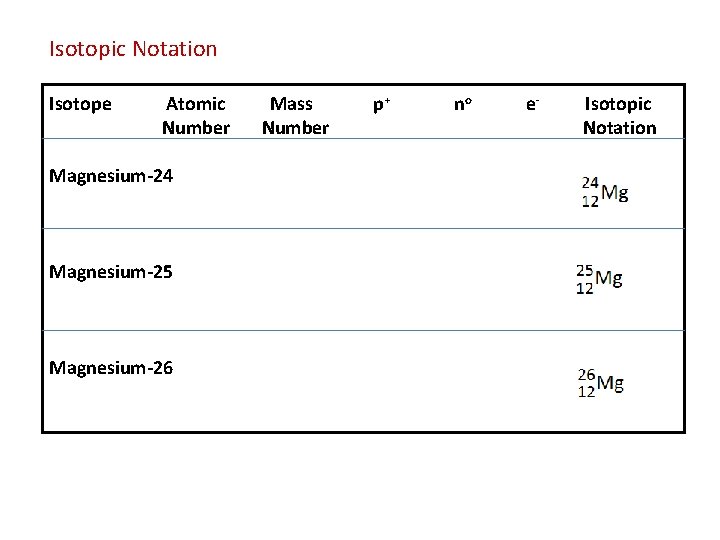
Isotopic Notation Isotope Atomic Number Magnesium-24 Magnesium-25 Magnesium-26 Mass Number p+ no e- Isotopic Notation
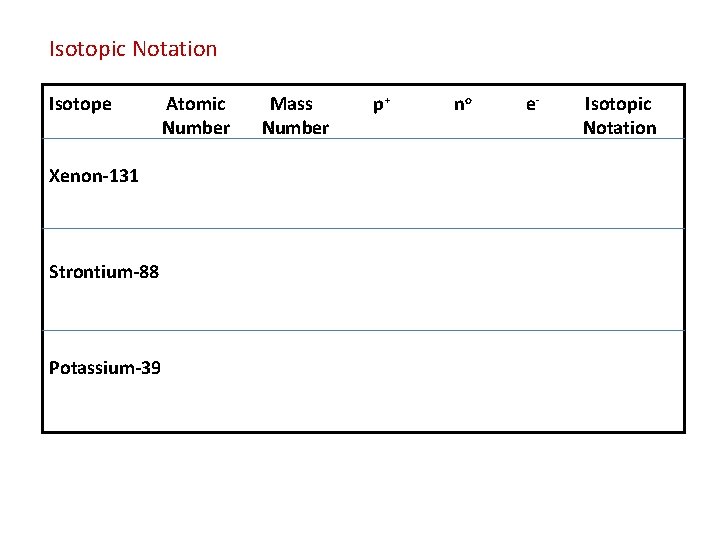
Isotopic Notation Isotope Xenon-131 Strontium-88 Potassium-39 Atomic Number Mass Number p+ no e- Isotopic Notation
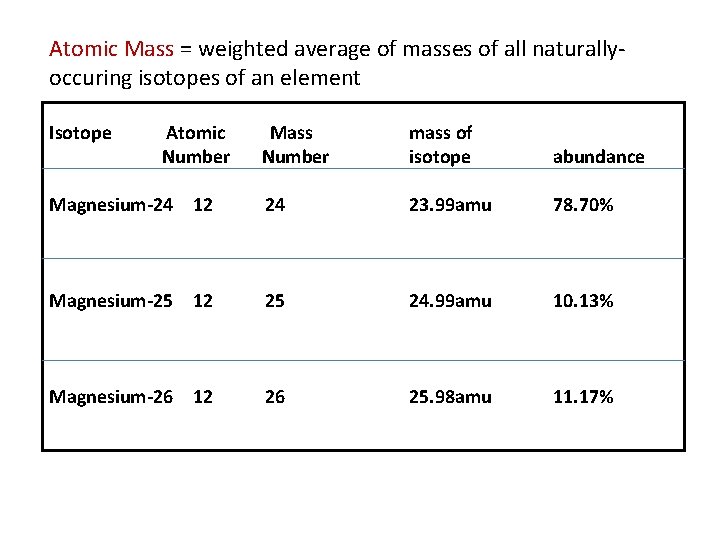
Atomic Mass = weighted average of masses of all naturallyoccuring isotopes of an element Isotope Atomic Number Mass Number mass of isotope abundance Magnesium-24 12 24 23. 99 amu 78. 70% Magnesium-25 12 25 24. 99 amu 10. 13% Magnesium-26 12 26 25. 98 amu 11. 17%

So, we now have 3 numbers related to the mass of an atom: Mass Number a counted number (p + n) Isotopic Mass a precise measure of the mass of an isotope Atomic Mass the weighted average of the masses of all naturally-occuring isotopes of an element Example: There are 2 naturally-occuring isotopes of copper. Calculate the Atomic Mass of copper, given the following: isotope abundance Cu-63 69. 09% Cu-65 30. 91% mass 62. 928 amu 64. 9278 amu
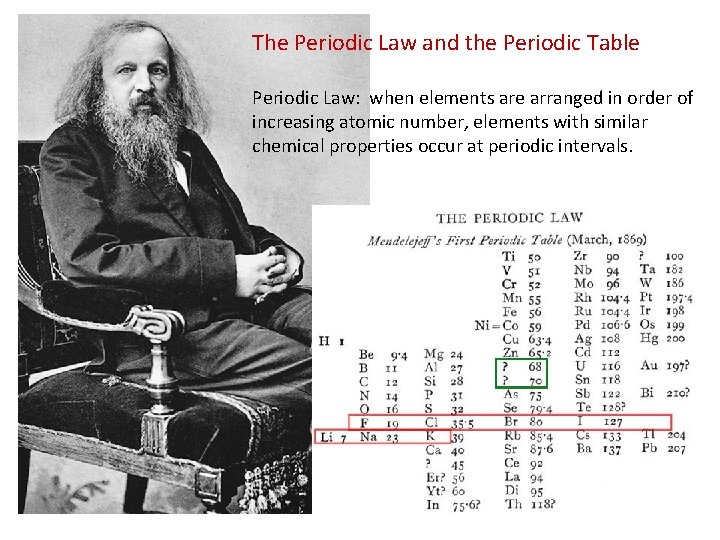
The Periodic Law and the Periodic Table Periodic Law: when elements are arranged in order of increasing atomic number, elements with similar chemical properties occur at periodic intervals.
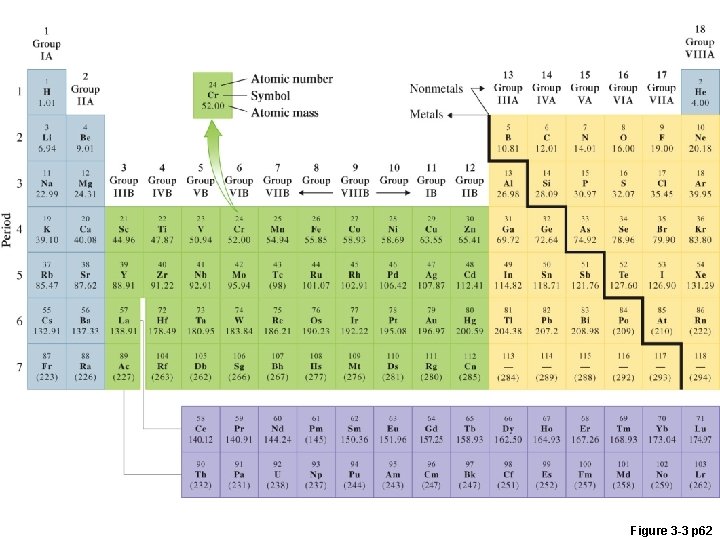
Figure 3 -3 p 62

Characteristics of: Metal Silver (Ag) Metalloid Antimony (Sb) Nonmetal Sulfur (S)
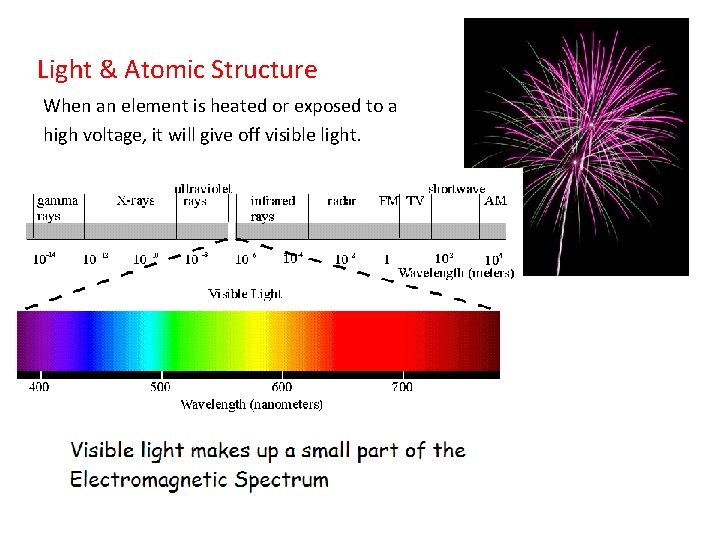
Light & Atomic Structure When an element is heated or exposed to a high voltage, it will give off visible light.

Emission Spectra “The energy of the electron is quantized” Neils Bohr
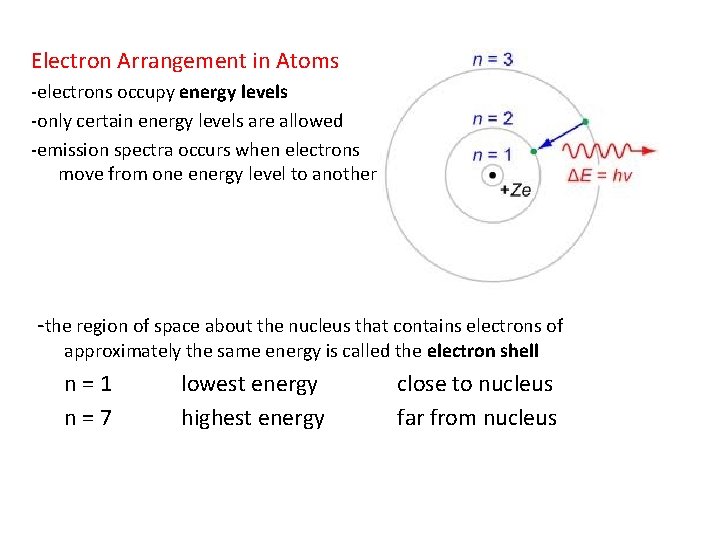
Electron Arrangement in Atoms -electrons occupy energy levels -only certain energy levels are allowed -emission spectra occurs when electrons move from one energy level to another -the region of space about the nucleus that contains electrons of approximately the same energy is called the electron shell n=1 n=7 lowest energy highest energy close to nucleus far from nucleus

Electron Sublevels & Orbitals -each electron shell has one or more sublevels (subshells) s, p, d, f -electrons in each sublevel occupy a 3 -dimensional space called an Orbital -Orbitals have unique 3 -dimensional shapes -each orbital can hold 2 electrons http: //dwb 4. unl. edu/chem. Anime/BOHRQD. html
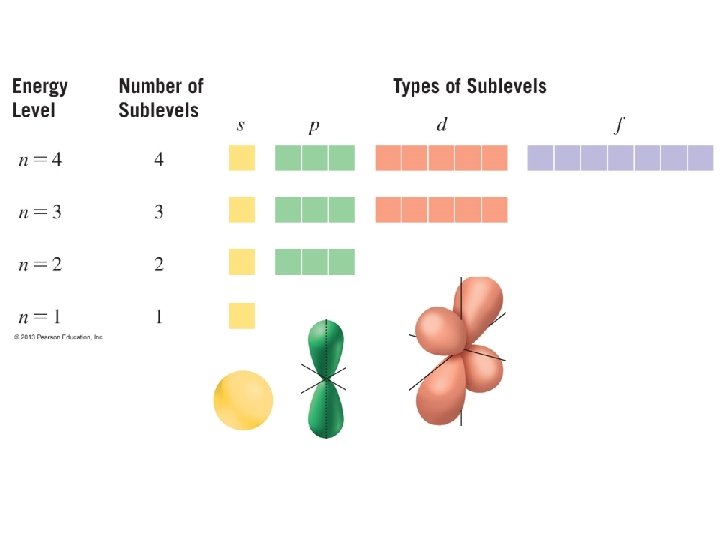

Each subshell contains orbitals of a particular shape. Orbitals of higher energy levels are larger and contain electrons with higher energy. Orbital shape represents a probability density for the location of electrons p orbital d orbital
With so many shells, subshells & orbitals available, how does an electron know where to go?
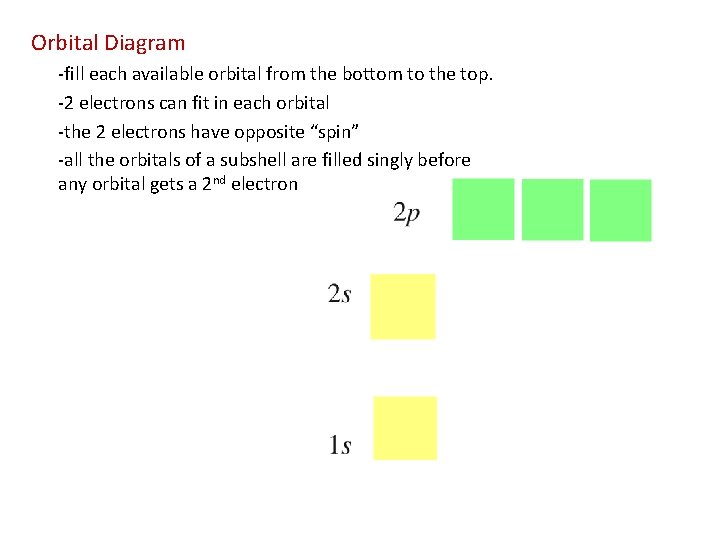
Orbital Diagram -fill each available orbital from the bottom to the top. -2 electrons can fit in each orbital -the 2 electrons have opposite “spin” -all the orbitals of a subshell are filled singly before any orbital gets a 2 nd electron

Electron configuration 3 Li 5 B
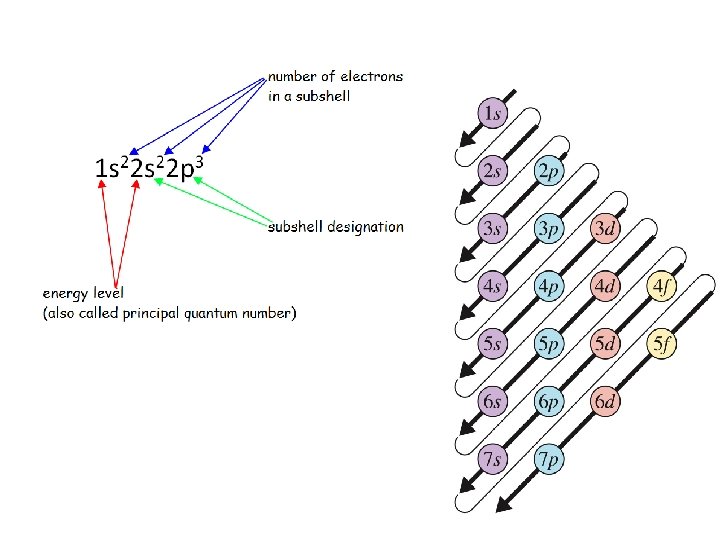
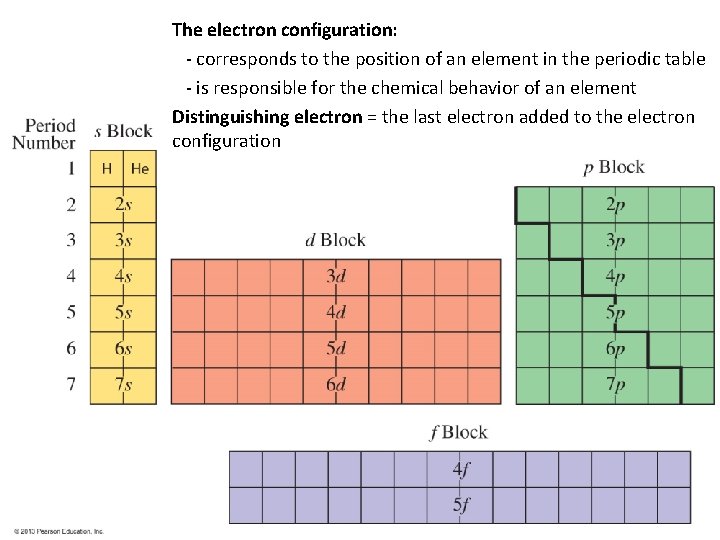
The electron configuration: - corresponds to the position of an element in the periodic table - is responsible for the chemical behavior of an element Distinguishing electron = the last electron added to the electron configuration
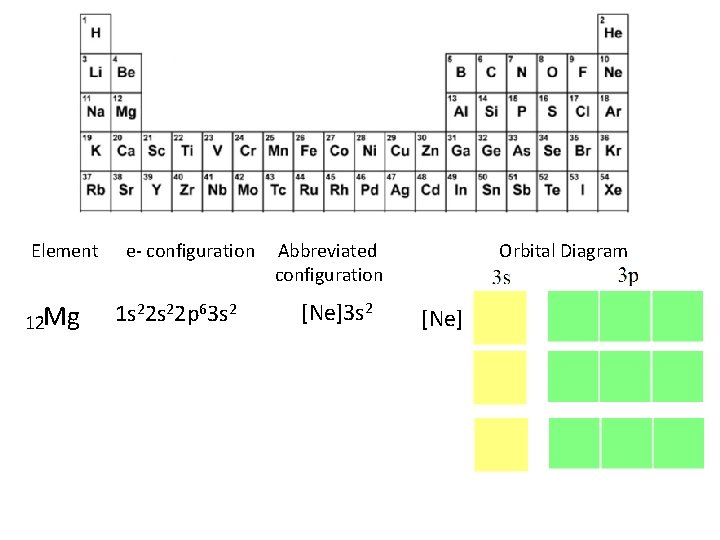
Element 12 Mg e- configuration 1 s 22 p 63 s 2 Abbreviated configuration [Ne]3 s 2 Orbital Diagram [Ne]
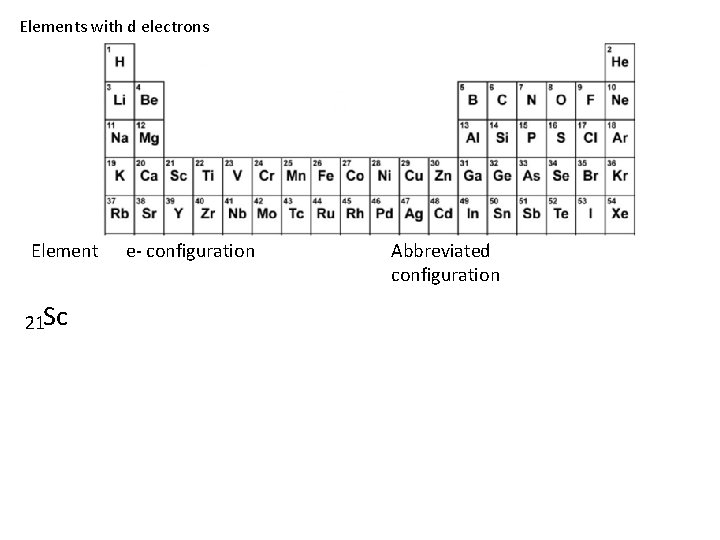
Elements with d electrons Element 21 Sc e- configuration Abbreviated configuration
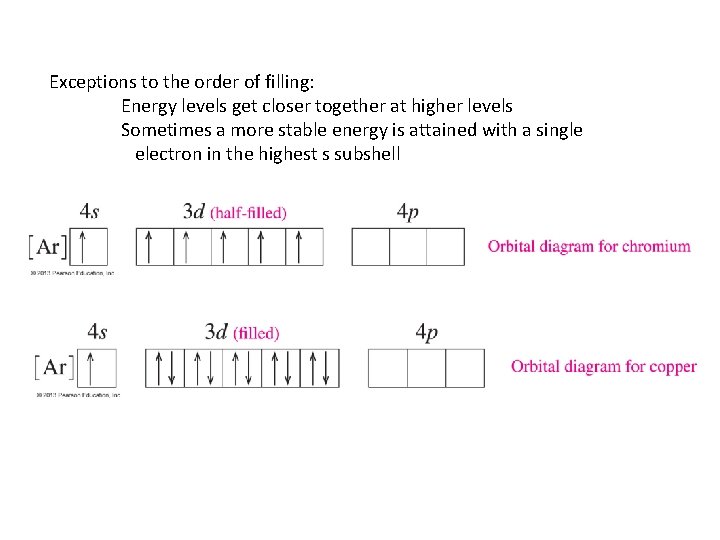
Exceptions to the order of filling: Energy levels get closer together at higher levels Sometimes a more stable energy is attained with a single electron in the highest s subshell
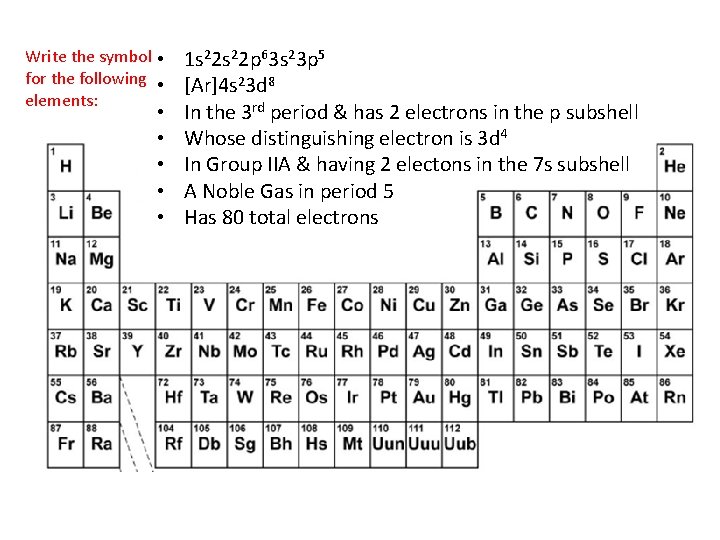
Write the symbol for the following elements: • • 1 s 22 p 63 s 23 p 5 [Ar]4 s 23 d 8 In the 3 rd period & has 2 electrons in the p subshell Whose distinguishing electron is 3 d 4 In Group IIA & having 2 electons in the 7 s subshell A Noble Gas in period 5 Has 80 total electrons
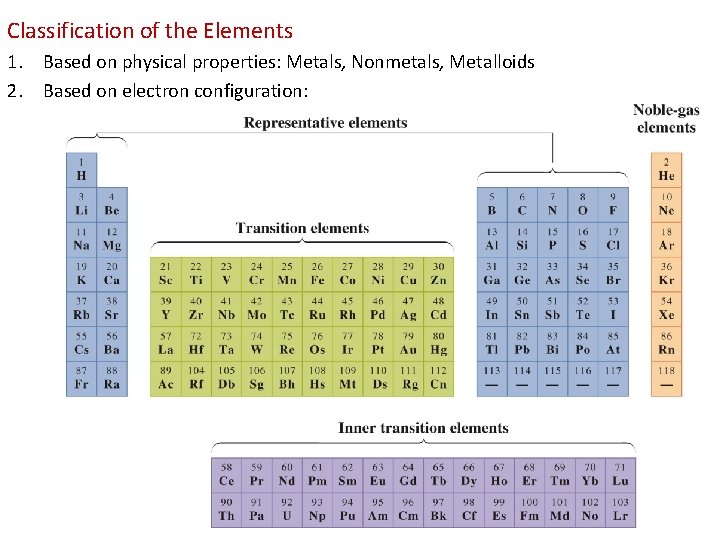
Classification of the Elements 1. Based on physical properties: Metals, Nonmetals, Metalloids 2. Based on electron configuration:
- Slides: 31