Chapter 29 Nuclear Physics Properties of Nuclei All
















- Slides: 39

Chapter 29 Nuclear Physics

Properties of Nuclei • All nuclei are composed of protons and neutrons (exception: ordinary hydrogen) • The atomic number, Z, equals the number of protons in the nucleus • The neutron number, N, is the number of neutrons in the nucleus • The mass number (not the same as the mass), A, is the number of nucleons in the nucleus: A = Z + N • Nucleon is a generic term used to refer to either a proton or a neutron

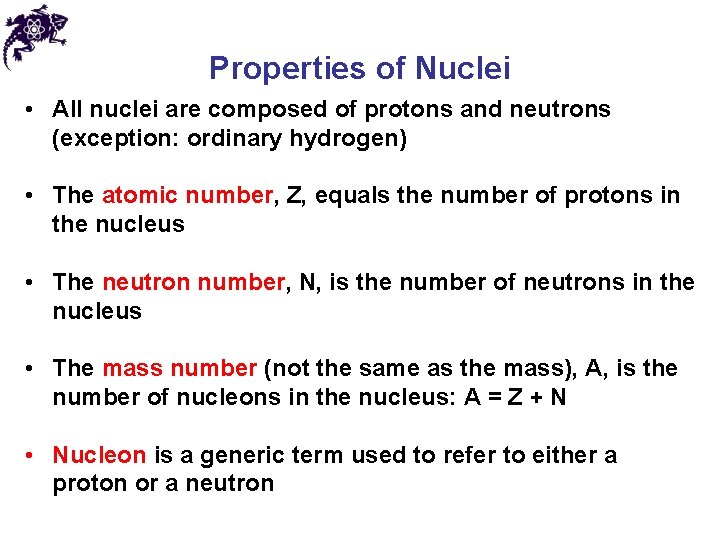
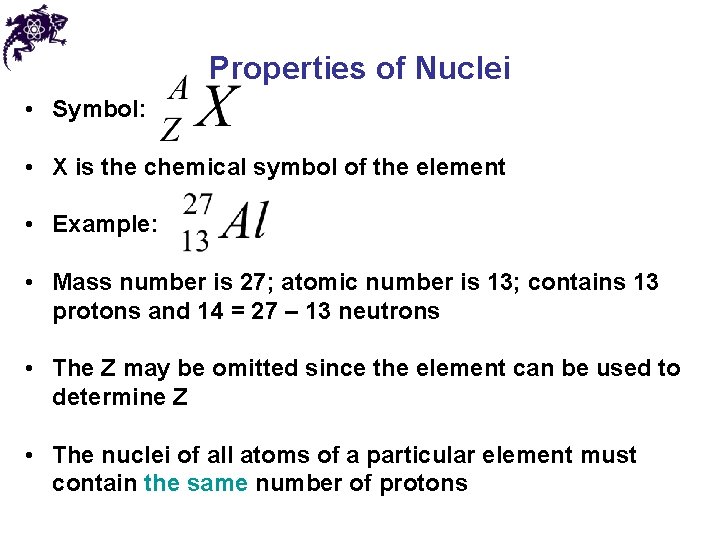
Properties of Nuclei • Symbol: • X is the chemical symbol of the element • Example: • Mass number is 27; atomic number is 13; contains 13 protons and 14 = 27 – 13 neutrons • The Z may be omitted since the element can be used to determine Z • The nuclei of all atoms of a particular element must contain the same number of protons

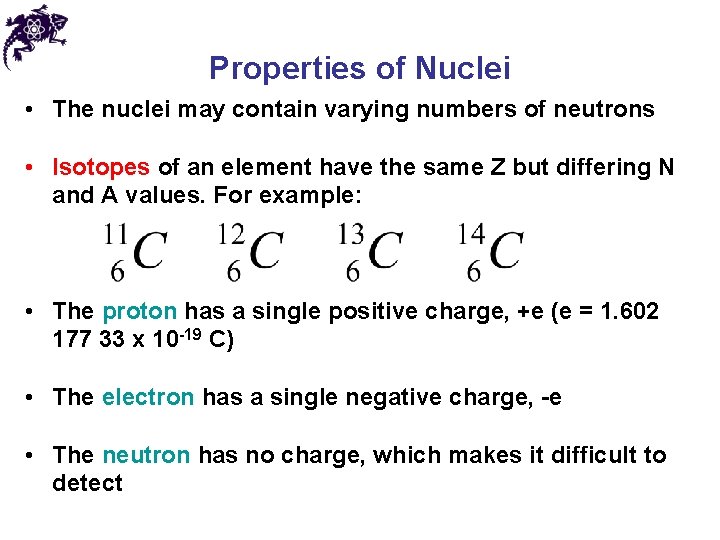
Properties of Nuclei • The nuclei may contain varying numbers of neutrons • Isotopes of an element have the same Z but differing N and A values. For example: • The proton has a single positive charge, +e (e = 1. 602 177 33 x 10 -19 C) • The electron has a single negative charge, -e • The neutron has no charge, which makes it difficult to detect

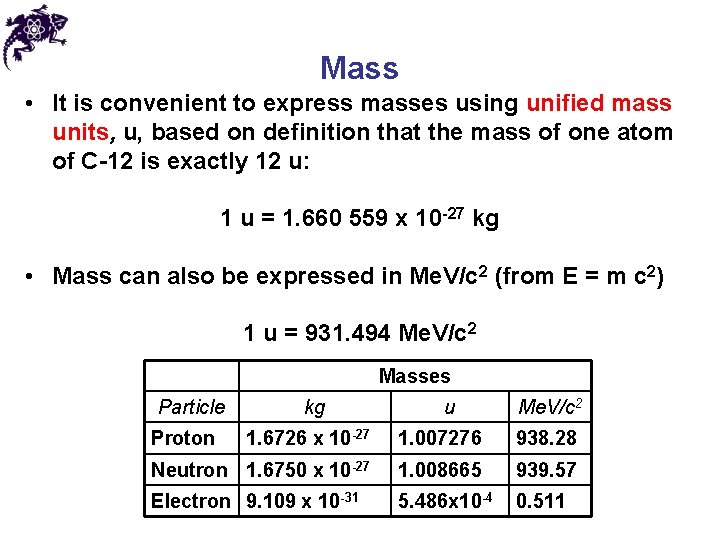
Mass • It is convenient to express masses using unified mass units, u, based on definition that the mass of one atom of C-12 is exactly 12 u: 1 u = 1. 660 559 x 10 -27 kg • Mass can also be expressed in Me. V/c 2 (from E = m c 2) 1 u = 931. 494 Me. V/c 2 Masses Particle Proton kg u Me. V/c 2 1. 6726 x 10 -27 1. 007276 938. 28 Neutron 1. 6750 x 10 -27 1. 008665 939. 57 Electron 9. 109 x 10 -31 5. 486 x 10 -4 0. 511

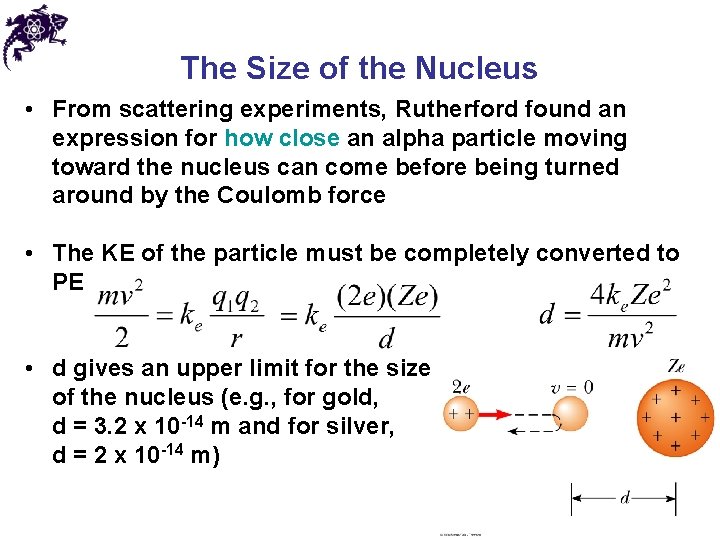
The Size of the Nucleus • From scattering experiments, Rutherford found an expression for how close an alpha particle moving toward the nucleus can come before being turned around by the Coulomb force • The KE of the particle must be completely converted to PE • d gives an upper limit for the size of the nucleus (e. g. , for gold, d = 3. 2 x 10 -14 m and for silver, d = 2 x 10 -14 m)

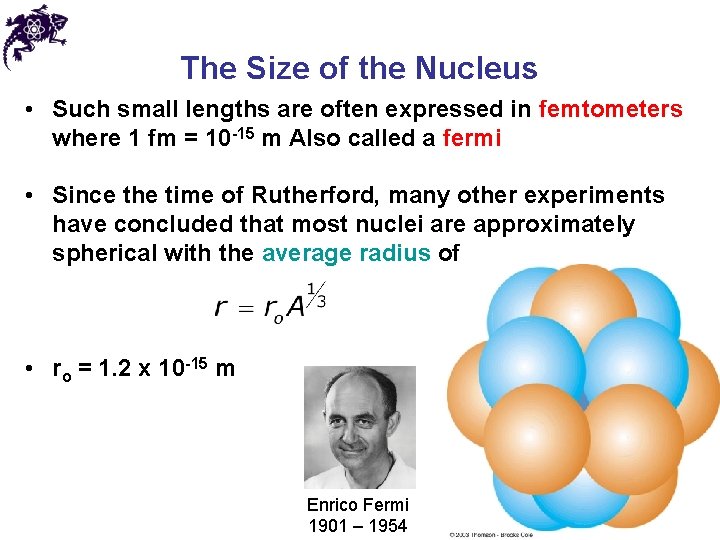
The Size of the Nucleus • Such small lengths are often expressed in femtometers where 1 fm = 10 -15 m Also called a fermi • Since the time of Rutherford, many other experiments have concluded that most nuclei are approximately spherical with the average radius of • ro = 1. 2 x 10 -15 m Enrico Fermi 1901 – 1954

Density of Nuclei • The volume of the nucleus (assumed to be spherical) is directly proportional to the total number of nucleons • This suggests that all nuclei have nearly the same density • Nucleons combine to form a nucleus as though they were tightly packed spheres

Nuclear Stability • There are very large repulsive electrostatic forces between protons • These forces should cause the nucleus to fly apart • The nuclei are stable because of the presence of another, short-range force, called the nuclear force • This is an attractive force that acts between all nuclear particles • The nuclear attractive force is stronger than the Coulomb repulsive force at the short ranges within the nucleus

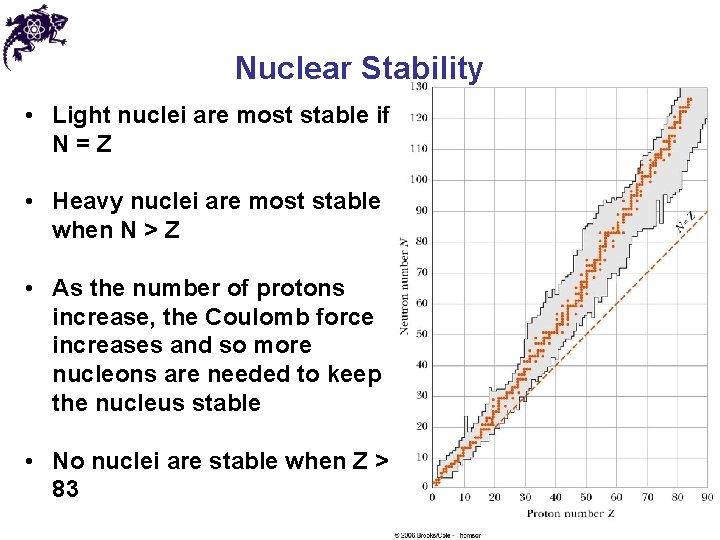
Nuclear Stability • Light nuclei are most stable if N=Z • Heavy nuclei are most stable when N > Z • As the number of protons increase, the Coulomb force increases and so more nucleons are needed to keep the nucleus stable • No nuclei are stable when Z > 83

Chapter 29 Problem 7 (a) Find the speed an alpha particle requires to come within 3. 2 × 10− 14 m of a gold nucleus. (b) Find the energy of the alpha particle in Me. V.

Binding Energy • The total energy of the bound system (the nucleus) is less than the combined energy of the separated nucleons • This difference in energy is called the binding energy of the nucleus • It can be thought of as the amount of energy you need to add to the nucleus to break it apart into separated protons and neutrons

Binding Energy

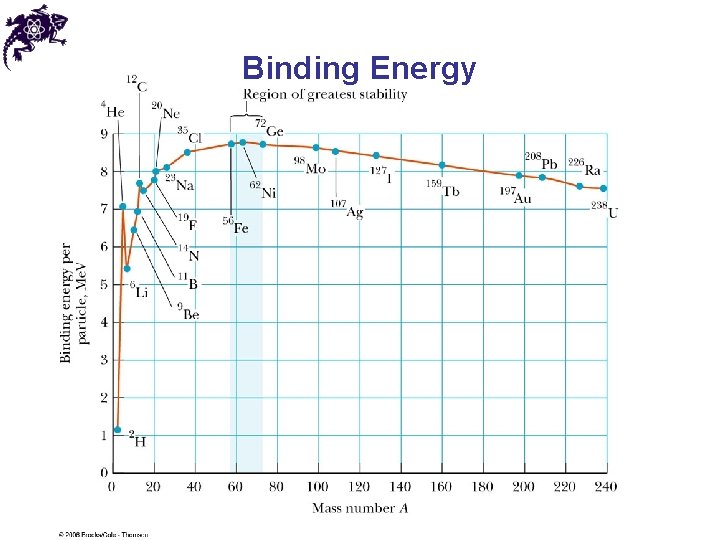
Binding Energy • Except for light nuclei, the binding energy is about 8 Me. V per nucleon • The curve peaks in the vicinity of A = 60 • Nuclei with mass numbers greater than or less than 60 are not as strongly bound as those near the middle of the periodic table • The curve is slowly varying at A > 40, suggesting that the nuclear force saturates • A particular nucleon can interact with only a limited number of other nucleons

Radioactivity • Radioactivity is the spontaneous emission of radiation • Radioactivity is the result of the decay (disintegration) of unstable nuclei • Three types of radiation can be emitted • 1) Alpha particles: 4 He nuclei) • 2) Beta particles: either electrons or positrons (a positron is the antiparticle of the electron, similar to the electron except its charge is +e) • 3) Gamma rays: high energy photons

Distinguishing Types of Radiation • A radioactive beam is directed into a region with a magnetic field • The gamma particles carry no charge and they are not deflected • The alpha particles are deflected upward • The beta particles (electrons) are deflected downward, whereas the positrons would be deflected upward

Penetrating Ability of Particles • Alpha particles: barely penetrate a piece of paper • Beta particles: can penetrate a few mm of aluminum • Gamma rays: can penetrate several cm of lead

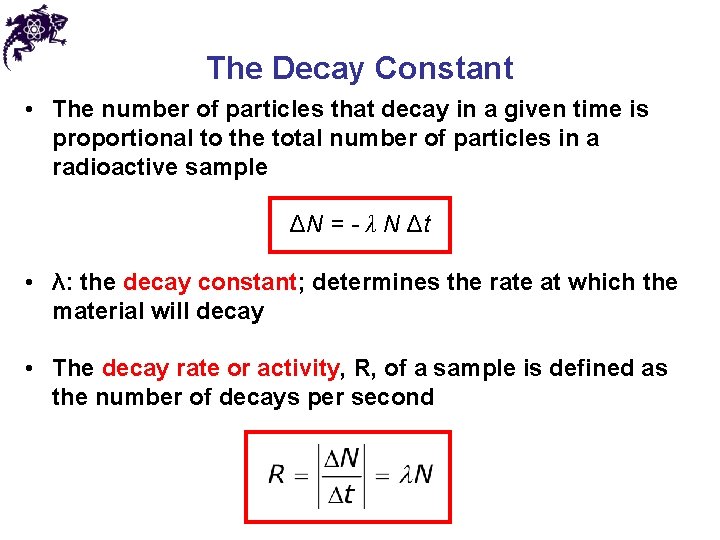
The Decay Constant • The number of particles that decay in a given time is proportional to the total number of particles in a radioactive sample ΔN = - λ N Δt • λ: the decay constant; determines the rate at which the material will decay • The decay rate or activity, R, of a sample is defined as the number of decays per second

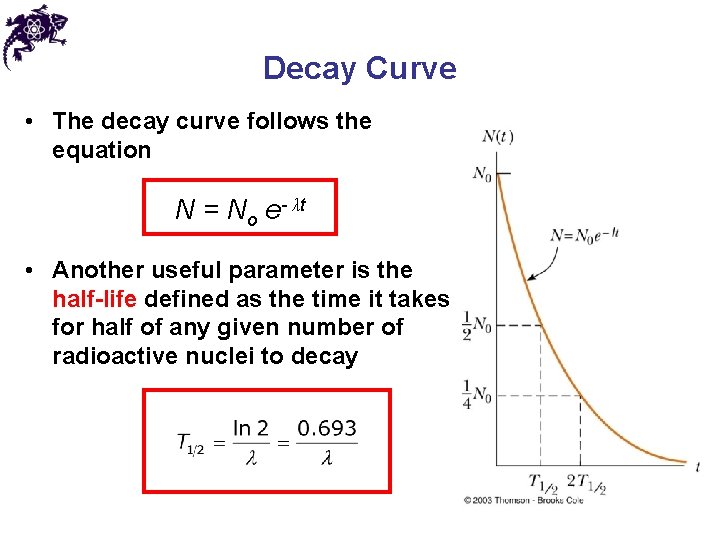
Decay Curve • The decay curve follows the equation N = No e- λt • Another useful parameter is the half-life defined as the time it takes for half of any given number of radioactive nuclei to decay

Decay Curve

Units • The unit of activity, R, is the Curie, Ci: 1 Ci = 3. 7 x 1010 decays/second • The most commonly used units of activity are the m. Ci and the µCi • The SI unit of activity is the Becquerel, Bq: 1 Bq = 1 decay/second (therefore, 1 Ci = 3. 7 x 1010 Bq) Maria Skłodowska-Curie 1867 – 1934 Antoine Henri Becquerel 1852 – 1908

Chapter 29 Problem 24 A building has become accidentally contaminated with radioactivity. The longest-lived material in the building is strontium-90. (The atomic mass of is 89. 907 7. ) If the building initially contained 5. 0 kg of this substance, and the safe level is less than 10. 0 counts/min, how long will the building be unsafe?

Decay – General Rules • When one element changes into another element, the process is called spontaneous decay or transmutation • The sum of the mass numbers, A, must be the same on both sides of the equation • The sum of the atomic numbers, Z, must be the same on both sides of the equation • Conservation of mass-energy and conservation of momentum must hold

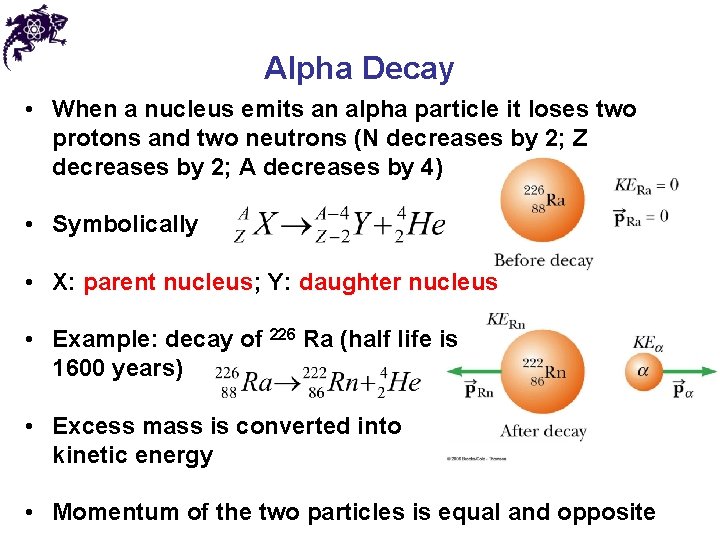
Alpha Decay • When a nucleus emits an alpha particle it loses two protons and two neutrons (N decreases by 2; Z decreases by 2; A decreases by 4) • Symbolically • X: parent nucleus; Y: daughter nucleus • Example: decay of 226 Ra (half life is 1600 years) • Excess mass is converted into kinetic energy • Momentum of the two particles is equal and opposite

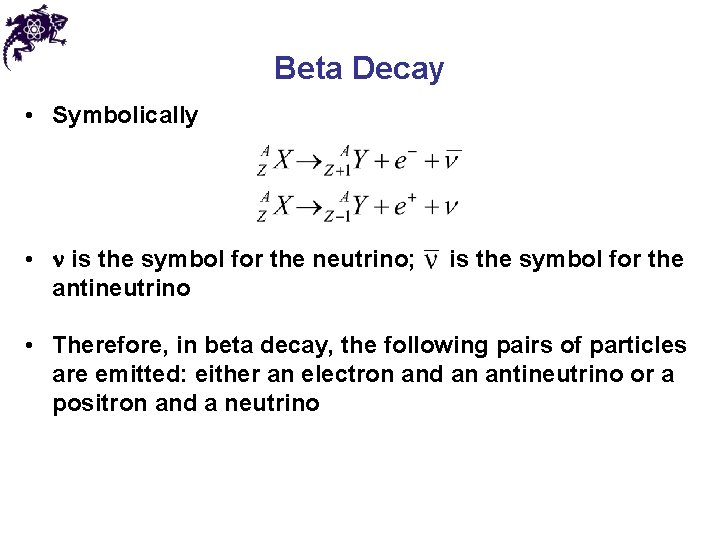
Beta Decay • During beta decay, the daughter nucleus has the same number of nucleons as the parent, but the atomic number is changed by one • Symbolically: • The emission of the electron is from the nucleus, which contains protons and neutrons • The process occurs when a neutron is transformed into a proton and an electron • The energy must be conserved: the energy released in the decay process should almost all go to kinetic energy of the electron (KEmax)

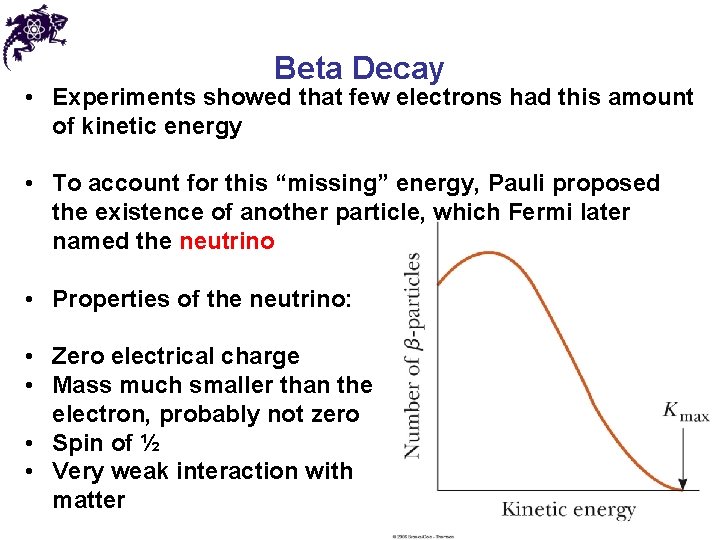
Beta Decay • Experiments showed that few electrons had this amount of kinetic energy • To account for this “missing” energy, Pauli proposed the existence of another particle, which Fermi later named the neutrino • Properties of the neutrino: • Zero electrical charge • Mass much smaller than the electron, probably not zero • Spin of ½ • Very weak interaction with matter

Beta Decay • Symbolically • is the symbol for the neutrino; antineutrino is the symbol for the • Therefore, in beta decay, the following pairs of particles are emitted: either an electron and an antineutrino or a positron and a neutrino

Gamma Decay • Gamma rays are given off when an excited nucleus “falls” to a lower energy state (similar to the process of electron “jumps” to lower energy states and giving off photons) • The gamma ray photons are very high in energy relative to light • The excited nuclear states result from “jumps” made by a proton or neutron • The excited nuclear states may be the result of violent collision or more likely of an alpha or beta emission

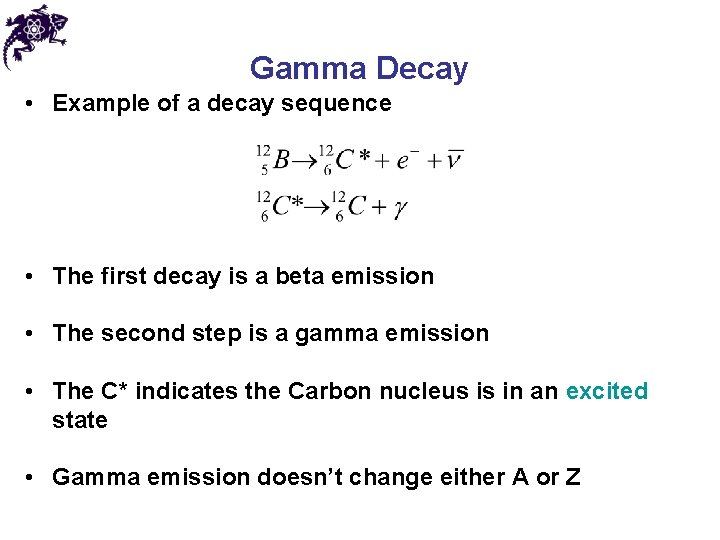
Gamma Decay • Example of a decay sequence • The first decay is a beta emission • The second step is a gamma emission • The C* indicates the Carbon nucleus is in an excited state • Gamma emission doesn’t change either A or Z

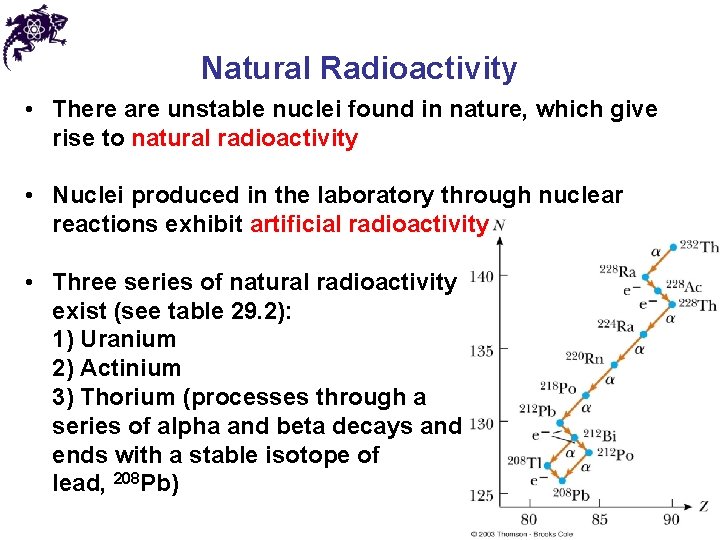
Natural Radioactivity • There are unstable nuclei found in nature, which give rise to natural radioactivity • Nuclei produced in the laboratory through nuclear reactions exhibit artificial radioactivity • Three series of natural radioactivity exist (see table 29. 2): 1) Uranium 2) Actinium 3) Thorium (processes through a series of alpha and beta decays and ends with a stable isotope of lead, 208 Pb)

Nuclear Reactions • Structure of nuclei can be changed by bombarding them with energetic particles • These changes are called nuclear reactions • As with nuclear decays, the atomic numbers and mass numbers must balance on both sides of the equation • E. g. , alpha particle colliding with nitrogen: • Balancing the equation allows for the identification of X, so the reaction is

Chapter 29 Problem 39 Identify the unknown particles X and X’ in the following nuclear reactions:

Answers to Even Numbered Problems Chapter 28: Problem 4 1. ρn/ρa = 8. 6 × 1013

Answers to Even Numbered Problems Chapter 28: Problem 8 a) 1. 9 × 10 -15 m b) 7. 4 × 10 -15 m

Answers to Even Numbered Problems Chapter 28: Problem 10 (a) 1. 11 Me. V/nucleon (b) 7. 07 Me. V/nucleon (c)(c) 8. 79 Me. V/nucleon (d)(d) 7. 57 Me. V/nucleon

Answers to Even Numbered Problems Chapter 28: Problem 18 (a)8. 06 d (b)(b) It is probably 13153 I

Answers to Even Numbered Problems Chapter 28: Problem 20 1. 2. 29 g

Answers to Even Numbered Problems Chapter 28: Problem 38 1. 4 4 He He, 2 2

Answers to Even Numbered Problems Chapter 28: Problem 42 a) 10 5 B b) – 2. 79 Me. V