Chapter 28 Quantum Theory Quantum Regime Draw physics









- Slides: 28

Chapter 28 Quantum Theory

Quantum Regime Draw physics diagram

Quantum Regime Macroscopic world explanations fail at the atomicscale world Newtonian mechanics Maxwell’s equations describing electromagnetism Atomic-scale = quantum regime “Quantum” refers to a very small amount of energy development of quantum theory began in early 1900 s

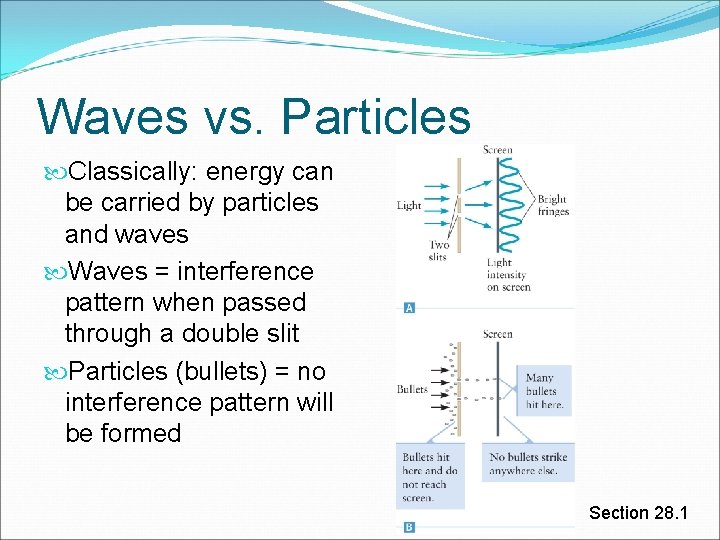
Waves vs. Particles Classically: energy can be carried by particles and waves Waves = interference pattern when passed through a double slit Particles (bullets) = no interference pattern will be formed Section 28. 1

Particles and Waves, Classical Waves exhibit inference; particles do not Particles deliver energy in discrete amounts The energy delivered by a wave is not discrete Wave energy = described by its intensity Energy absorbed depends on: intensity absorption time Section 28. 1

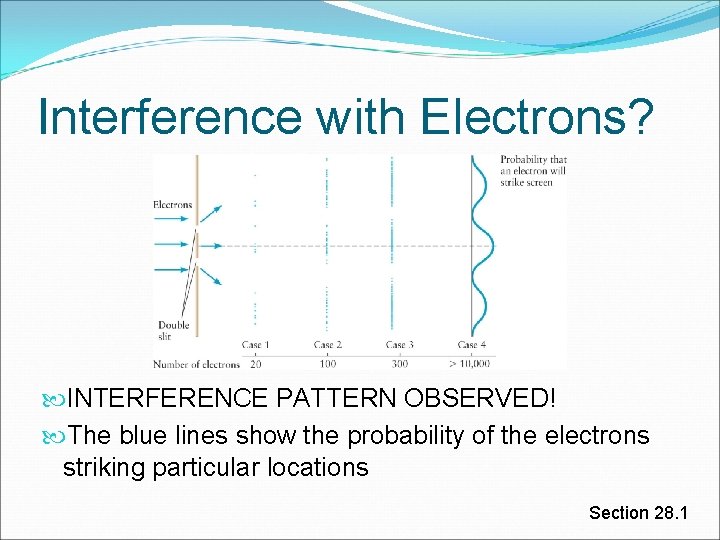
Interference with Electrons? Quantum regime – extremely strange All human intuition is wrong! Lets use electrons in a double slit experiment Shoot them through ONE AT A TIME! What do you think happens? *long pause till someone answers* Section 28. 1

Interference with Electrons? INTERFERENCE PATTERN OBSERVED! The blue lines show the probability of the electrons striking particular locations Section 28. 1

Interference with Electrons, cont. The probability curve of the electrons has same form as light intensity in the double-slit interference experiment This shows that electrons undergo constructive and destructive interference Also shows particle-like behavior since the electrons arrive one at a time at the screen Q: How can this happen? A: It just does. Section 28. 1

Particles and Waves, Quantum All objects, including light and electrons, can exhibit interference (waves) All objects, including light and electrons, carry energy in discrete amounts (particles) These discrete “parcels” are called quanta Wave-particle duality Section 28. 1

WPD Formal Definition The notion that the properties of both classical waves and classical particles are present at the same time is also called wave-particle duality To understand QM sometimes we must think of the objects in question as Waves (electron interference) Particles (photoelectric effect) Section 28. 3

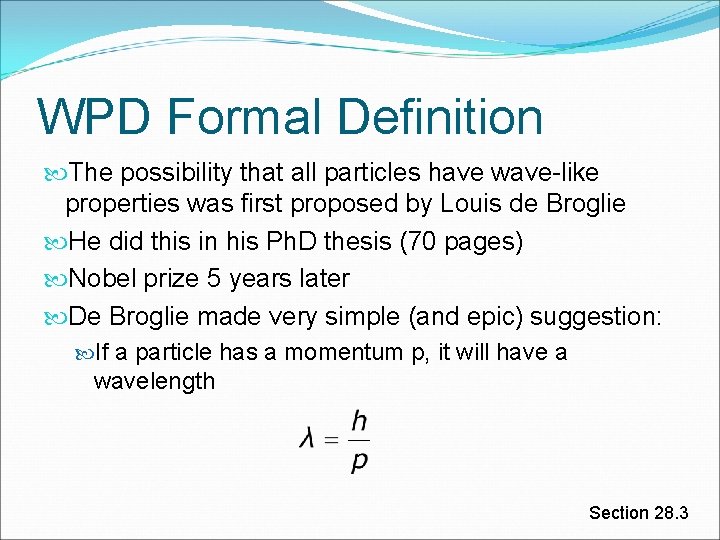
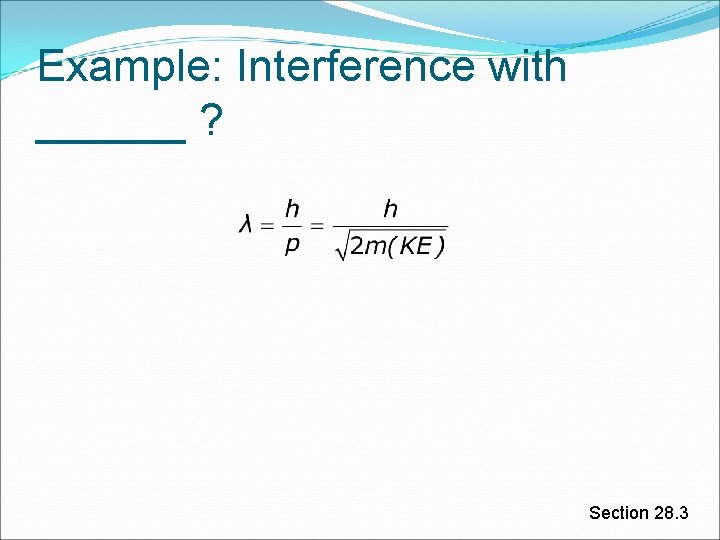
WPD Formal Definition The possibility that all particles have wave-like properties was first proposed by Louis de Broglie He did this in his Ph. D thesis (70 pages) Nobel prize 5 years later De Broglie made very simple (and epic) suggestion: If a particle has a momentum p, it will have a wavelength Section 28. 3

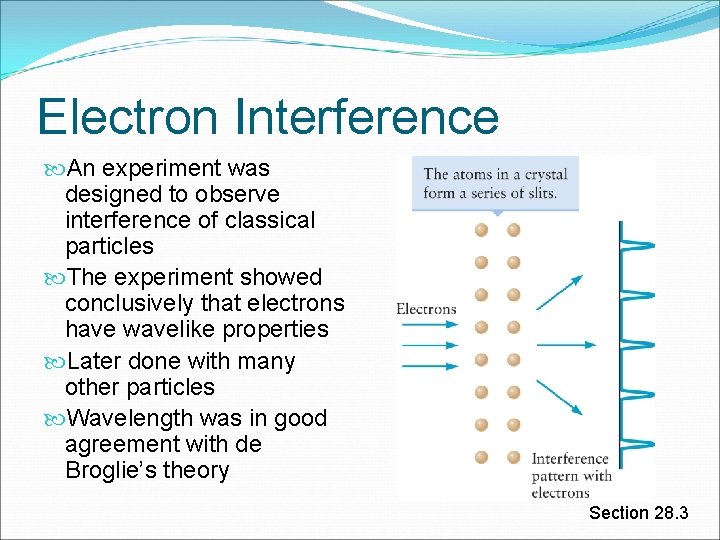
Electron Interference An experiment was designed to observe interference of classical particles The experiment showed conclusively that electrons have wavelike properties Later done with many other particles Wavelength was in good agreement with de Broglie’s theory Section 28. 3

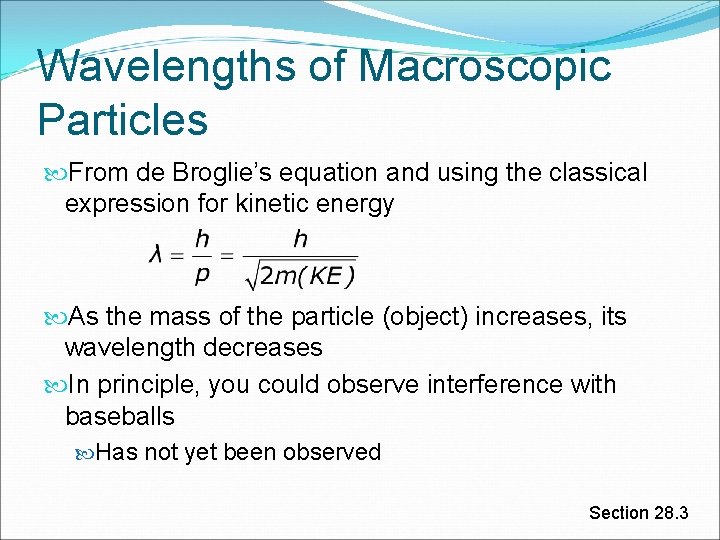
Wavelengths of Macroscopic Particles From de Broglie’s equation and using the classical expression for kinetic energy As the mass of the particle (object) increases, its wavelength decreases In principle, you could observe interference with baseballs Has not yet been observed Section 28. 3

Example: Interference with ______ ? Section 28. 3

The Nature of Quanta 3 principles that are thought to always hold: conservation of energy conservation of momentum conservation of charge The energy and momentum of a photon come in discrete quantized units Electric charge also comes in quantized units Electrons and photons are particle-waves Example? Section 28. 8

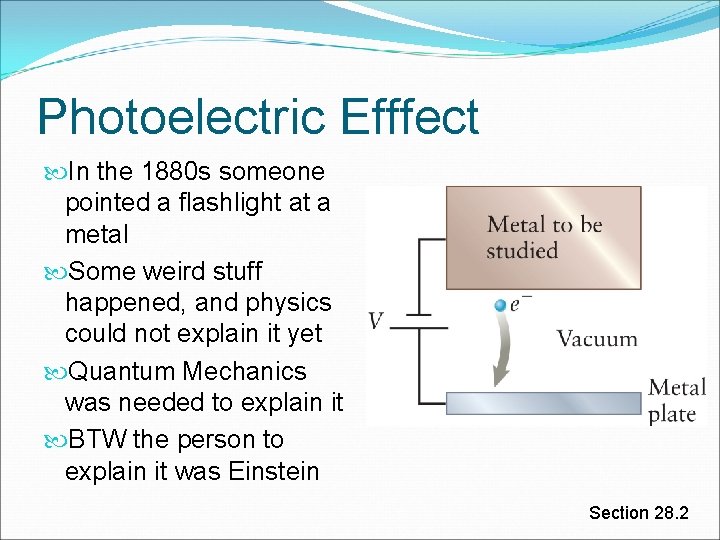
Photoelectric Efffect In the 1880 s someone pointed a flashlight at a metal Some weird stuff happened, and physics could not explain it yet Quantum Mechanics was needed to explain it BTW the person to explain it was Einstein Section 28. 2

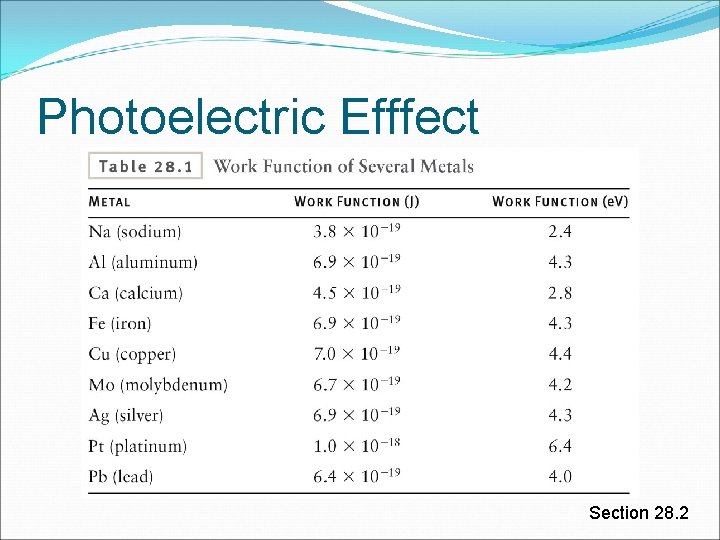
Photoelectric Efffect What’s a metal? Think of it as a sea of electrons Electrons are free to move around within the metal (this is why metals conduct) Electrons are bound to the metal Need energy to be removed from the metal (atom) This energy is the work function Different metals – different work function Measure work function with voltmeter Wc = e. V Section 28. 2

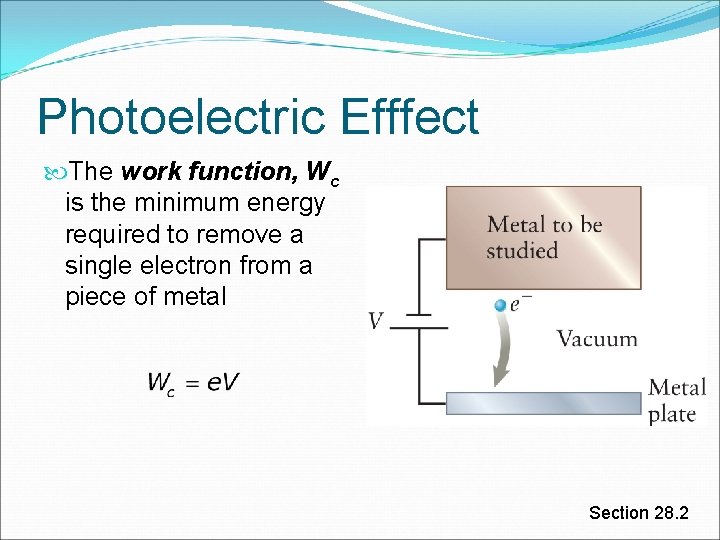
Photoelectric Efffect The work function, Wc is the minimum energy required to remove a single electron from a piece of metal Section 28. 2

Photoelectric Efffect Section 28. 2

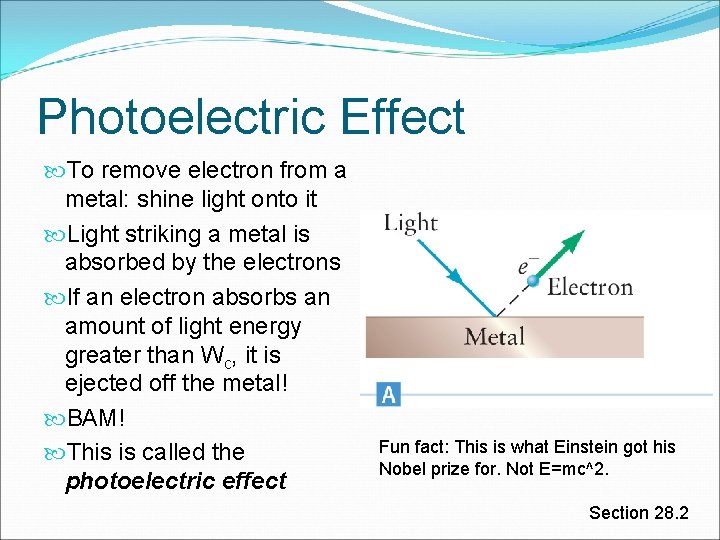
Photoelectric Effect To remove electron from a metal: shine light onto it Light striking a metal is absorbed by the electrons If an electron absorbs an amount of light energy greater than Wc, it is ejected off the metal! BAM! This is called the photoelectric effect Fun fact: This is what Einstein got his Nobel prize for. Not E=mc^2. Section 28. 2

Light Review Light has Frequency Wavelength Travels at c Intensity (how bright it is) Energy Is a particle or a wave? Both! #nofilter #Just. Light. Things #wave #particle #lolcats

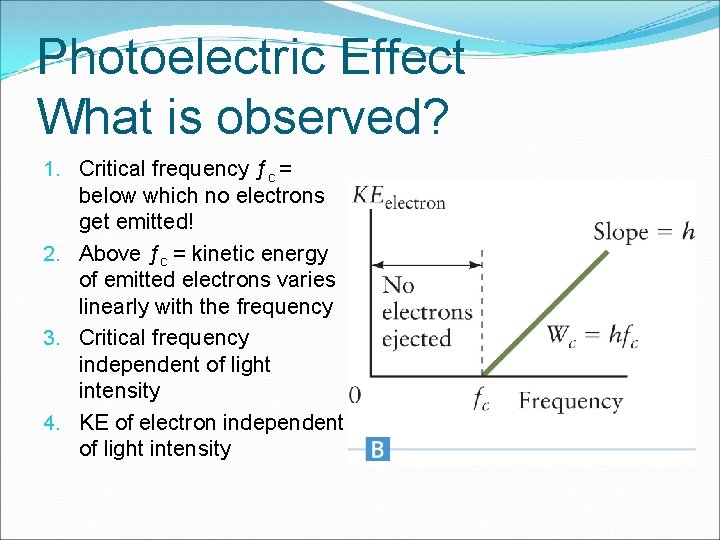
Photoelectric Effect What is observed? 1. Critical frequency ƒc = below which no electrons get emitted! 2. Above ƒc = kinetic energy of emitted electrons varies linearly with the frequency 3. Critical frequency independent of light intensity 4. KE of electron independent of light intensity

Photoelectric Effect Classical Explanation Fails Critical frequency is independent of the light intensity Classically, the energy is proportional to the intensity It should always be possible to eject electrons by increasing the intensity Below the critical frequency there, are no ejected electrons no matter how great the light intensity KE of an ejected electron is independent of the light intensity Classically, increasing the intensity will cause ejected electrons to have a higher KE Experiments actually show the electron kinetic energy depends on the light’s frequency NOT intensity Section 28. 2

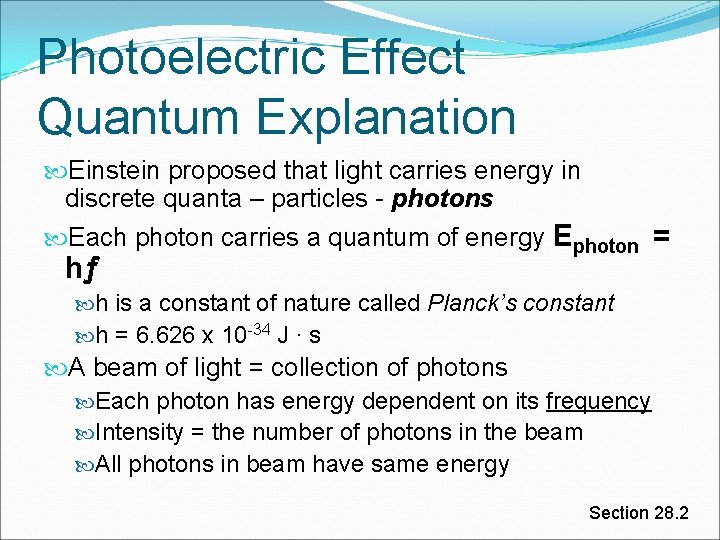
Photoelectric Effect Quantum Explanation Einstein proposed that light carries energy in discrete quanta – particles - photons Each photon carries a quantum of energy Ephoton = hƒ h is a constant of nature called Planck’s constant h = 6. 626 x 10 -34 J ∙ s A beam of light = collection of photons Each photon has energy dependent on its frequency Intensity = the number of photons in the beam All photons in beam have same energy Section 28. 2

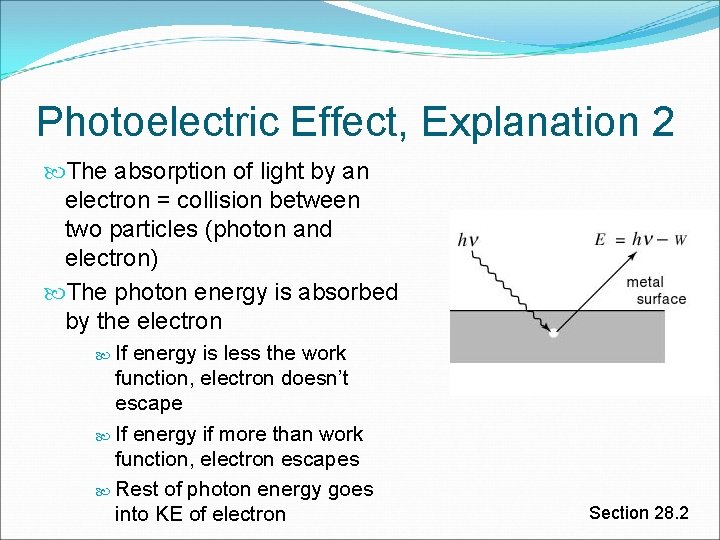
Photoelectric Effect, Explanation 2 The absorption of light by an electron = collision between two particles (photon and electron) The photon energy is absorbed by the electron If energy is less the work function, electron doesn’t escape If energy if more than work function, electron escapes Rest of photon energy goes into KE of electron Section 28. 2

Explanation 2, cont. KE of ejected electrons depends on frequency (not intensity) Critical Frequency -> photons whose energy is equal to the work function h ƒc = W c The electron is just ejected and would have no kinetic energy If the photon has a higher energy, the difference goes into kinetic energy of the ejected electron KEelectron = h ƒ - h ƒc = h ƒ - Wc This linear relationship is what was found experimentally Section 28. 2

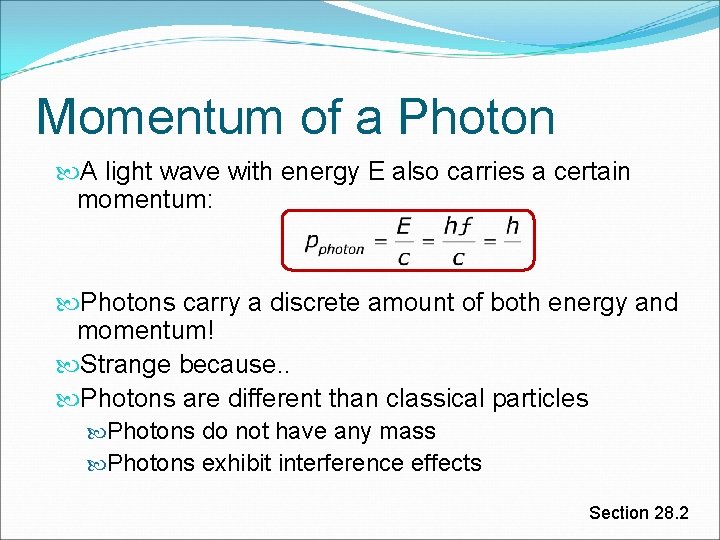
Momentum of a Photon A light wave with energy E also carries a certain momentum: Photons carry a discrete amount of both energy and momentum! Strange because. . Photons are different than classical particles Photons do not have any mass Photons exhibit interference effects Section 28. 2

Photoelectric Example We take potassium (W=2. 21 e. V) and shine on it a light with wavelength 600 nm. What happens? Make this light 10 times as bright. What happens now? Take a light of 250 nm. What happens? Make this light 10 times as bright. What happens now? Section 28. 2