Chapter 26 Ozone Depletion OzoneThe Basics A form

Chapter 26 Ozone Depletion

Ozone…The Basics • A form of oxygen in which three atoms of oxygen occur together (O 3) • Chemically active and has a short average lifetime in the atmosphere • Forms a natural layer high in the atmosphere that protects us from harmful ultraviolet radiation from the sun • A pollutant when present in the lower atmosphere – Remember…ozone is a secondary pollutant.
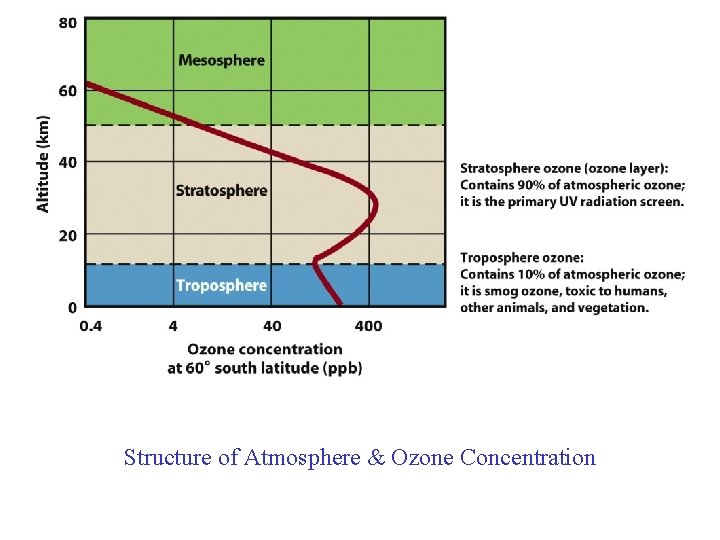
Structure of Atmosphere & Ozone Concentration
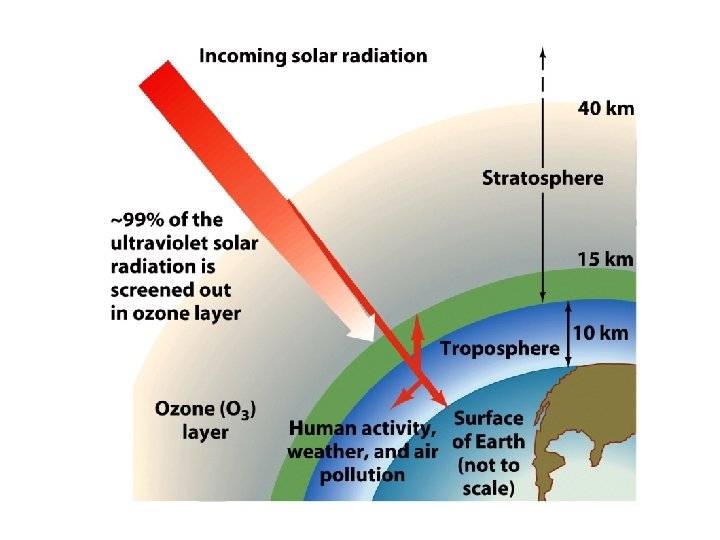

Ultraviolet Radiation and Ozone • Ozone Shield: Stratospheric ozone layer that absorbs ultraviolet radiation Ultraviolet A: – Longest length, least energetic – Can cause some damage to living cells – Transmitted to earth Ultraviolet B: – Intermediate wavelength – Cause of Ozone Problem because O 3 absorbs it Ultraviolet C: – Shortest wavelength/ Most energetic
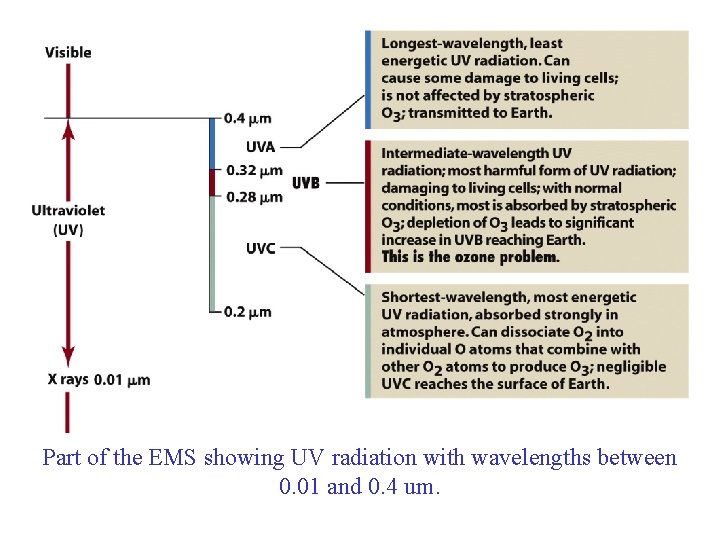
Part of the EMS showing UV radiation with wavelengths between 0. 01 and 0. 4 um.

Measurement of Stratospheric Ozone • Dobson Unit – Commonly used to measure the concentration of ozone – 1 DU is = to 1 ppb O 3 – Measurements of O 3 began in 1970
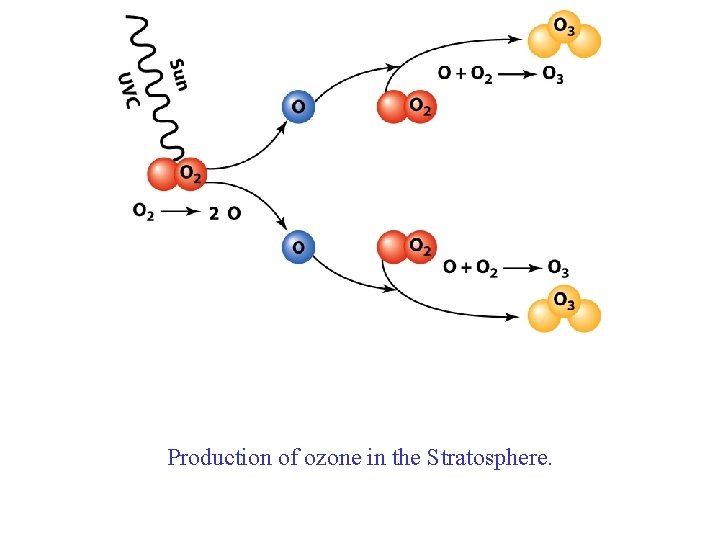
Production of ozone in the Stratosphere.
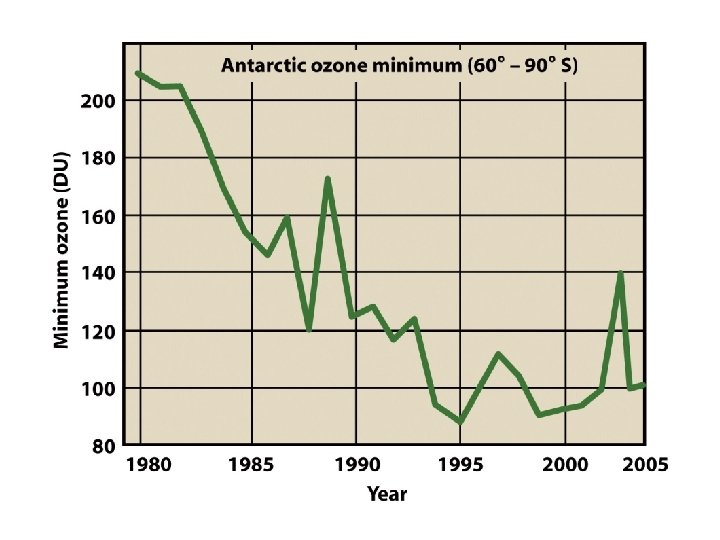

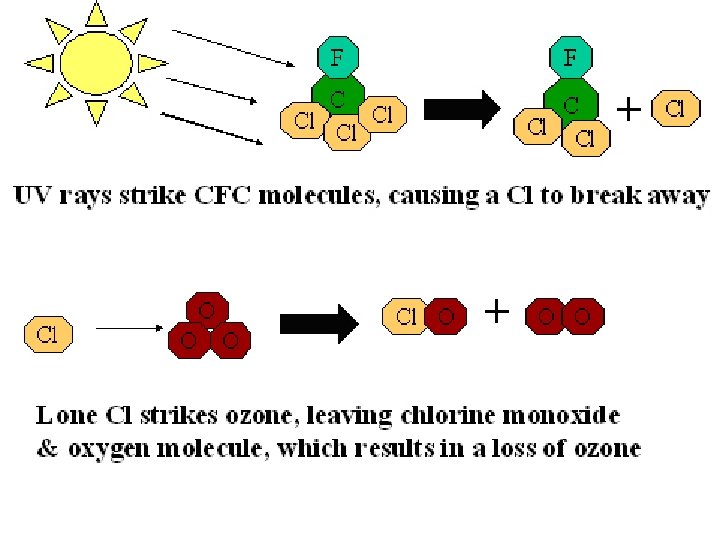
Ozone Depletion and CFCs • Ozone depleted due to emission of CFCs • CFCs are stable and have a long residence time in the atmosphere • In stratosphere, release chlorine and enter into catalytic chain reaction that depletes ozone • More UV radiation reaches lower atmosphere

Polar Stratospheric Clouds • Clouds that form in the stratosphere during the polar winter • Polar Vortex – Artic air masses that in the winter become isolated from the rest of the atmosphere and circulate about the pole – Rotates counterclockwise because of the rotation of the earth in the Southern Hemisphere
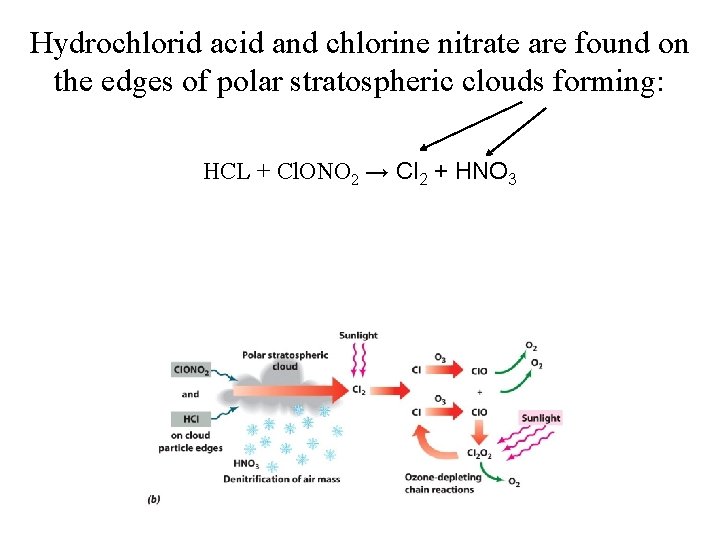
Hydrochlorid acid and chlorine nitrate are found on the edges of polar stratospheric clouds forming: HCL + Cl. ONO 2 → Cl 2 + HNO 3

Management Issues • Key issue is whether ozone depletion is natural or human-induced • Montreal Protocol • Collection and Reuse of CFCs • Substitutes for CFCs – HFCs and HCFCs • Short-Term Adaptation to Ozone Depletion

Any Questions?
- Slides: 15