Chapter 26 Functional Groups and Organic Reactions Charles





























































- Slides: 76

Chapter 26 “Functional Groups and Organic Reactions” Charles Page High School Dr. Stephen L. Cotton

Section 26. 1 - Introduction to Functional Groups l OBJECTIVES: –Define a functional group, and give several examples.

Section 26. 1 - Introduction to Functional Groups l OBJECTIVES: –Describe halocarbons, and the substitution reactions they undergo.

Functional Groups l Most organic chemistry involves substituents – often contain O, N, S, or P – also called “functional groups”- they are the chemically functional part of the molecule, and are the non-hydrocarbon part

Functional Groups l Functional group - a specific arrangement of atoms in an organic compound, that is capable of characteristic chemical reactions. – What is the best way to classify organic compounds? By their functional groups.

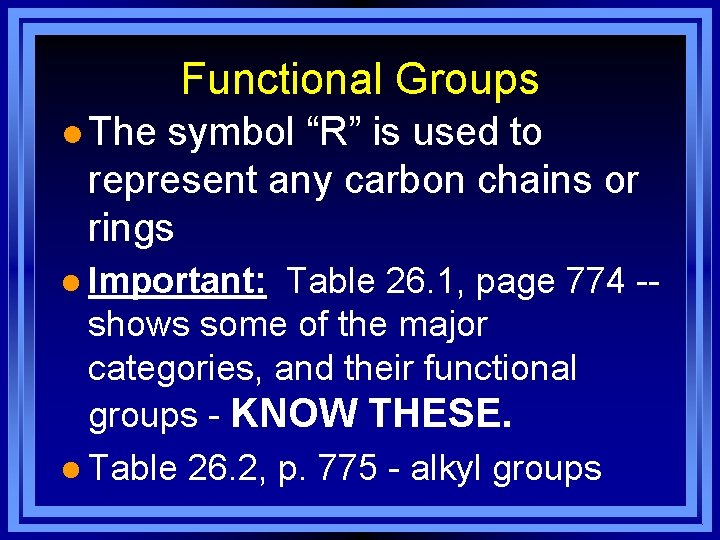
Functional Groups l The symbol “R” is used to represent any carbon chains or rings l Important: Table 26. 1, page 774 -- shows some of the major categories, and their functional groups - KNOW THESE. l Table 26. 2, p. 775 - alkyl groups

Halogen Substituents l Halocarbons - class of organic compounds containing covalently bonded fluorine, chlorine, bromine, or iodine – General formula: R-X l Naming? Name parent as normal, add the halogen as a substituent (or prefix) - Examples on page 774

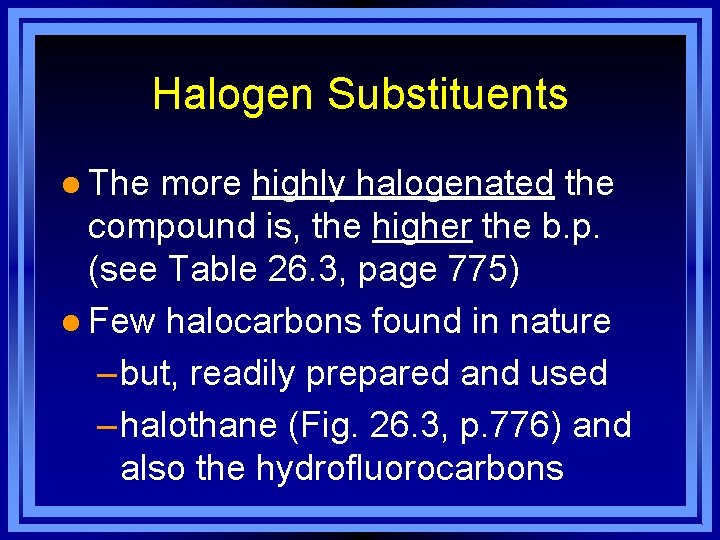
Halogen Substituents l The more highly halogenated the compound is, the higher the b. p. (see Table 26. 3, page 775) l Few halocarbons found in nature – but, readily prepared and used – halothane (Fig. 26. 3, p. 776) and also the hydrofluorocarbons

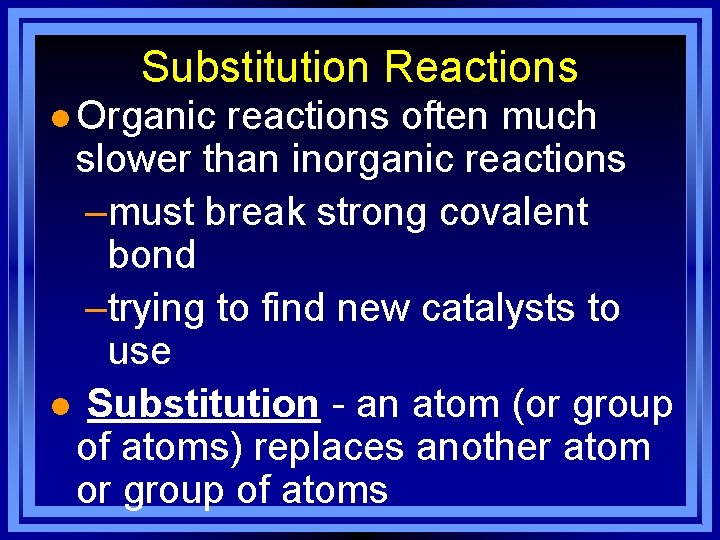
Substitution Reactions l Organic reactions often much slower than inorganic reactions –must break strong covalent bond –trying to find new catalysts to use l Substitution - an atom (or group of atoms) replaces another atom or group of atoms

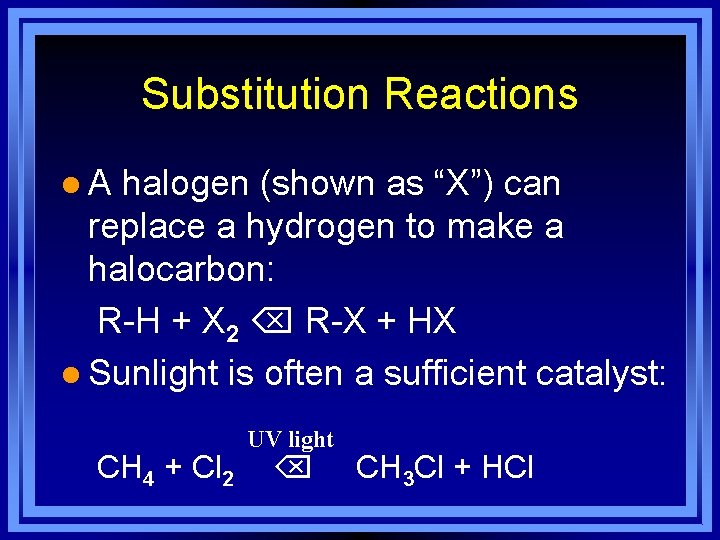
Substitution Reactions l A halogen (shown as “X”) can replace a hydrogen to make a halocarbon: R-H + X 2 R-X + HX l Sunlight is often a sufficient catalyst: UV light CH 4 + Cl 2 CH 3 Cl + HCl

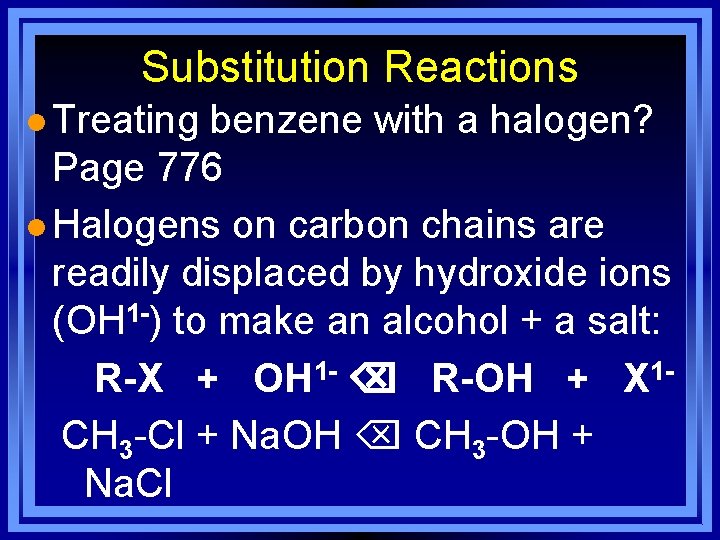
Substitution Reactions l Treating benzene with a halogen? Page 776 l Halogens on carbon chains are readily displaced by hydroxide ions (OH 1 -) to make an alcohol + a salt: R-X + OH 1 - R-OH + X 1 CH 3 -Cl + Na. OH CH 3 -OH + Na. Cl

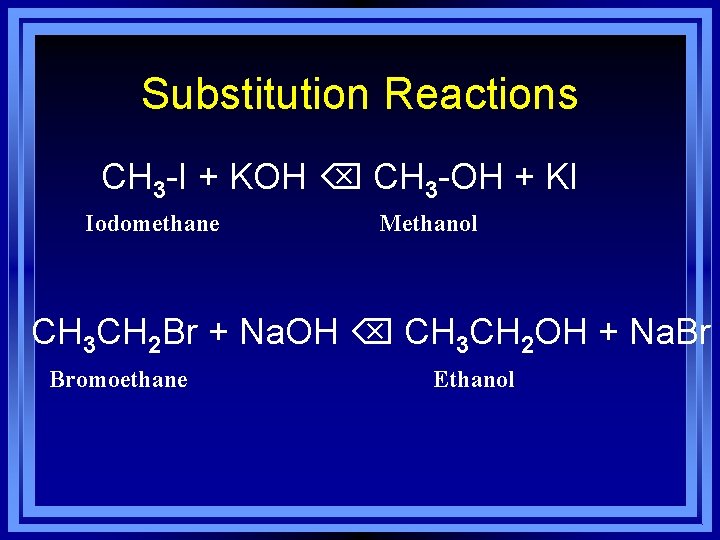
Substitution Reactions CH 3 -I + KOH CH 3 -OH + KI Iodomethane Methanol CH 3 CH 2 Br + Na. OH CH 3 CH 2 OH + Na. Br Bromoethane Ethanol

Section 26. 2 Alcohols and Ethers l OBJECTIVES: –Describe the structures and naming of alcohols and ethers.

Section 26. 2 Alcohols and Ethers l OBJECTIVES: –Define an addition reaction, and give several examples.

Section 26. 2 Alcohols and Ethers l OBJECTIVES: –Compare the properties of alcohols and ethers.

Alcohols l Alcohols - a class of organic compounds with an -OH group – The -OH functional group in alcohols is called a “hydroxyl” group; thus R-OH is the formula l How is this different from the hydroxide ion? (covalent bonding with the carbon- not ionic with a metal like bases)

Alcohols l Arranged into categories according to the number of R groups attached to the carbon with the hydroxyl – 1 R group: primary alcohol – 2 R groups: secondary alcohol – 3 R groups: tertiary alcohol l Note drawings on page 778

Alcohols l Both IUPAC and common names l For IUPAC: –drop the -e ending of the parent alkane name; add ending of -ol, number the position of -OH –parent is the longest chain that contains the carbon with the hydroxyl attached.

Alcohols l The hydroxyl is given the lowest position number l Alcohols containing 2, 3, and 4 of the -OH substituents are named diols, triols, and tetrols respectively –Examples on page 779

Alcohols l Common names: –similar to halocarbons, meaning name the alkyl group followed by the word alcohol –One carbon alcohol = methyl alcohol

Alcohols l More than one -OH substituents are called glycols (ethylene glycol? ) l ** Examples on page 779 ** l Phenols - compounds in which a hydroxyl group is attached directly to an aromatic ring. Cresol is the common name of o, m, and p isomers of methylphenol

Properties of Alcohols l Much like water, alcohols are capable of hydrogen bonding between molecules – this means they will boil at a higher temp. than alkanes and halocarbons with a comparable number of atoms

Properties of Alcohols l Alcohols are derivates of water; the -OH comes from water, and thus are somewhat soluble l Alcohols of up to 4 carbons are soluble in all proportions; more than 4 carbons are usually less soluble, because…?

Properties of Alcohols l Many aliphatic alcohols used in laboratories, clinics, and industry – Isopropyl alcohol (2 -propanol) is rubbing alcohol; used as antiseptic, and a base for perfume, creams, lotions, and other cosmetics l Ethylene glycol (1, 2 -ethanediol) - commonly sold as antifreeze

Properties of Alcohols l Glycerol (1, 2, 3 -propanetriol) - used as a moistening agent in cosmetics, foods, and drugs; also a component of fats and oils l Ethyl alcohol (ethanol) used in the intoxicating beverages; an important industrial solvent

Properties of Alcohols l Denatured alcohol- means it has been made poisonous by the addition of other chemicals, often methyl alcohol (methanol, or wood alcohol). As little as 10 m. L of methanol has been known to cause permanent blindness, and 30 ml has resulted in death!!!

Addition Reactions l Carbon-carbon single bond is not easy to break l In double bonded alkenes, it is easier to break a bond l Addition reaction- substance is added at the double or triple bond location, after it is broken

Addition Reactions l Addition of water to an alkene is a hydration reaction - usually occurs with heat and an acid (such as HCl or H 2 SO 4 acting as a catalyst) l Note sample at bottom of page 781 for the formation of ethanol from ethene + water

Addition Reactions l If a halogen is added in an addition reaction, the result is a halocarbon that is disubstituted - top page 782 l The addition of bromine is often used as a test for saturation - p. 782 l Addition of a hydrogen halide? called monosubstituted halocarbon

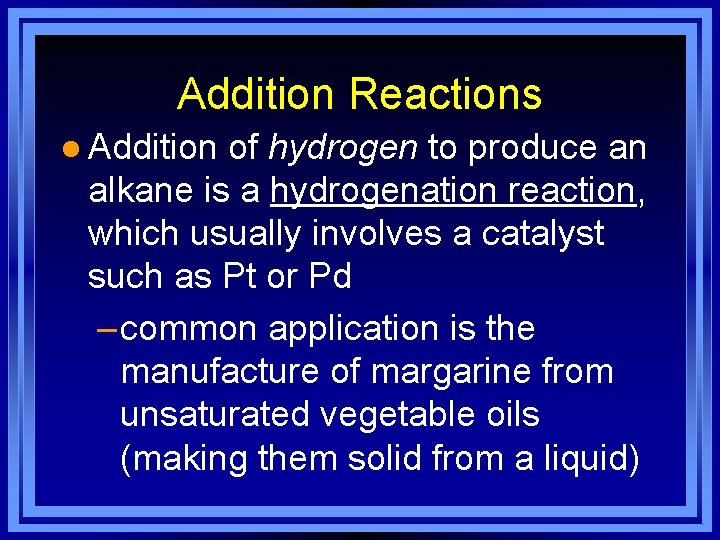
Addition Reactions l Addition of hydrogen to produce an alkane is a hydrogenation reaction, which usually involves a catalyst such as Pt or Pd – common application is the manufacture of margarine from unsaturated vegetable oils (making them solid from a liquid)

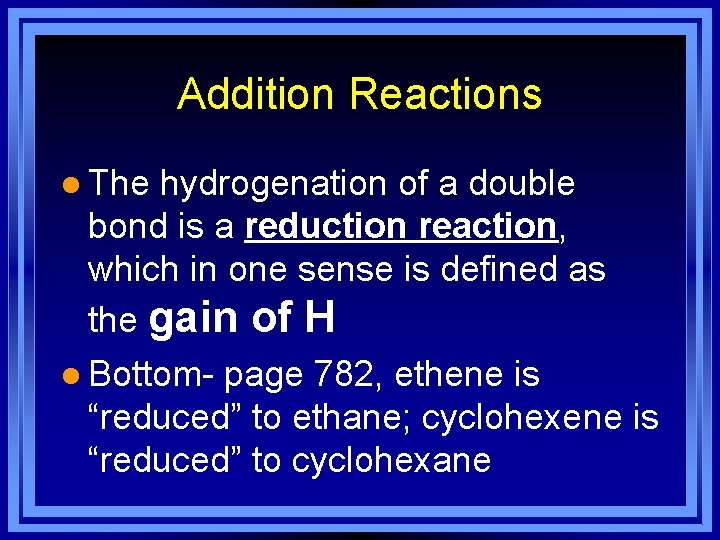
Addition Reactions l The hydrogenation of a double bond is a reduction reaction, which in one sense is defined as the gain of H l Bottom- page 782, ethene is “reduced” to ethane; cyclohexene is “reduced” to cyclohexane

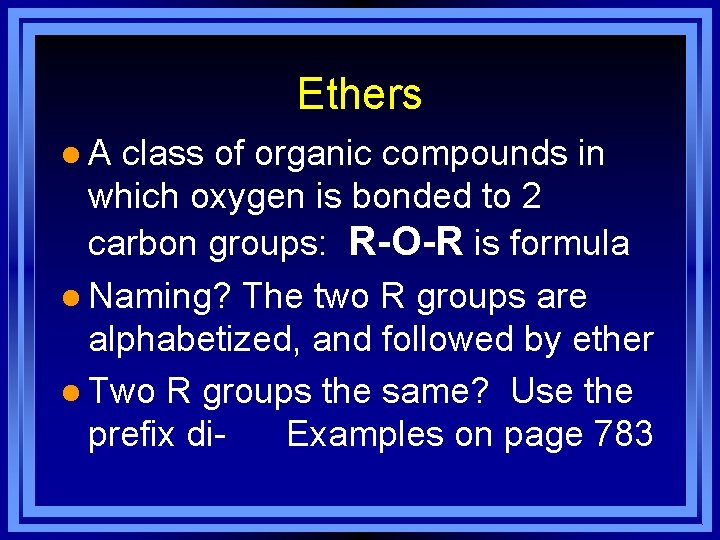
Ethers l A class of organic compounds in which oxygen is bonded to 2 carbon groups: R-O-R is formula l Naming? The two R groups are alphabetized, and followed by ether l Two R groups the same? Use the prefix di- Examples on page 783

Ethers l Diethyl ether is the one commonly called just “ether” – was the first reliable general anesthetic – dangerous- highly flammable, also causes nausea l ethers are fairly soluble in water l Note the LINK on page 784

Section 26. 3 Carbonyl Compounds l OBJECTIVES: –Distinguish among the carbonyl groups of aldehydes, ketones, carboxylic acids, and esters.

Section 26. 3 Carbonyl Compounds l OBJECTIVES: –Describe the reactions of compounds that contain the carbonyl functional group.

Aldehydes and Ketones l Review: – alcohol has an oxygen bonded to a carbon group and a hydrogen – ether has an oxygen bonded to two carbon groups l An oxygen can also be bonded to a single carbon by a double bond

Aldehydes and Ketones l The C=O group is called the “carbonyl group” – it is the functional group in both aldehydes and ketones l Aldehydes - carbonyl group always joined to at least one hydrogen (meaning it is always on the end!)

Aldehydes and Ketones l Ketones - the carbon of the carbonyl group is joined to two other carbons (meaning it is never on the end) l Structures - middle of page 785

Aldehydes and Ketones l Naming? – Aldehydes: identify longest chain containing the carbonyl group, then the -e ending replaced by -al, such as methanal, etc. – Ketones: longest chain w/carbonyl, then new ending of -one; number it l propanone, 2 -pentanone, 3 -pentanone

Aldehydes and Ketones l Table 26. 4, page 786 examples l Neither can form intermolecular hydrogen bonds, thus a much lower b. p. than corresponding alcohols l wide variety have been isolated from plants and animals; possible fragrant odor or taste; many common names

Aldehydes and Ketones l Benzaldehyde l Cinnamaldehyde l Vanillin l Methanal (common: formaldehyde) – 40% in water is formalin, a preservative

Aldehydes and Ketones l Propanone (common: acetone) is a good solvent; miscible with water in all proportions l why is it a good substance used in nail-polish removers? (a powerful solvent-able to dissolve both polar & nonpolar)

Carboxylic Acids l Also have a carbonyl group (C=O), but is also attached to a hydroxyl group (-OH) = “carboxyl” group l general formula: R-COOH – weak acids (ionize slightly) l Named by replacing -e with -oic and followed by the word acid l methanoic acid; ethanoic acid

Carboxylic Acids l Abundant and widely distributed in nature, many having a Greek or Latin word describing their origin – acetic acid (ethanoic acid) from acetum, meaning vinegar – many that were isolated from fats are called fatty acids

Esters l General formula: RCOOR l Derivatives of the carboxylic acids, in which the -OH from the carboxyl group is replaced by an -OR from an alcohol: carboxylic acid + alcohol ester + water l many esters have pleasant, fruity odors- banana, pineapple, perfumes

Esters l Although polar, they do not form hydrogen bonds (reason: there is no hydrogen bonded to a highly electronegative atom!) – thus, much lower b. p. than the hydrogen-bonded carboxylic acids they came from

Esters l Can be prepared from a carboxylic acid an alcohol; usually a trace of mineral acid added as catalyst (because acids are dehydrating agents) l Note equation on bottom p. 790

Esters l Naming? It has 2 words: – 1 st: alkyl attached to single bonded oxygen from alcohol – 2 nd: take the acid name, remove the -ic acid, add -ate l example on top of page 791

Oxidation- Reduction Reactions l All of the previous classes of organic compounds are related by oxidation and reduction reactions l What is oxidation-reduction? – Oxidation: the gain of oxygen, loss of hydrogen, or loss of e-1 – Reduction: the loss of oxygen, gain of hydrogen, or gain of e-1

Oxidation- Reduction Reactions l Oxidation and reduction reactions (sometimes called redox) are coupled- one does not occur without the other l The number of Oxygen and Hydrogen attached to Carbon indicates the degree of oxidation

Oxidation- Reduction Reactions l The fewer the # of H on a C-C bond, the more oxidized the bond – Thus, a triple bond is more oxidized than a double bond a single bond l An alkane is oxidized (loss of H) to an alkene, and then to an alkyne

Oxidation- Reduction Reactions l Loss of hydrogen is called a dehydrogenation reaction –may require strong heating and a catalyst l Note equations on page 791

Oxidation- Reduction Reactions l Methane can be oxidized in steps to carbon dioxide (top page 792): methane methanol methanal methanoic acid CO 2 l the more reduced (more H) a carbon compound, the more energy it can release upon oxidation

Oxidation- Reduction Reactions l Alcohols can also be oxidized into other products l “Dr. Al K. Hall Mr. Al D. Hyde” l Equations top of page 793 l Preparing aldehydes from a primaryf alcohol is a problem, because they are then easily oxidized to carboxylic acids

Oxidation- Reduction Reactions l Benedict’s test and Fehling’s test are commonly used for aldehyde detection - margin p. 793

Section 26. 4 Polymerization l OBJECTIVES: –Define polymer and monomer.

Section 26. 4 Polymerization l OBJECTIVES: –Name and describe the uses of some important addition and condensation polymers.

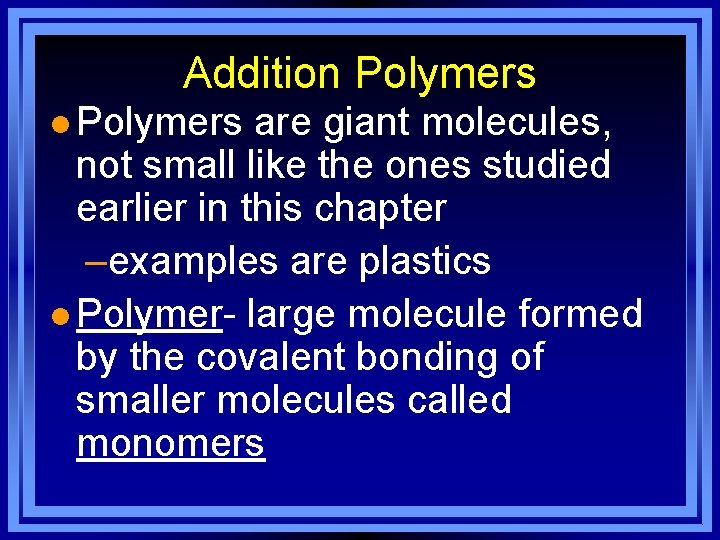
Addition Polymers l Polymers are giant molecules, not small like the ones studied earlier in this chapter –examples are plastics l Polymer- large molecule formed by the covalent bonding of smaller molecules called monomers

Polymers from Monomers

Addition Polymers l An addition polymer forms when unsaturated monomers react to form a polymer – ethene will form polyethylene, shown on page 795 – polyethylene is easy to clean, chemically resistant- milk bottles, plastic wrap, refrigerator dishes

High Density Polyethylene

Addition Polymers l Polypropylene is a stiffer polymer, used in utensils and containers l Polystyrene is formed from styrene (phenylethene), and is a poor heat conductor (styrofoam – Dow Chemical) – molded coffee cups and picnic coolers, insulates homes l Polyvinyl chloride (PVC) used for pipes in plumbing

Addition Polymers l Polytetrafluoroethene (PTFE, or “Teflon”) is very resistant to heat and chemical corrosion –found on nonstick cookware; coating on bearings and bushings used in chemical reactors

Condensation Polymers l Condensation polymers are formed by the head-to-tail joining of monomer units –usually accompanied by the loss of water from the reacting monomers, and forming water as a product

Condensation Polymers l Ex: polyethylene terephthalate (PETE) – Dacron , Fortrel , Polyesters: permanent press clothing, tire cords – Sheets of polyester called Mylar , used as magnetic tape in tape recorders and computers, as well as balloons – Nylon: carpet, fishing line, hosiery

Condensation Polymers l Examples: – aromatic rings form Nomex , which is a poor electrical conductor; makes parts for electrical fixtures; flame resistant clothing for race car drivers; flame resistant building materials – Kevlar : strong and flame resistant

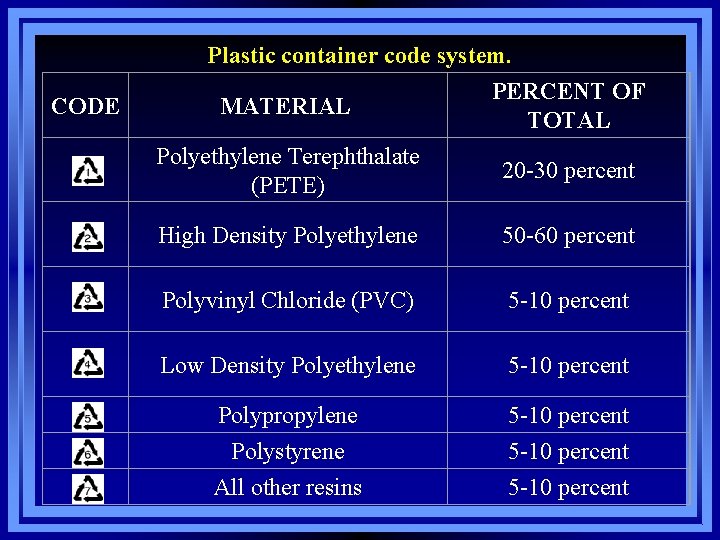
Plastic container code system. CODE MATERIAL PERCENT OF TOTAL Polyethylene Terephthalate (PETE) 20 -30 percent High Density Polyethylene 50 -60 percent Polyvinyl Chloride (PVC) 5 -10 percent Low Density Polyethylene 5 -10 percent Polypropylene 5 -10 percent Polystyrene All other resins 5 -10 percent

What Do the Numbers Mean? 1 -- PETE (Polyethylene terephthalate) • PET is used in the production of soft drink bottles, peanut butter jars. . . • PET can be recycled into fiberfill for sleeping bags, carpet fibers, rope, pillows. . .

What Do the Numbers Mean? 2 -- HDPE (High-density polyethylene) • HDPE is found in milk jugs, butter tubs, detergent bottles, motor oil bottles. . . • HDPE can be recycled into flower pots, trash cans, traffic barrier cones, detergent bottles. . .

What Do the Numbers Mean? 3 -- V (Polyvinyl chloride) • PVC is used in shampoo bottles, cooking oil bottles, fast food service items. . . • PVC can be recycled into drainage and irrigation pipes. . .

What Do the Numbers Mean? 4 -- LDPE (Low-density polyethylene) • LDPE is found in grocery bags, bread bags, shrink wrap, margarine tub tops. . . • LDPE can be recycled into new grocery bags. . .

What Do the Numbers Mean? 5 -- PP (Polypropylene) • PP is used in most yogurt containers, straws, pancake syrup bottles, bottle caps. . • PP can be recycled into plastic lumber, car battery cases, manhole steps. . .

What Do the Numbers Mean? 6 -- PS (Polystyrene) • PS is found in disposable hot cups, packaging materials (peanuts), and meat trays. . . • PS can be recycled into plastic lumber, cassette tape boxes, flower pots. . .

What Do the Numbers Mean? 7 -- Other • This is usually a mixture of various plastics, like squeeze ketchup bottles, "microwaveable" dishes. . .

Timeline of Plastics 1862 – First man-made plastic 1866 – Celluloid makes it’s debut 1891 – Rayon is discovered 1907 – Bakelite is invented 1913 – Cellophane causes the plastics craze

Timeline of Plastics 1926 – PVC is invented 1933 – Polyethylene is discovered 1933 – Saran makes it’s debut 1938 – Teflon is discovered 1939 – Nylon stockings hit market 1957 – Here comes velcro