CHAPTER 26 FLUID ELECTROLYTE AND ACIDBASE BALANCE Identify

CHAPTER 26 FLUID, ELECTROLYTE, AND ACID-BASE BALANCE
• Identify the body’s main fluid compartments 1 • Define plasma osmolality and identify two ways in which plasma osmolality is maintained 2 CHAPTER OBJECTIVE S • Identify the six ions most important to the function of the body 3 • Define buffer and discuss the role of buffers in the body 4 • Explain why bicarbonate must be conserved rather than reabsorbed in the kidney 5 6
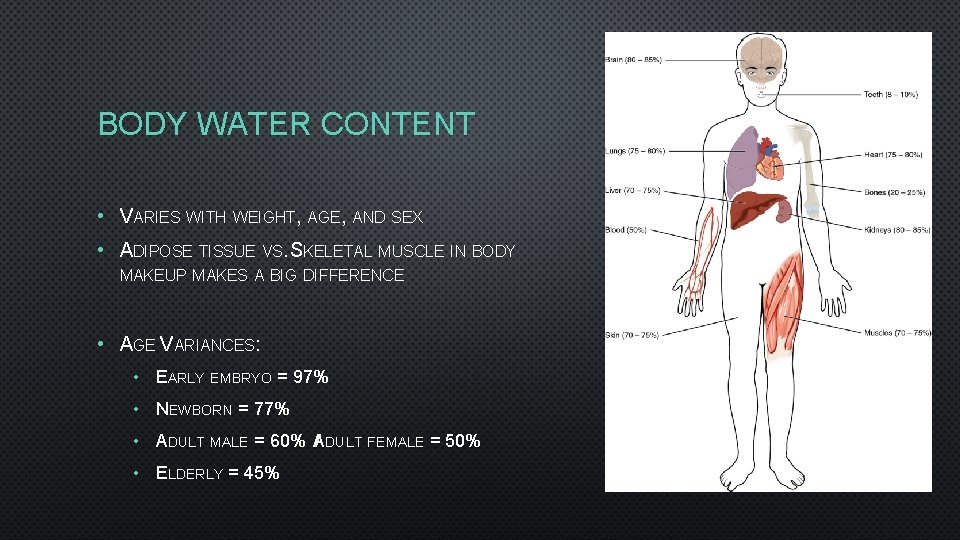
BODY WATER CONTENT • VARIES WITH WEIGHT, AGE, AND SEX • ADIPOSE TISSUE VS. SKELETAL MUSCLE IN BODY MAKEUP MAKES A BIG DIFFERENCE • AGE VARIANCES: • EARLY EMBRYO = 97% • NEWBORN = 77% • ADULT MALE = 60% A / DULT FEMALE = 50% • ELDERLY = 45%
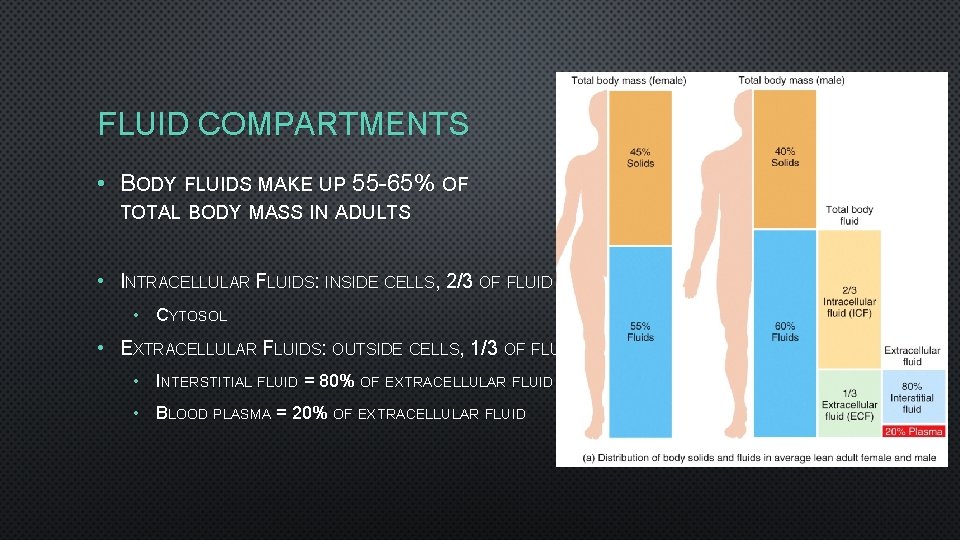
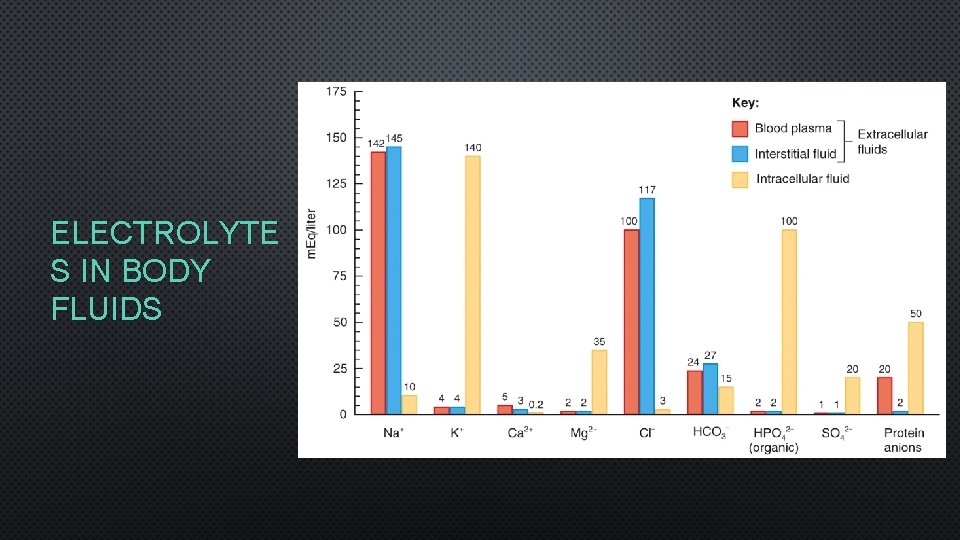
FLUID COMPARTMENTS • BODY FLUIDS MAKE UP 55 -65% OF TOTAL BODY MASS IN ADULTS • INTRACELLULAR FLUIDS: INSIDE CELLS, 2/3 OF FLUID • CYTOSOL • EXTRACELLULAR FLUIDS: OUTSIDE CELLS, 1/3 OF FLUID • INTERSTITIAL FLUID = 80% OF EXTRACELLULAR FLUID • BLOOD PLASMA = 20% OF EXTRACELLULAR FLUID
FLUID COMPARTMENT SEPARATION • PLASMA MEMBRANES: SEPARATE INTRACELLULAR FROM INTERSTITIALFLUID • BLOOD VESSEL WALLS: SEPARATE INTERSTITIAL FLUID FROM BLOOD PLASMA • CAPILLARY WALLS: ARE THIN ENOUGH TO ALLOW EXCHANGE OF WATER AND SOLUTES • FILTRATION, REABSORPTION, DIFFUSION, AND OSMOSIS ALLOW CONTINUOUS EXCHANGE OF WATER AND SOLUTES AMONG BODY FLUID COMPARTMENTS • ELECTROLYTE BALANCE IS CLOSELY RELATED TO FLUID BALANCE
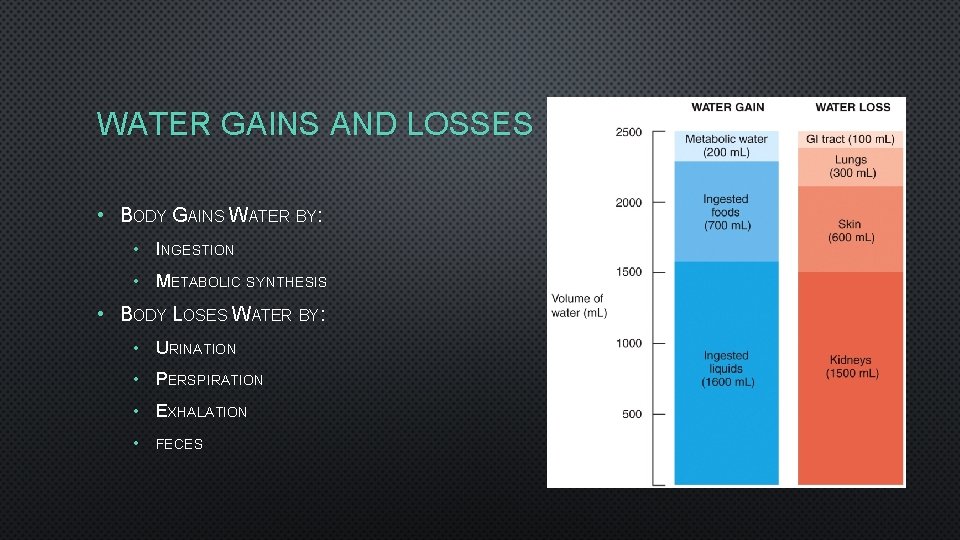
WATER GAINS AND LOSSES • BODY GAINS WATER BY: • INGESTION • METABOLIC SYNTHESIS • BODY LOSES WATER BY: • URINATION • PERSPIRATION • EXHALATION • FECES
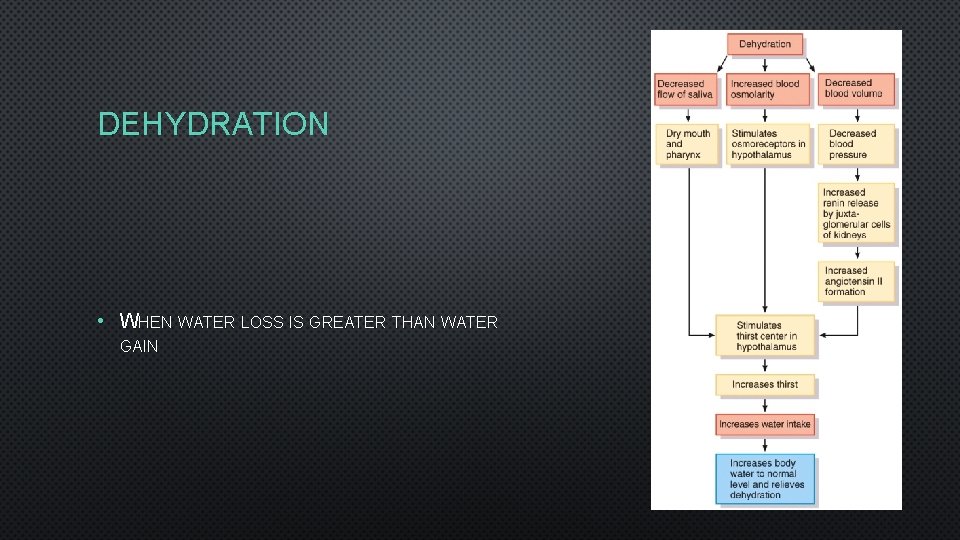
DEHYDRATION • WHEN WATER LOSS IS GREATER THAN WATER GAIN
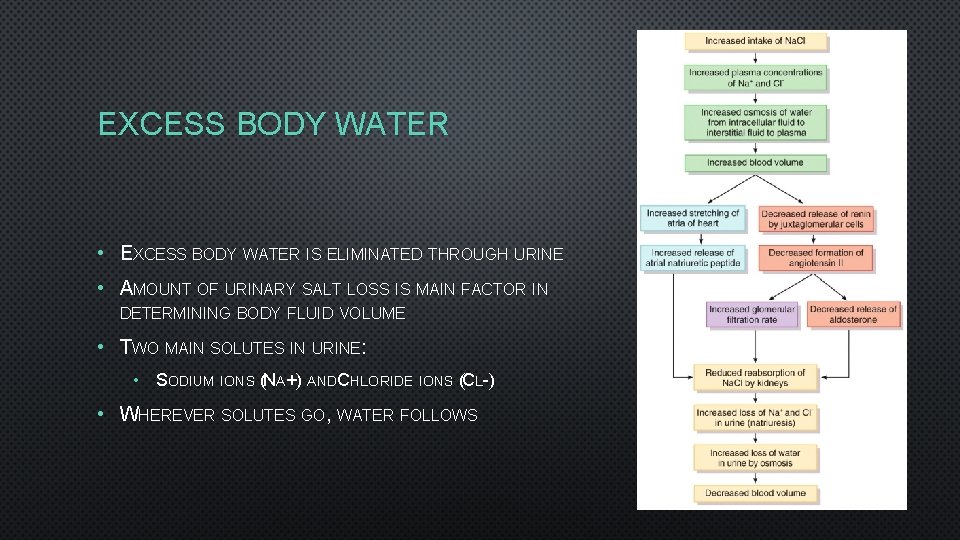
EXCESS BODY WATER • EXCESS BODY WATER IS ELIMINATED THROUGH URINE • AMOUNT OF URINARY SALT LOSS IS MAIN FACTOR IN DETERMINING BODY FLUID VOLUME • TWO MAIN SOLUTES IN URINE: • SODIUM IONS (NA+) ANDCHLORIDE IONS (CL-) • WHEREVER SOLUTES GO, WATER FOLLOWS

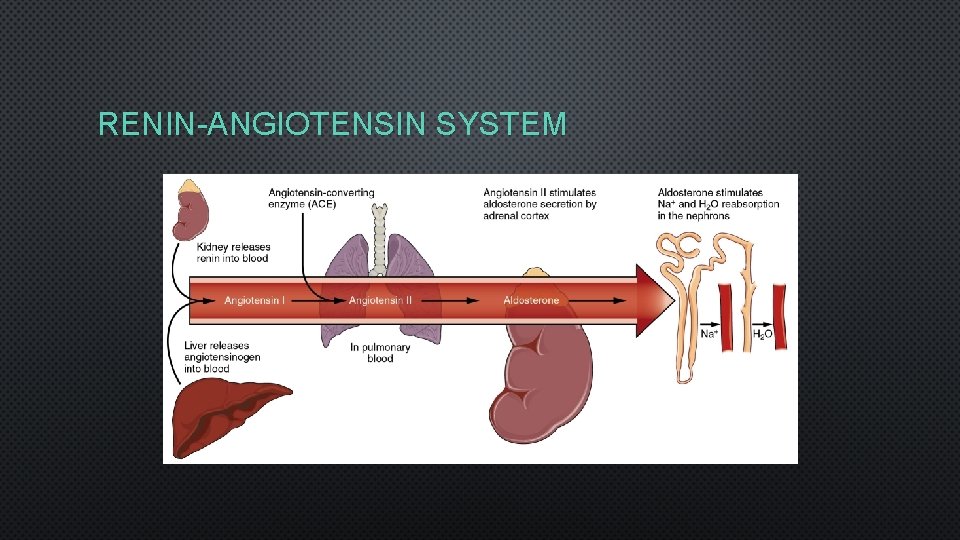
HORMONAL CONTROL • 3 HORMONES CONTROL RENAL NA+ AND CL-: • ANGIOTENSIN II • ALDOSTERONE • ATRIAL NATRIURETIC PEPTIDE (ANP) • HORMONE REGULATING WATER LOSS: • ANTIDIURETIC HORMONE (ADH)
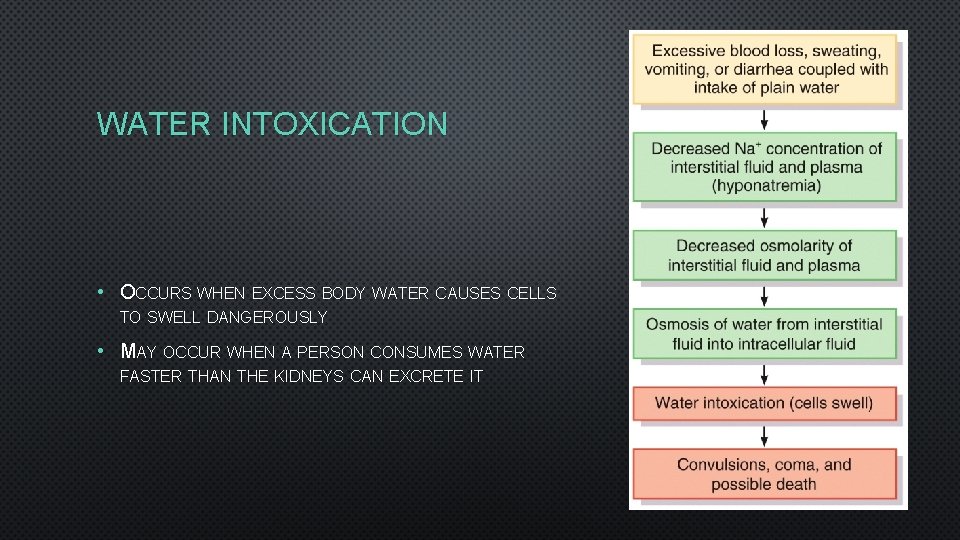
WATER INTOXICATION • OCCURS WHEN EXCESS BODY WATER CAUSES CELLS TO SWELL DANGEROUSLY • MAY OCCUR WHEN A PERSON CONSUMES WATER FASTER THAN THE KIDNEYS CAN EXCRETE IT
ELECTROLYTES • IONS FORMED WHEN ELECTROLYTES DISSOCIATE AND DISSOLVE • CONTROL OSMOSIS OF WATER BETWEEN FLUID COMPARTMENTS • HELP MAINTAIN THE ACID-BASE BALANCE • CARRY ELECTRICAL CURRENT • SERVE AS COFACTORS • CATIONS: SODIUM (Na+), POTASSIUM (K+), HYDROGEN H ( +), MAGNESIUM ( Mg 2+), CALCIUM (Ca 2+) • ANIONS: CHLORIDE (Cl-), BICARBONATE (HCO 3 -), PHOSPHATE (PO 43 -), SULFATESO ( 42 -) • CONCENTRATION OF IONS IS EXPRESSED IN UNITS OF MILLIEQUIVALENTS PER LITER(m. Eq/liter)
ELECTROLYTE S IN BODY FLUIDS
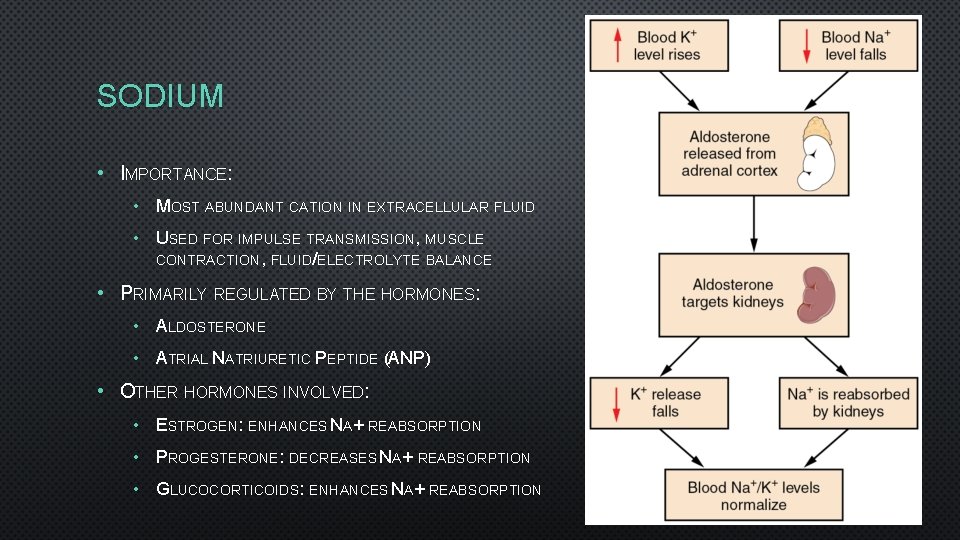
SODIUM • IMPORTANCE: • MOST ABUNDANT CATION IN EXTRACELLULAR FLUID • USED FOR IMPULSE TRANSMISSION, MUSCLE CONTRACTION, FLUID/ELECTROLYTE BALANCE • PRIMARILY REGULATED BY THE HORMONES: • ALDOSTERONE • ATRIAL NATRIURETIC PEPTIDE (ANP) • OTHER HORMONES INVOLVED: • ESTROGEN: ENHANCES NA+ REABSORPTION • PROGESTERONE: DECREASES NA+ REABSORPTION • GLUCOCORTICOIDS: ENHANCES NA+ REABSORPTION
RENIN-ANGIOTENSIN SYSTEM

SODIUM • NORMAL VALUE: 136 -148 m. Eq/liter • DEFICIENCY = HYPONATREMIA • DUE TO DECREASED SODIUM INTAKE; INCREASED SODIUM LOSS THROUGH VOMITING, DIARRHEA, ALDOSTERONE DEFICIENCY, OR DIURETIC DRUGS; OR EXCESSIVE WATER INTAKE • SIGNS/SYMPTOMS: MUSCULAR WEAKNESS, DIZZINESS, HEADACHE, HYPOTENSION, TACHYCARDIA, SHOCK, MENTAL CONFUSION, STUPOR, COMA • EXCESS = HYPERNATREMIA • DUE TO DEHYDRATION, WATER DEPRIVATION, EXCESSIVE SODIUM INTAKE • CAUSES HYPERTONICITY OF EXTRACELLULAR FLUIDS WHICH PULLS WATER OUT OF CELLS • SIGNS/SYMPTOMS: INTENSE THIRST, HYPERTENSION, EDEMA, AGITATION, CONVULSIONS

CHLORIDE • IMPORTANCE: • MAJOR EXTRACELLULAR ANION • HELPS REGULATE OSMOTIC PRESSURE BETWEEN COMPARTMENTS • FORMS HCL (HYDROCHLORIC ACID) IN THE STOMACH • PRIMARILY REGULATED BY THE HORMONES: • ALDOSTERONE: INCREASES BLOOD CHLORIDE LEVELS • ATRIAL NATRIURETIC PEPTIDE (ANP): DECREASES BLOOD CHLORIDE LEVELS

CHLORIDE • NORMAL VALUE: 95 -105 m. Eq/liter • DEFICIENCY = HYPOCHLOREMIA • DUE TO EXCESSIVE VOMITING, OVERHYDRATION, ALDOSTERONE DEFICIENCY, CONGESTIVE HEART FAILURE, THERAPY WITH CERTAIN DIURETICS SUCH AS LASIX • SIGNS/SYMPTOMS: MUSCLE SPASMS, METABOLIC ALKALOSIS, SHALLOW RESPIRATION, HYPOTENSION, TETANY • EXCESS = HYPERCHLOREMIA • DUE TO DEHYDRATION, EXCESSIVE CHLORIDE INTAKE, SEVERE RENAL FAILURE, HYPERALDOSTERONISM, CERTAIN TYPES OF ACIDOSIS, AND SOME DRUGS • SIGNS/SYMPTOMS: LETHARGY, WEAKNESS, METABOLIC ACIDOSIS, RAPID DEEP BREATHING

POTASSIUM • IMPORTANCE: • MOST ABUNDANT CATION IN INTRACELLULAR FLUID • INVOLVED IN FLUID VOLUME, IMPULSE CONDUCTION, MUSCLE CONTRACTION, AND REGULATING H P • POTASSIUM MOVE OPPOSITE OF SODIUM • PRIMARILY REGULATED BY THE HORMONES: • ALDOSTERONE: • ATRIAL NATRIURETIC PEPTIDE (ANP)

POTASSIUM • NORMAL VALUE: 3. 5 -5. 0 m. Eq/liter • DEFICIENCY = HYPOKALEMIA • DUE TO EXCESSIVE LOSS FROM VOMITING OR DIARRHEA, POTASSIUM INTAKE, HYPERALDOSTERONISM, KIDNEY DISEASE, THERAPY WITH SOME DIURETICS • SIGNS/SYMPTOMS: MUSCLE FATIGUE, FLACCID PARALYSIS, MENTAL CONFUSION, INCREASED URINE OUTPUT, SHALLOW RESPIRATION, CHANGES INECG (INCLUDING FLATTENING TOF WAVE) • EXCESS = HYPERKALEMIA • DUE TO EXCESSIVE POTASSIUM INTAKE, RENAL FAILURE, ALDOSTERONE DEFICIENCY, CRUSHING INJURIES TO BODY TISSUES, TRANSFUSION OF HEMOLYZED BLOOD • SIGNS/SYMPTOMS: IRRITABILITY, NAUSEA, VOMITING, DIARRHEA, MUSCULAR WEAKNESS, CAN CAUSE DEATH BY INDUCING VENTRICULAR FIBRILLATION

CALCIUM • IMPORTANCE: • MOST ABUNDANT MINERAL IN THE BODY, STRUCTURAL COMPONENT OF BONES AND TEETH • USED FOR BLOOD COAGULATION, NEUROTRANSMITTER RELEASE, MUSCLE TONE, EXCITABILITY OF NERVES AND MUSCLES • PRIMARILY REGULATED BY THE HORMONES: • PARATHYROID HORMONE (PTH): RAISES BLOOD CALCIUM LEVELS • INCREASED OSTEOCLAST ACTIVITY • INCREASED ABSORPTION IN THE INTESTINES • INCREASED REABSORPTION IN THE KIDNEYS • CALCITONIN: LOWERS BLOOD CALCIUM • DECREASED OSTEOCLAST ACTIVITY • DECREASED ABSORPTION IN THE INTESTINES • DECREASED REABSORPTION IN THE KIDNEYS

CALCIUM • NORMAL VALUE: TOTAL: 9. 0 -10. 5 m. Eq/liter / IONIZED: 4. 5 -5. 5 m. Eq/liter • DEFICIENCY = HYPOCALCEMIA • DUE TO INCREASED CALCIUM LOSS, REDUCED CALCIUM INTAKE, ELEVATED PHOSPHATE LEVELS OR HYPOPARATHYROIDISM • SIGNS/SYMPTOMS: NUMBNESS AND TINGLING OF FINGERS, HYPERACTIVE REFLEXES, MUSCLE CRAMPS, TETANY, CONVULSIONS, BONE FRACTURES, SPASMS OF LARYNGEAL MUSCLES THAT CAN CAUSE DEATH BY ASPHYXIATION • EXCESS = HYPERCALCEMIA • DUE TO HYPERPARATHYROIDISM, SOME CANCERS, EXCESSIVE INTAKE OF VITAMIND, PAGET’S DISEASE • SIGNS/SYMPTOMS: LETHARGY, WEAKNESS, ANOREXIA, NAUSEA, VOMITING, POLYURIA, ITCHING, BONE PAIN, DEPRESSION, CONFUSION, PARESTHESIA, STUPOR, COMA

PHOSPHATE • IMPORTANCE: • COMPONENT OF BONE AND TEETH, CALCIUM PHOSPHATE SALT • USED IN THE BUFFER SYSTEM • REGULATED OPPOSITE OFCALCIUM MOVEMENTS • PRIMARILY REGULATED BY THE HORMONES: • PARATHYROID HORMONE (PTH): DECREASES PLASMA PHOSPHATE CONCENTRATIONS • CALCITONIN: INCREASES PLASMA PHOSPHATE CONCENTRATIONS

PHOSPHATE • NORMAL VALUE: 1. 7 -2. 6 m. Eq/liter • DEFICIENCY = HYPOPHOSPHATEMIA • DUE TO INCREASED URINARY LOSSES, DECREASED INTESTINAL ABSORPTION, OR INCREASED UTILIZATION • SIGNS/SYMPTOMS: CONFUSION, SEIZURES, COMA, CHEST AND MUSCLE PAIN, NUMBNESS AND TINGLING OF FINGERS, DECREASED COORDINATION, MEMORY LOSS, LETHARGY • EXCESS = HYPERPHOSPHATEMIA • DUE TO INCREASED INTAKE OF PHOSPHATES OR DESTRUCTION OF BODY CELLS, OR WHEN KIDNEYS FAIL TO EXCRETE EXCESS PHOSPHATE AS IN RENAL FAILURE • SIGNS/SYMPTOMS: ANOREXIA, NAUSEA, VOMITING, MUSCULAR WEAKNESS, HYPERACTIVE REFLEXES, TETANY, AND TACHYCARDIA

MAGNESIUM • IMPORTANCE: • AN INTRACELLULAR CATION • ACTIVATES ENZYMES INVOLVED IN CARBOHYDRATE AND PROTEIN METABOLISM • USED IN MYOCARDIAL FUNCTION, TRANSMISSION IN THE CENTRAL NERVOUS SYSTEM, AND OPERATION OF THE SODIUM PUMP

MAGNESIUM • NORMAL VALUE: 1. 3 -2. 1 m. Eq/liter • DEFICIENCY = HYPOMAGNESEMIA • DUE TO INADEQUATE INTAKE OR EXCESSIVE LOSS IN URINE OR FECES, ALCOHOLISM, MALNUTRITION, DIABETES MELLITUS, DIURETIC THERAPY • SIGNS/SYMPTOMS: WEAKNESS, IRRITABILITY, TETANY, DELIRIUM, CONVULSIONS, CONFUSION, ANOREXIA, NAUSEA, VOMITING, PARESTHESIA, AND CARDIAC ARRHYTHMIAS • EXCESS = HYPERMAGNESEMIA • DUE TO RENAL FAILURE OR INCREASED INTAKE (SUCH AS ANTACIDS) OCCURS IN ALDOSTERONE DEFICIENCY AND HYPOTHYROIDISM • SIGNS/SYMPTOMS: HYPOTENSION, MUSCULAR WEAKNESS OR PARALYSIS, NAUSEA, VOMITING, ALTERED MENTAL FUNCTIONING

BICARBONATE • IMPORTANCE: • MAJOR MEMBER OF THE PLASMA ACID-BASE BUFFER SYSTEM • KIDNEYS REABSORB OR SECRETE IT FOR FINAL ACID-BASE BALANCE
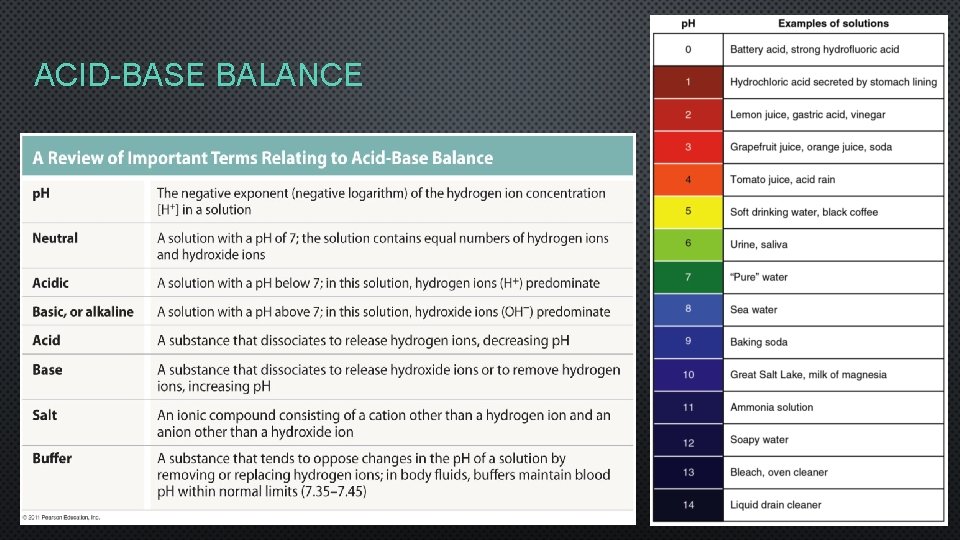
ACID-BASE BALANCE
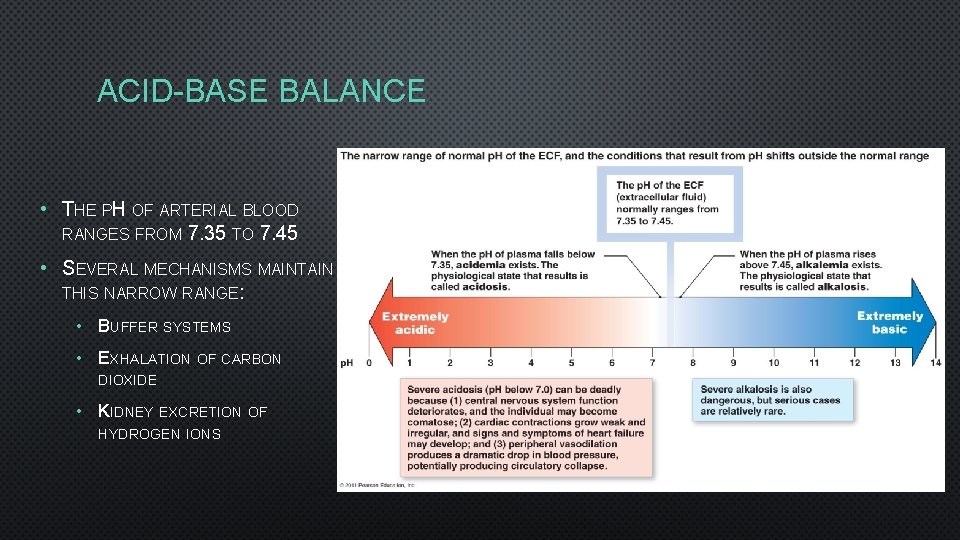
ACID-BASE BALANCE • THE PH OF ARTERIAL BLOOD RANGES FROM 7. 35 TO 7. 45 • SEVERAL MECHANISMS MAINTAIN THIS NARROW RANGE: • BUFFER SYSTEMS • EXHALATION OF CARBON DIOXIDE • KIDNEY EXCRETION OF HYDROGEN IONS

ACID-BASE BALANCE • STRONG ACID: COMPLETELY IONIZES (DISSOCIATES), LIKE HYDROCHLORIC ACID • WEAK ACID: PARTIALLY IONIZES LEAVING MUCH ACID STILL IN SOLUTION, LIKE CARBONIC ACID (H 2 CO 3)
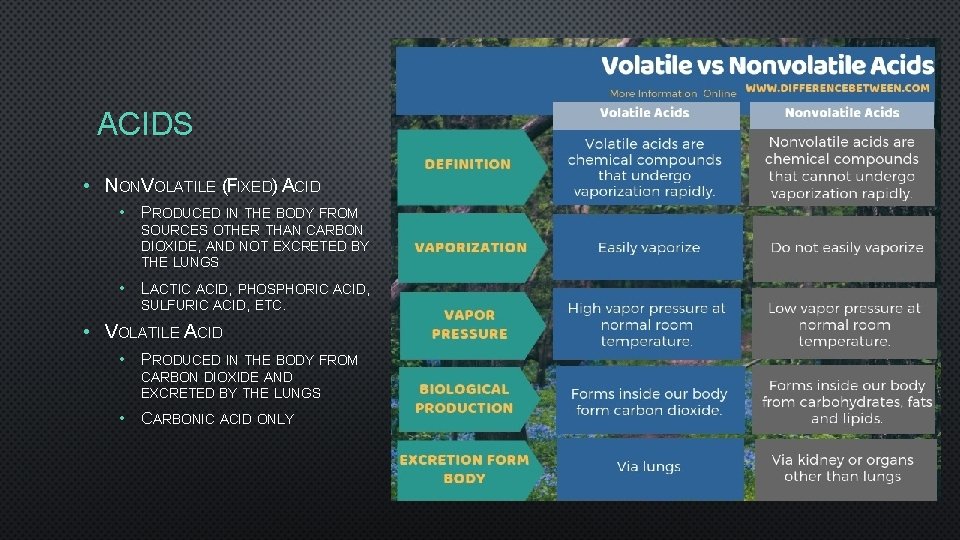
ACIDS • NONVOLATILE (FIXED) ACID • PRODUCED IN THE BODY FROM SOURCES OTHER THAN CARBON DIOXIDE, AND NOT EXCRETED BY THE LUNGS • LACTIC ACID, PHOSPHORIC ACID, SULFURIC ACID, ETC. • VOLATILE ACID • PRODUCED IN THE BODY FROM CARBON DIOXIDE AND EXCRETED BY THE LUNGS • CARBONIC ACID ONLY
THREE MAJOR CHEMICAL BUFFER SYSTEMS • PHOSPHATE BUFFER SYSTEM: • IMPORTANT ROLE IN BUFFERING THE PH OFICF AND URINE • PROTEIN BUFFER SYSTEMS: • USED IN BUFFERING PH IN BOTHECF AND ICF, INTERACTS WITH OTHER 2 SYSTEMS • INCLUDES HEMOGLOBIN (RBCS), AMINO ACID BUFFERS (ALL PROTEINS), ANDPLASMA PROTEIN BUFFERS • CARBONIC ACID-BICARBONATE BUFFER SYSTEM: • MOST IMPORTANT IN ECF
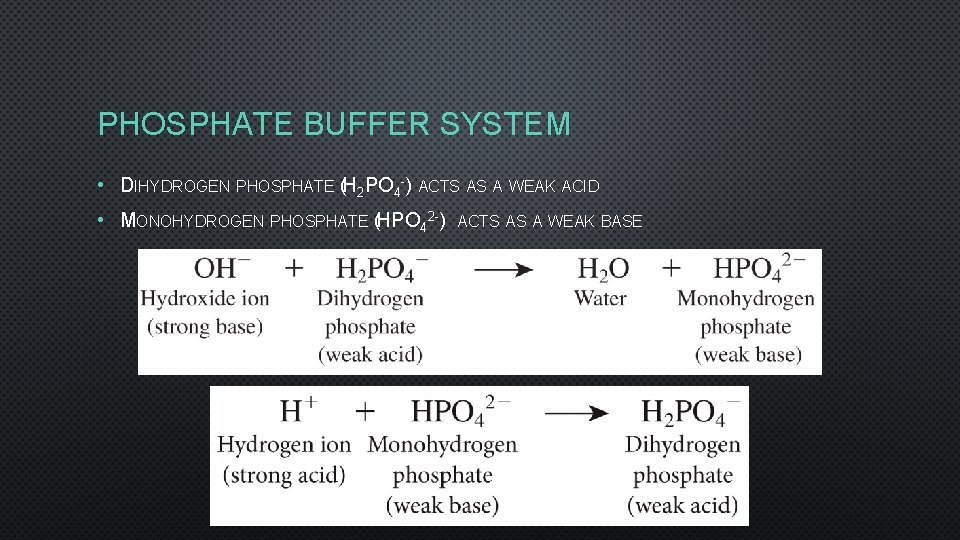
PHOSPHATE BUFFER SYSTEM • DIHYDROGEN PHOSPHATE (H 2 PO 4 -) ACTS AS A WEAK ACID • MONOHYDROGEN PHOSPHATE (HPO 42 -) ACTS AS A WEAK BASE
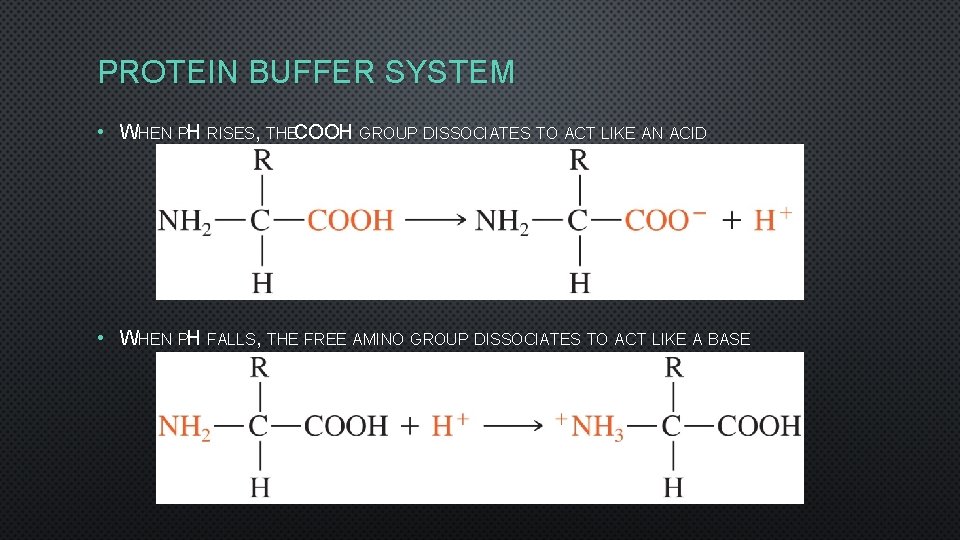
PROTEIN BUFFER SYSTEM • WHEN PH RISES, THECOOH GROUP DISSOCIATES TO ACT LIKE AN ACID • WHEN PH FALLS, THE FREE AMINO GROUP DISSOCIATES TO ACT LIKE A BASE
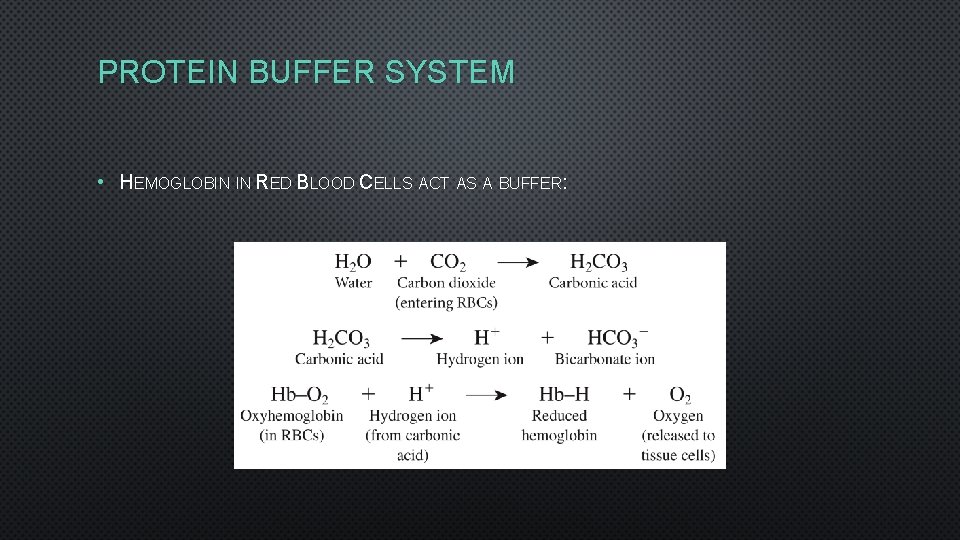
PROTEIN BUFFER SYSTEM • HEMOGLOBIN IN RED BLOOD CELLS ACT AS A BUFFER:
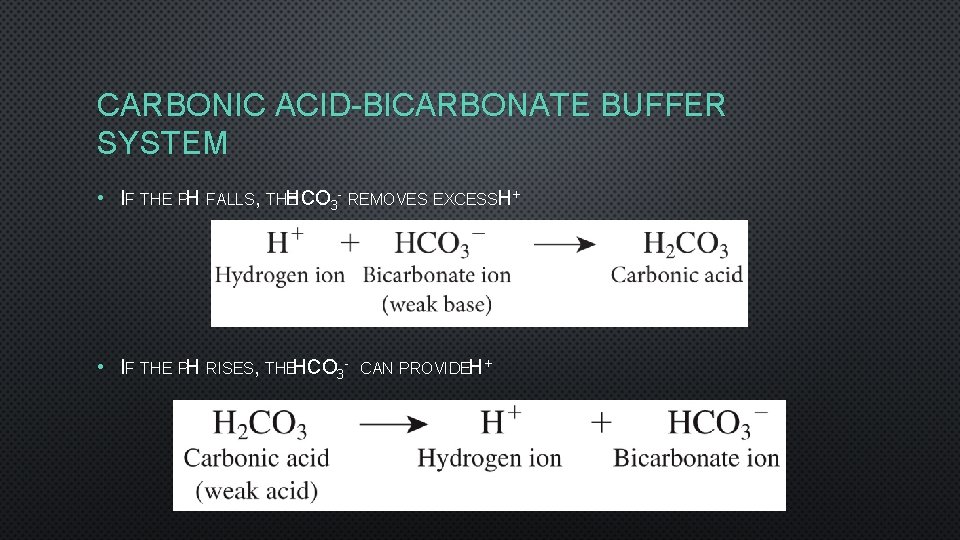
CARBONIC ACID-BICARBONATE BUFFER SYSTEM • IF THE PH FALLS, THE HCO 3 - REMOVES EXCESSH+ • IF THE PH RISES, THEHCO 3 - CAN PROVIDEH+
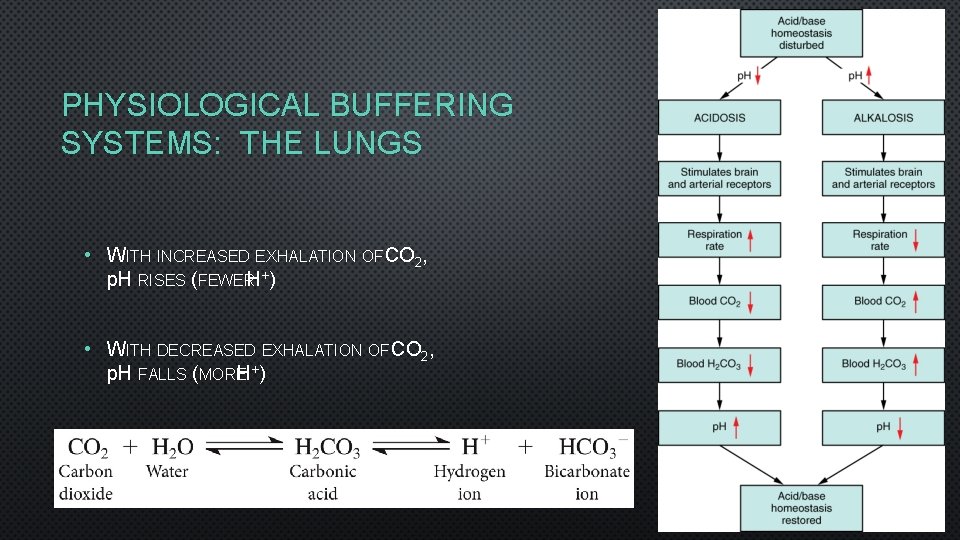
PHYSIOLOGICAL BUFFERING SYSTEMS: THE LUNGS • WITH INCREASED EXHALATION OF CO 2, p. H RISES (FEWERH+) • WITH DECREASED EXHALATION OF CO 2, p. H FALLS (MOREH+)

PHYSIOLOGICAL BUFFERING SYSTEM: THE KIDNEYS • EXCRETING H+ IN THE URINE REMOVES NONVOLATILE ACIDS • THE PROXIMAL CONVOLUTED TUBULES AND COLLECTING DUCTS SECRETE H+ INTO THE TUBULAR FLUID
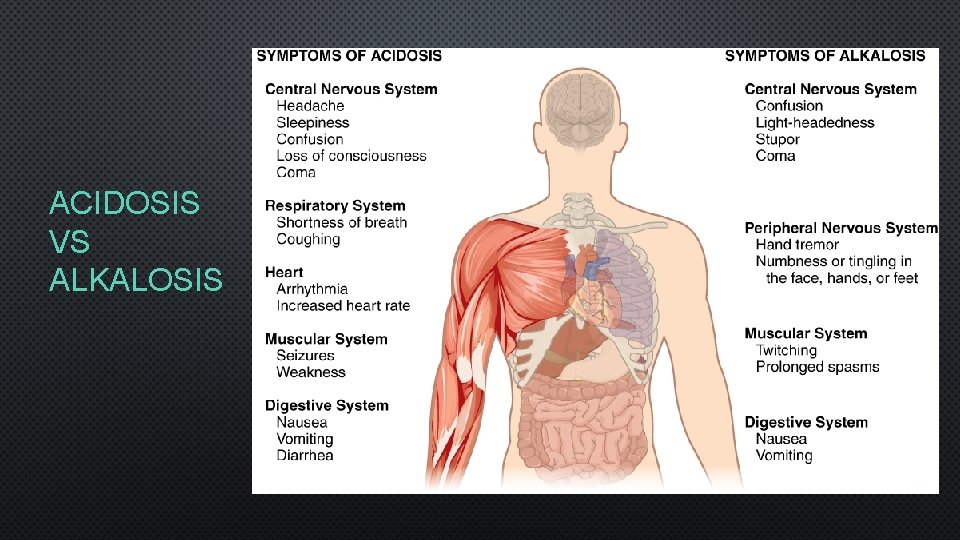
ACIDOSIS VS ALKALOSIS

METABOLIC ACIDOSIS/ALKALOSIS • METABOLIC ACIDOSIS • DECREASED HCO 3 - → INCREASED H+ → DECREASED PH • CAUSES: DIARRHEA, KETOSIS, RENAL DYSFUNCTION • COMPENSATIONS: HYPERVENTILATION AND KIDNEY FUNCTION • METABOLIC ALKALOSIS • INCREASED HCO 3 - → DECREASED H+ → INCREASED PH • CAUSES: VOMITING, DIURETICS, ALKALINE DRUG USE • COMPENSATIONS: HYPOVENTILATION AND KIDNEY FUNCTION

RESPIRATORY ACIDOSIS/ALKALOSIS • RESPIRATORY ACIDOSIS • INCREASED CO 2 → INCREASED H+ → DECREASED PH • CAUSES: HYPOVENTILATION • COMPENSATIONS: KIDNEY FUNCTION → INCREASED EXCRETION OFH+ OR BY INCREASED REABSORPTION OF HCO 3 - • RESPIRATORY ALKALOSIS • DECREASED CO 2 → DECREASED H+ → INCREASED PH • CAUSES: HYPERVENTILATION • COMPENSATIONS: KIDNEY FUNCTION → DECREASED EXCRETION OFH+ OR BY DECREASED REABSORPTION OF HCO 3 -
- Slides: 40