Chapter 24 Problem 20 A standard solution containing
Chapter 24, Problem 20 A standard solution containing 5. 2 x 10 -8 M iodoacetone and 1. 8 x 10 -7 M p-dichlorobenzene (an internal standard) gave peak areas of 397 and 837, respectively, in a gas chromatogram. A 3. 00 m. L unknown solution of iodoacetone was treated with 0. 100 m. L of 9. 0 x 10 -6 M p-dichlorobenzene and the mixture was diluted to 10. 00 m. L. Gas chromatography gave peak areas of 1135 and 357 for iodoacetone and p-dichlorobenzene, respectively. Find the concentration of iodoacetone in the 3. 00 m. L of original unknown. This problem requires knowledge of Gas Chromatography. If you haven’t read Chapter 24, now would be a good time. Oregon State University Chemistry 324 Chapter 24 Problem 20 Page 1

Do You Remember Doing Extractions In Organic Lab? • Compounds will partition (distribute) themselves between polar and non-polar solvents • Compounds with mostly polar character will be found mostly in the polar solvent (water) • Compounds with mostly non-polar character will be found mostly in the non-polar solvent (chloroform) Differences in solubility can be used to physically separate two compounds Oregon State University Chemistry 324 Chapter 24 Problem 20 Page 2
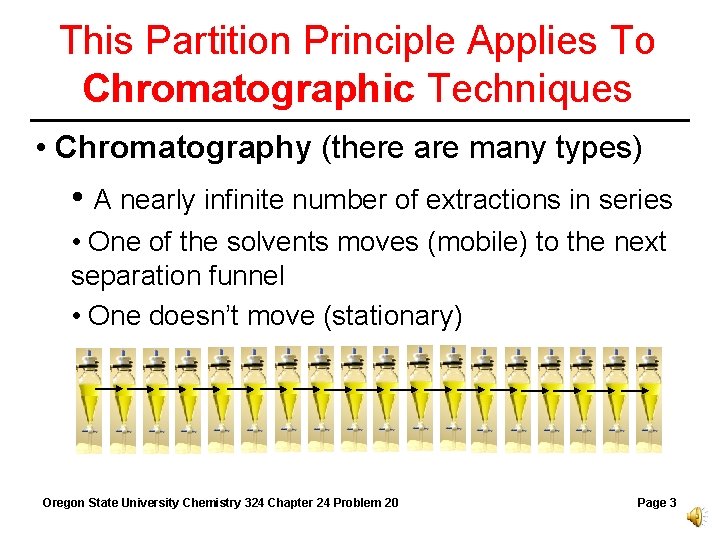
This Partition Principle Applies To Chromatographic Techniques • Chromatography (there are many types) • A nearly infinite number of extractions in series • One of the solvents moves (mobile) to the next separation funnel • One doesn’t move (stationary) Oregon State University Chemistry 324 Chapter 24 Problem 20 Page 3
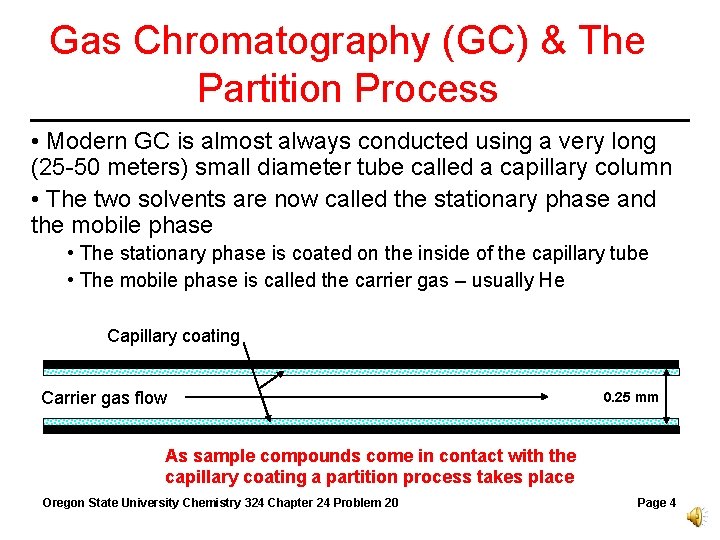
Gas Chromatography (GC) & The Partition Process • Modern GC is almost always conducted using a very long (25 -50 meters) small diameter tube called a capillary column • The two solvents are now called the stationary phase and the mobile phase • The stationary phase is coated on the inside of the capillary tube • The mobile phase is called the carrier gas – usually He Capillary coating Carrier gas flow 0. 25 mm As sample compounds come in contact with the capillary coating a partition process takes place Oregon State University Chemistry 324 Chapter 24 Problem 20 Page 4
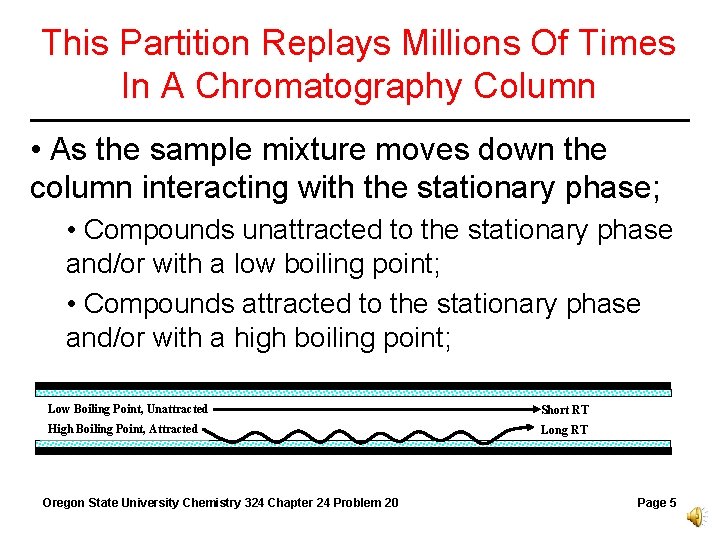
This Partition Replays Millions Of Times In A Chromatography Column • As the sample mixture moves down the column interacting with the stationary phase; • Compounds unattracted to the stationary phase and/or with a low boiling point; • Compounds attracted to the stationary phase and/or with a high boiling point; Low Boiling Point, Unattracted Short RT High Boiling Point, Attracted Long RT Oregon State University Chemistry 324 Chapter 24 Problem 20 Page 5
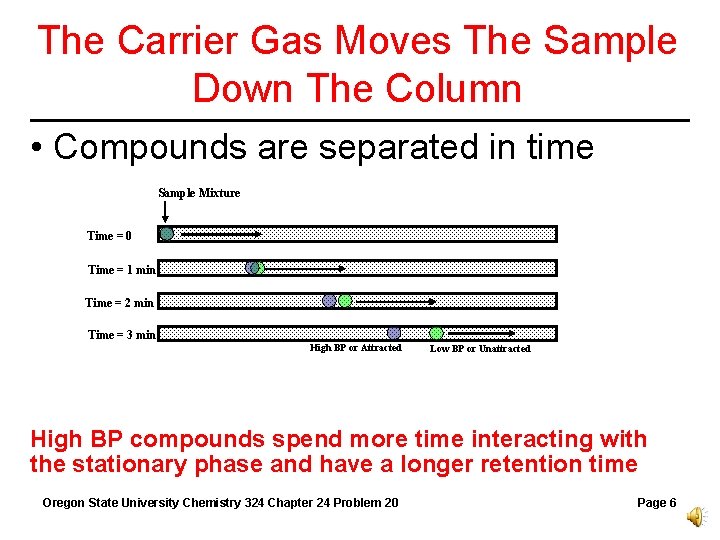
The Carrier Gas Moves The Sample Down The Column • Compounds are separated in time Sample Mixture Time = 0 Time = 1 min Time = 2 min Time = 3 min High BP or Attracted Low BP or Unattracted High BP compounds spend more time interacting with the stationary phase and have a longer retention time Oregon State University Chemistry 324 Chapter 24 Problem 20 Page 6
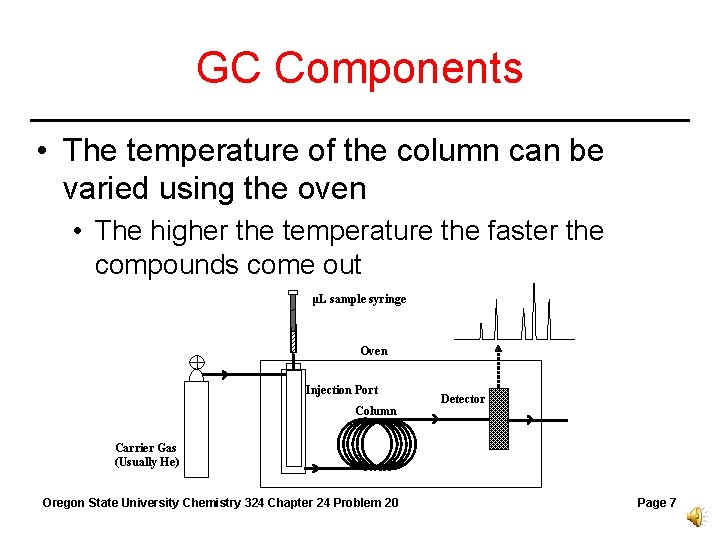
GC Components • The temperature of the column can be varied using the oven • The higher the temperature the faster the compounds come out μL sample syringe Oven Injection Port Column Detector Carrier Gas (Usually He) Oregon State University Chemistry 324 Chapter 24 Problem 20 Page 7
Back To The Problem A standard solution containing 5. 2 x 10 -8 M iodoacetone and 1. 8 x 10 -7 M p-dichlorobenzene (an internal standard) gave peak areas of 397 and 837, respectively, in a gas chromatogram. • What’s an internal standard? • And why do we need one? Many (most) analytical techniques have different sensitivities for different compounds Oregon State University Chemistry 324 Chapter 24 Problem 20 Page 8
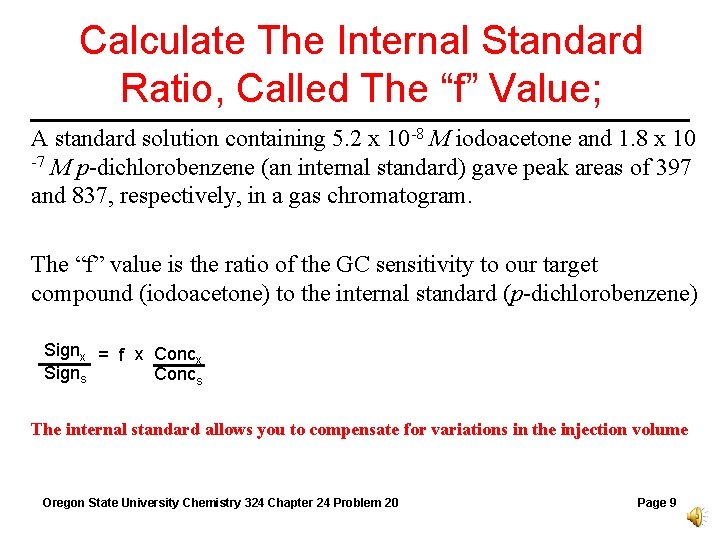
Calculate The Internal Standard Ratio, Called The “f” Value; A standard solution containing 5. 2 x 10 -8 M iodoacetone and 1. 8 x 10 -7 M p-dichlorobenzene (an internal standard) gave peak areas of 397 and 837, respectively, in a gas chromatogram. The “f” value is the ratio of the GC sensitivity to our target compound (iodoacetone) to the internal standard (p-dichlorobenzene) Signx = f x Concx Signs Concs The internal standard allows you to compensate for variations in the injection volume Oregon State University Chemistry 324 Chapter 24 Problem 20 Page 9
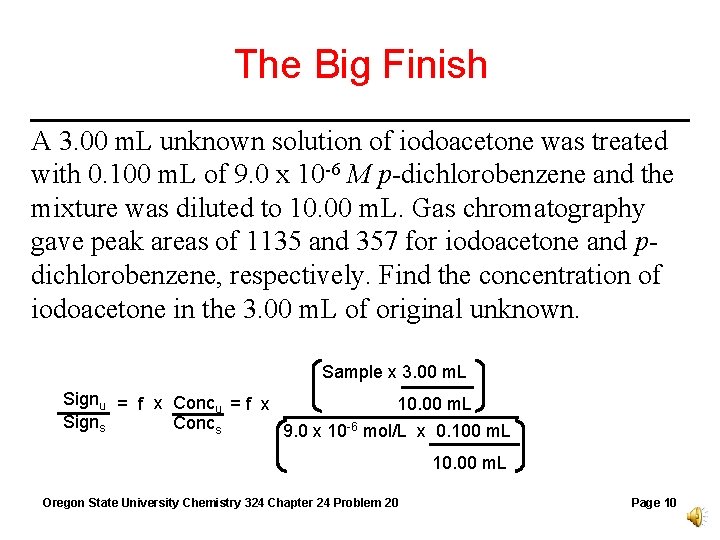
The Big Finish A 3. 00 m. L unknown solution of iodoacetone was treated with 0. 100 m. L of 9. 0 x 10 -6 M p-dichlorobenzene and the mixture was diluted to 10. 00 m. L. Gas chromatography gave peak areas of 1135 and 357 for iodoacetone and pdichlorobenzene, respectively. Find the concentration of iodoacetone in the 3. 00 m. L of original unknown. Sample x 3. 00 m. L Signu = f x Concu = f x 10. 00 m. L Signs Concs 9. 0 x 10 -6 mol/L x 0. 100 m. L 10. 00 m. L Oregon State University Chemistry 324 Chapter 24 Problem 20 Page 10
- Slides: 10