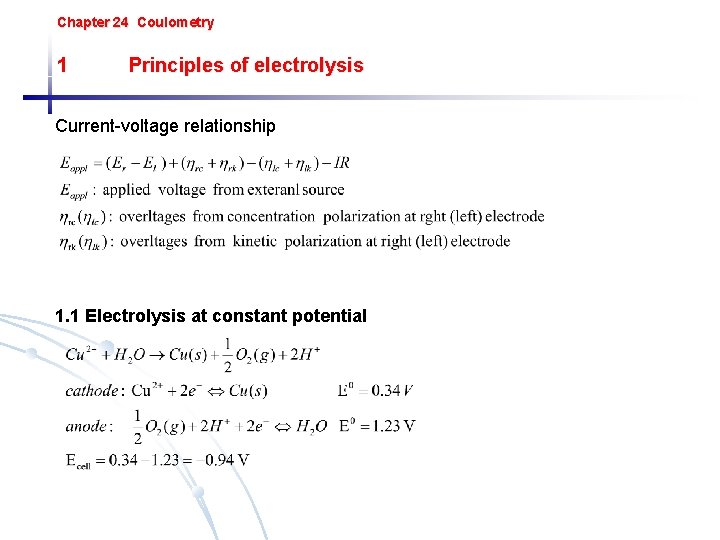
Chapter 24 Coulometry 1 Principles of electrolysis Currentvoltage
![Containing more than one metal ions Example: [Cu 2+] = 1. 0 M, [Ag+] Containing more than one metal ions Example: [Cu 2+] = 1. 0 M, [Ag+]](https://slidetodoc.com/presentation_image_h2/f8af3c7d91a90dc1639cca448039d25f/image-3.jpg)
![Notes: 1) For {univalent} [M+] decreases from 0. 1 M to 10 -6 M, Notes: 1) For {univalent} [M+] decreases from 0. 1 M to 10 -6 M,](https://slidetodoc.com/presentation_image_h2/f8af3c7d91a90dc1639cca448039d25f/image-4.jpg)


- Slides: 11

Chapter 24 Coulometry 1 Principles of electrolysis Current-voltage relationship 1. 1 Electrolysis at constant potential

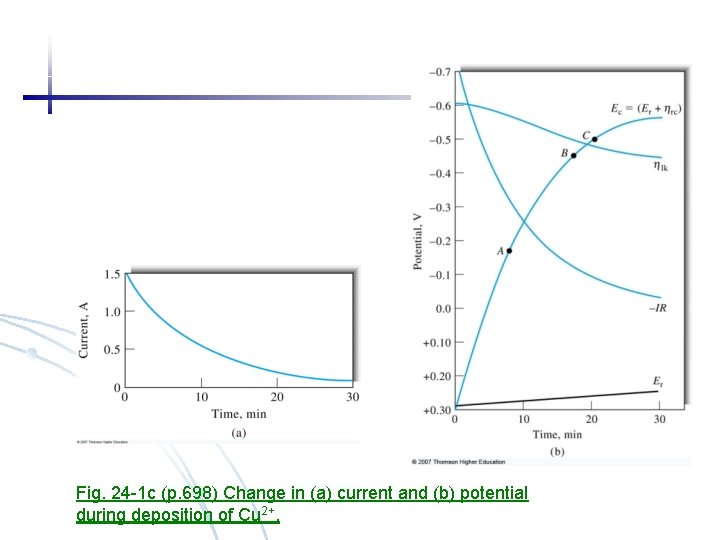
Fig. 24 -1 c (p. 698) Change in (a) current and (b) potential during deposition of Cu 2+.
![Containing more than one metal ions Example Cu 2 1 0 M Ag Containing more than one metal ions Example: [Cu 2+] = 1. 0 M, [Ag+]](https://slidetodoc.com/presentation_image_h2/f8af3c7d91a90dc1639cca448039d25f/image-3.jpg)
Containing more than one metal ions Example: [Cu 2+] = 1. 0 M, [Ag+] = 0. 1 M in 0. 05 M H 2 SO 4
![Notes 1 For univalent M decreases from 0 1 M to 10 6 M Notes: 1) For {univalent} [M+] decreases from 0. 1 M to 10 -6 M,](https://slidetodoc.com/presentation_image_h2/f8af3c7d91a90dc1639cca448039d25f/image-4.jpg)
Notes: 1) For {univalent} [M+] decreases from 0. 1 M to 10 -6 M, Er changes by =0. 30 V -Separation requires 0. 30 -V difference 2) For {divalent} [M 2+] decreases from 0. 1 M to 10 -6 M, Er changes by 0. 0592/2 log(1/10 -6)-0. 0592/2 log(1/0. 1)=0. 15 V -Selectivity: separate any elements doesn’t deposit within this 0. 15 V potential range; 3) For {trivalent} [M 3+] decreases from 0. 1 M to 10 -6 M, Er changes by 0. 10 V

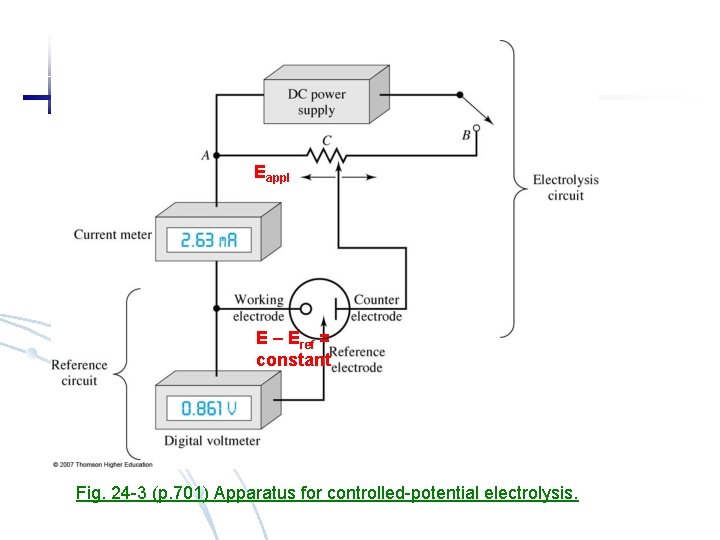
4) In electrolysis, even Eappl holds constant, both Er and El are changing, and I decreases because of concentration polarization. result in crude separation. Controlled potential electrolysis: measure the potential of working electrode against a reference electrode whose potential is known and holds constant. Eappl is adjusted such the potential difference between working electrode and references hold constant. Working electrode: place where redox occurs Reference electrode: constant potential reference Counter electrode: inert material (Hg, Pt) plays no part in redox but completes circuit Supporting electrolyte: alkali metal salt does not react with electrodes but has conductivity

Eappl E – Eref = constant Fig. 24 -3 (p. 701) Apparatus for controlled-potential electrolysis.

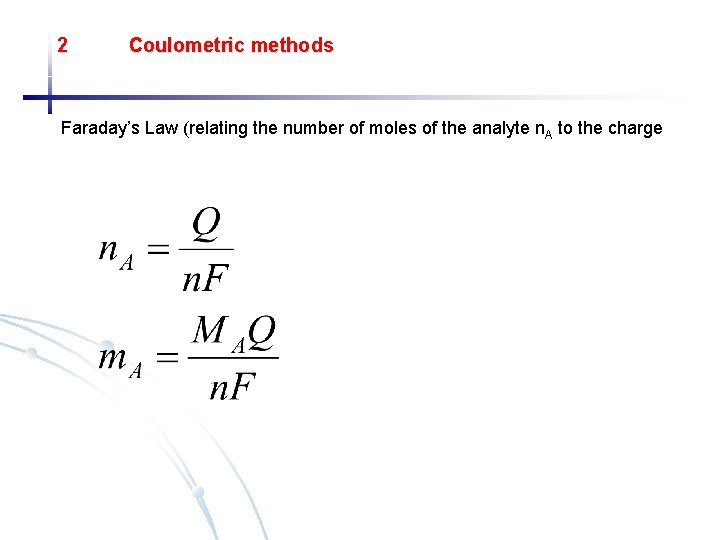
2 Coulometric methods Faraday’s Law (relating the number of moles of the analyte n. A to the charge

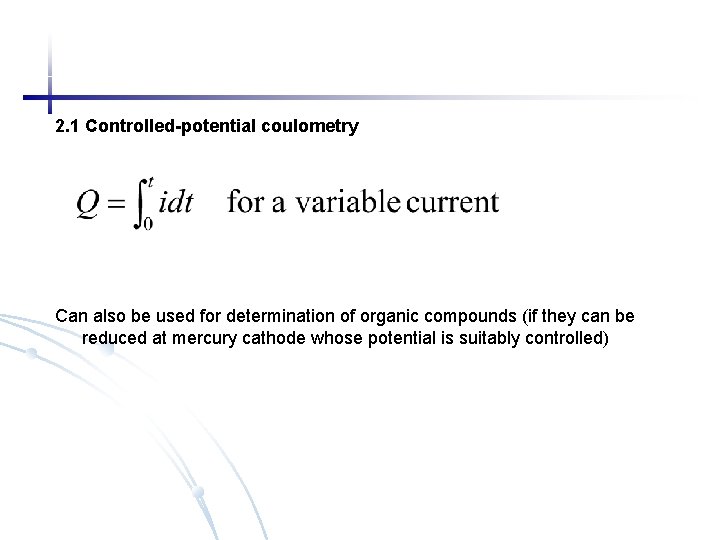
2. 1 Controlled-potential coulometry Can also be used for determination of organic compounds (if they can be reduced at mercury cathode whose potential is suitably controlled)

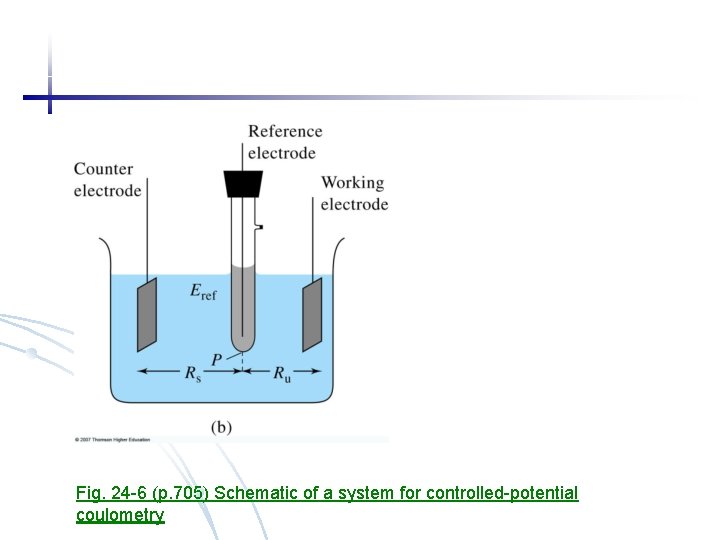
Fig. 24 -6 (p. 705) Schematic of a system for controlled-potential coulometry

2. 2 Coulometric titration – controlled current coulometry Notes: 1. current efficiency = 100% 2. need a end-point detection (color changes, potentiometric, photometric measurement) Karl Fisher determination of water 2 I- I 2 + 2 e. I 2 + SO 2 + 2 H 2 O 2 HI + H 2 SO 4 2 HI + H 2 O + SO 2 + 3 C 5 H 5 N 2(C 5 H 5 N+H)I- + C 5 H 5 N. SO 3 + CH 3 OH (C 5 H 5 N+H)O. SO 2. OCH 3

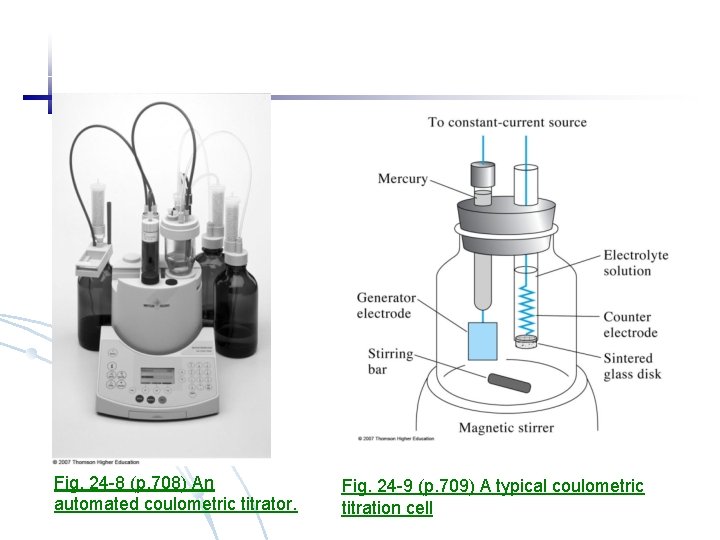
Fig. 24 -8 (p. 708) An automated coulometric titrator. Fig. 24 -9 (p. 709) A typical coulometric titration cell