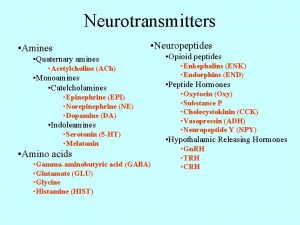
Chapter 23 The Chemistry of Amines Amines 2


- Slides: 30

Chapter 23 The Chemistry of Amines

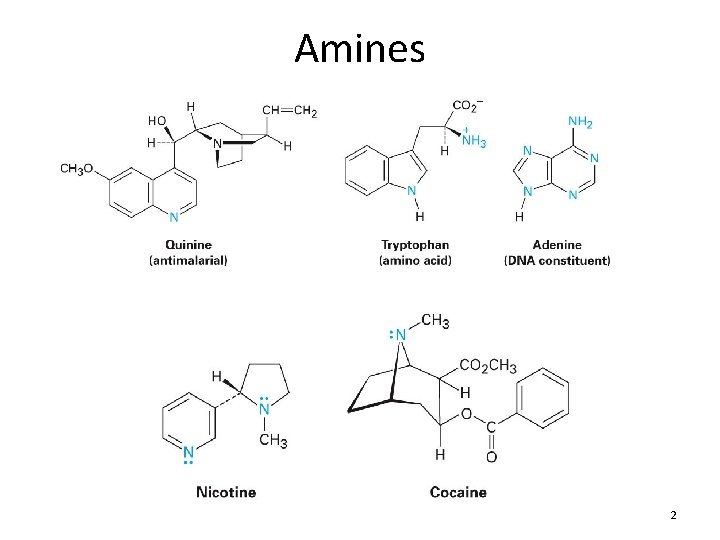
Amines 2

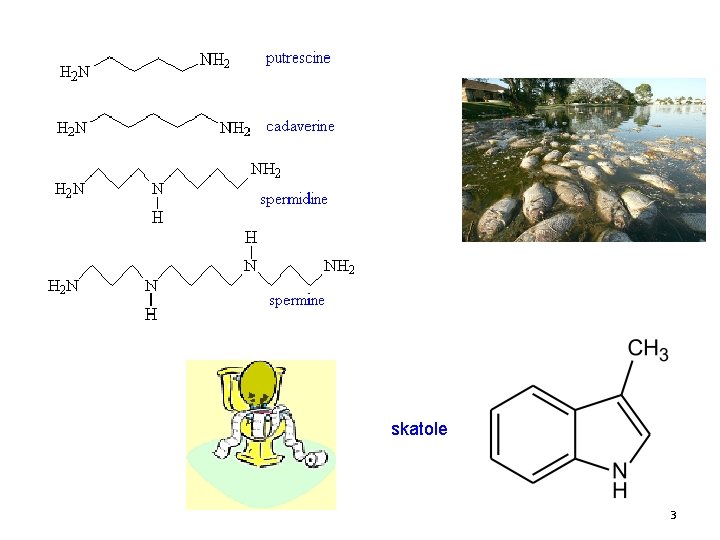
skatole 3

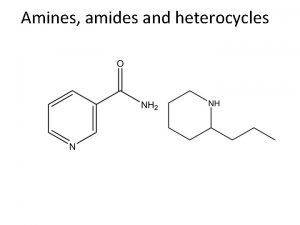
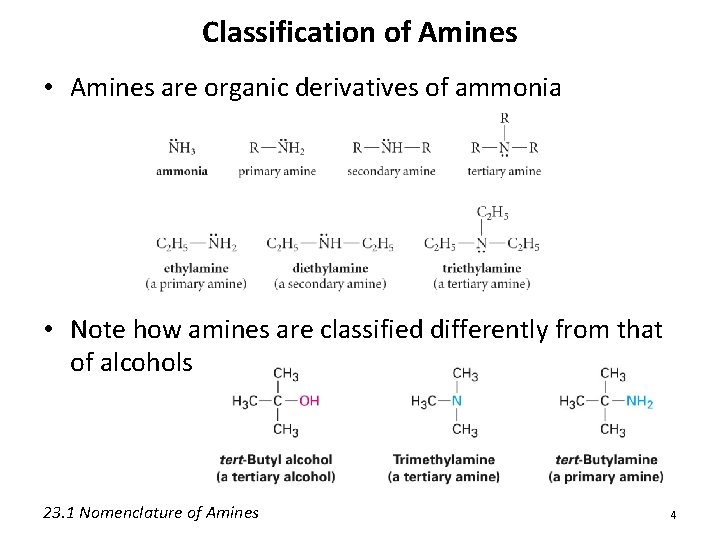
Classification of Amines • Amines are organic derivatives of ammonia • Note how amines are classified differently from that of alcohols 23. 1 Nomenclature of Amines 4

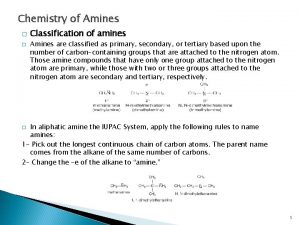
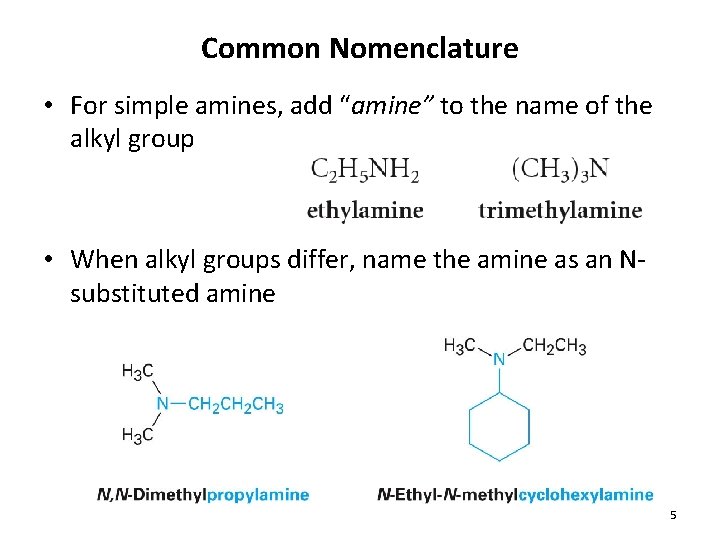
Common Nomenclature • For simple amines, add “amine” to the name of the alkyl group • When alkyl groups differ, name the amine as an Nsubstituted amine 5

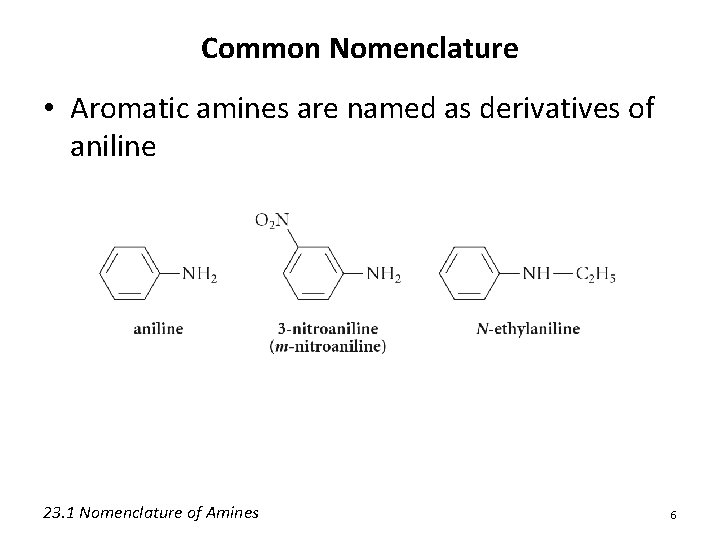
Common Nomenclature • Aromatic amines are named as derivatives of aniline 23. 1 Nomenclature of Amines 6

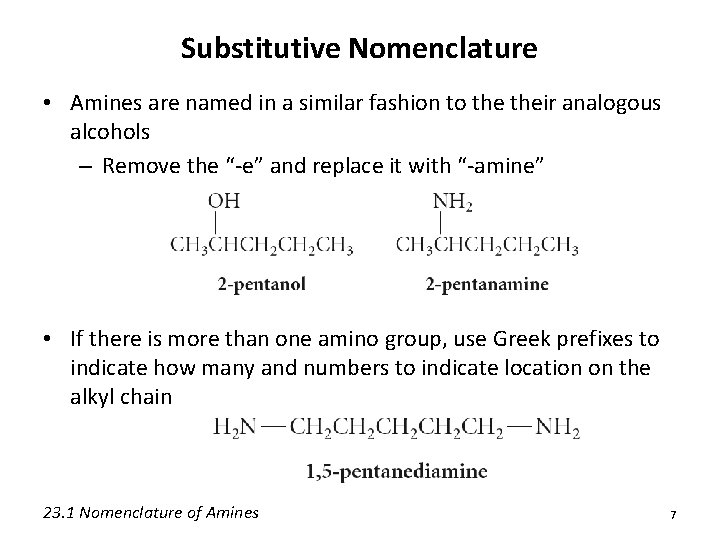
Substitutive Nomenclature • Amines are named in a similar fashion to their analogous alcohols – Remove the “-e” and replace it with “-amine” • If there is more than one amino group, use Greek prefixes to indicate how many and numbers to indicate location on the alkyl chain 23. 1 Nomenclature of Amines 7

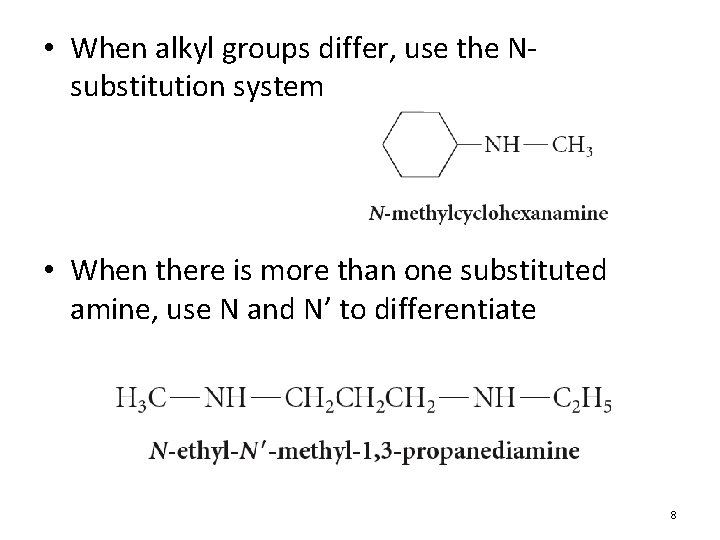
• When alkyl groups differ, use the Nsubstitution system • When there is more than one substituted amine, use N and N’ to differentiate 8

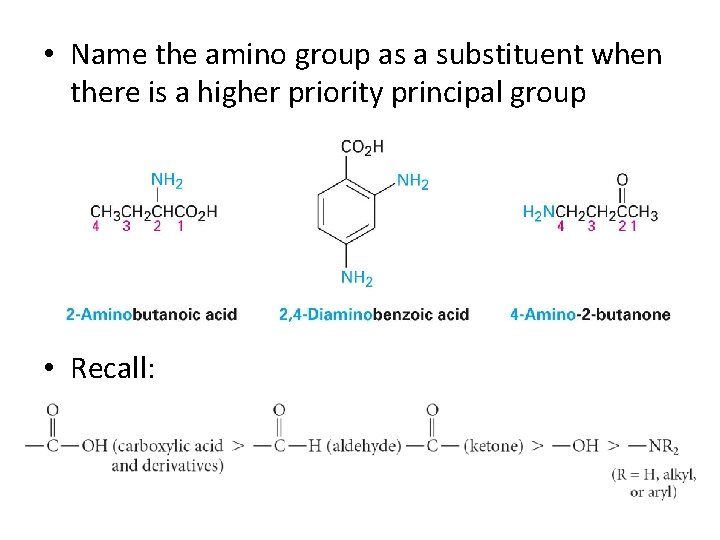
• Name the amino group as a substituent when there is a higher priority principal group • Recall:

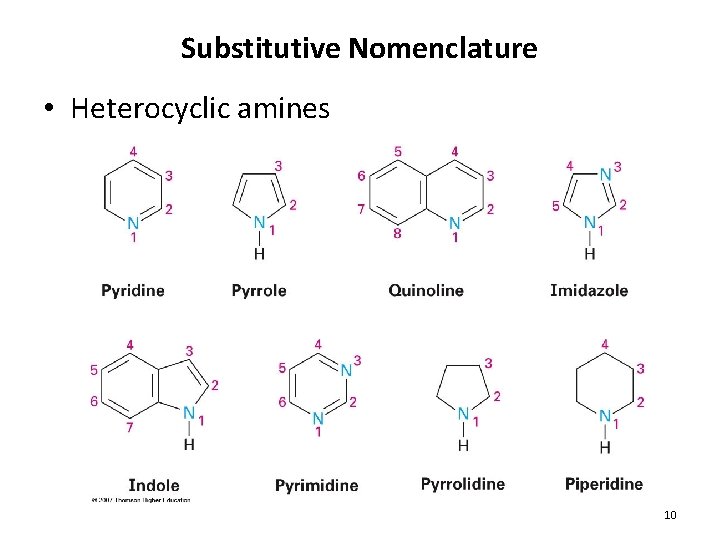
Substitutive Nomenclature • Heterocyclic amines 10

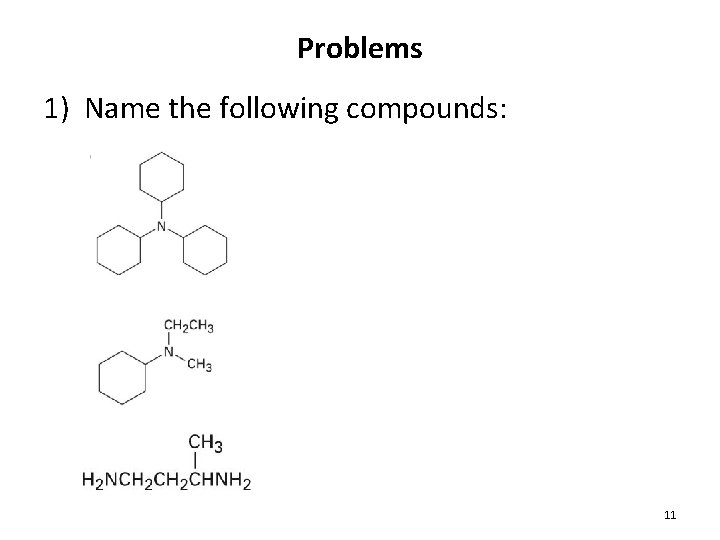
Problems 1) Name the following compounds: 11

2) Draw the following molecules • 2 -propen-1 -amine • N-methyl-N’-propyl-1, 4 -butanediamine • p-aminophenol 12

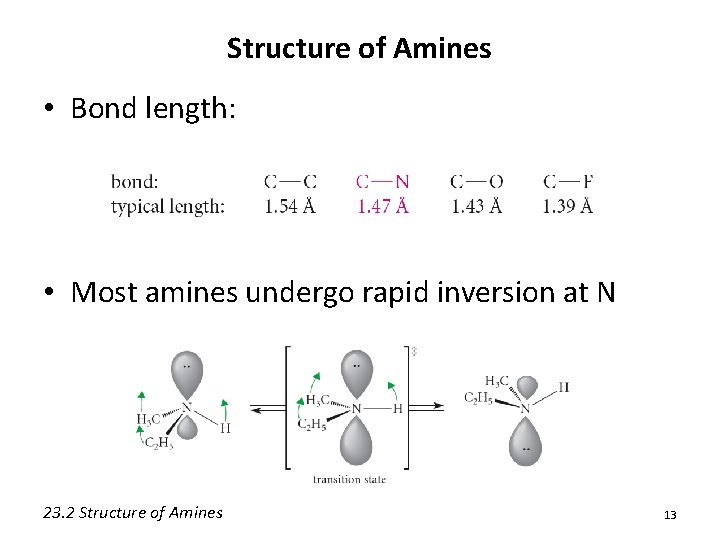
Structure of Amines • Bond length: • Most amines undergo rapid inversion at N 23. 2 Structure of Amines 13

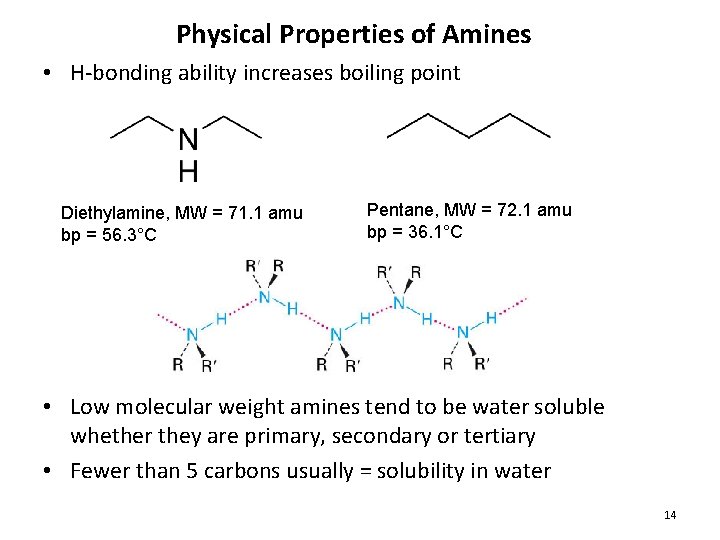
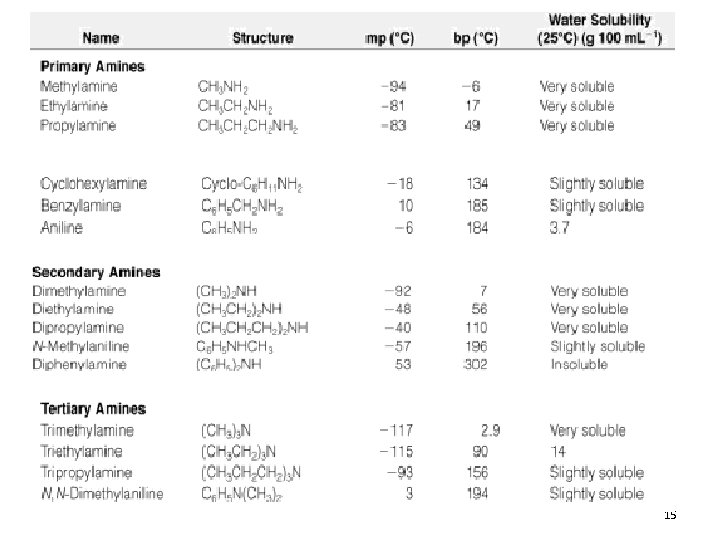
Physical Properties of Amines • H-bonding ability increases boiling point Diethylamine, MW = 71. 1 amu bp = 56. 3°C Pentane, MW = 72. 1 amu bp = 36. 1°C • Low molecular weight amines tend to be water soluble whether they are primary, secondary or tertiary • Fewer than 5 carbons usually = solubility in water 14

15

Basicity of Amines • Amines react with acids to form ammonium salts 16

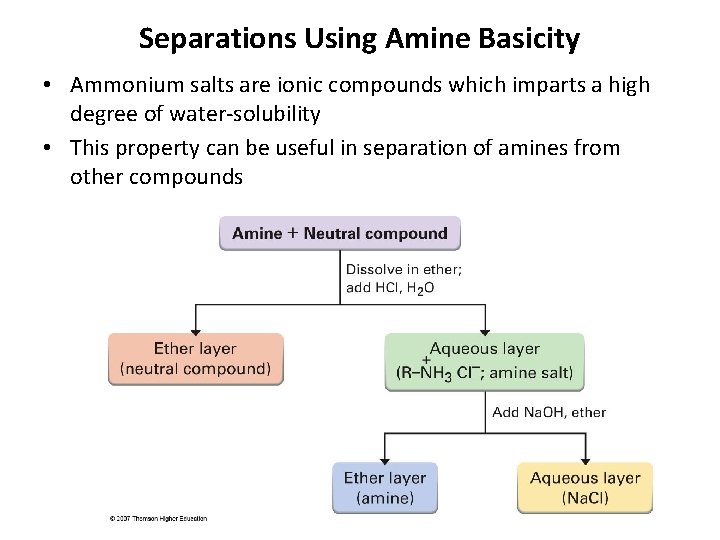
Separations Using Amine Basicity • Ammonium salts are ionic compounds which imparts a high degree of water-solubility • This property can be useful in separation of amines from other compounds

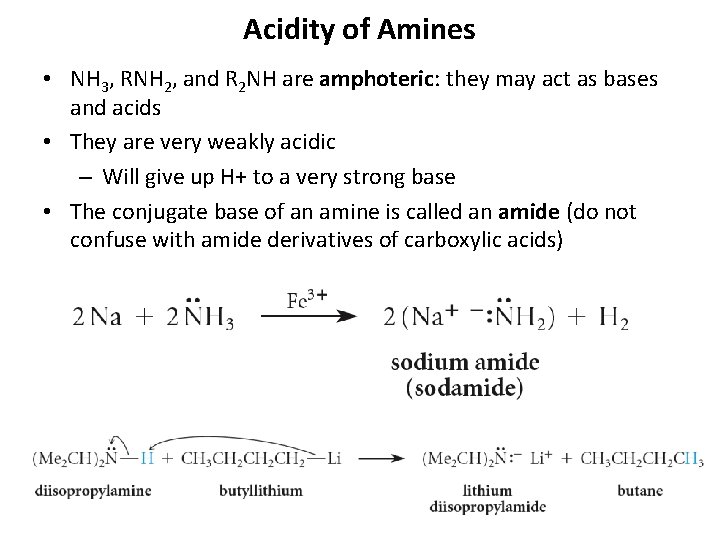
Acidity of Amines • NH 3, RNH 2, and R 2 NH are amphoteric: they may act as bases and acids • They are very weakly acidic – Will give up H+ to a very strong base • The conjugate base of an amine is called an amide (do not confuse with amide derivatives of carboxylic acids) 18

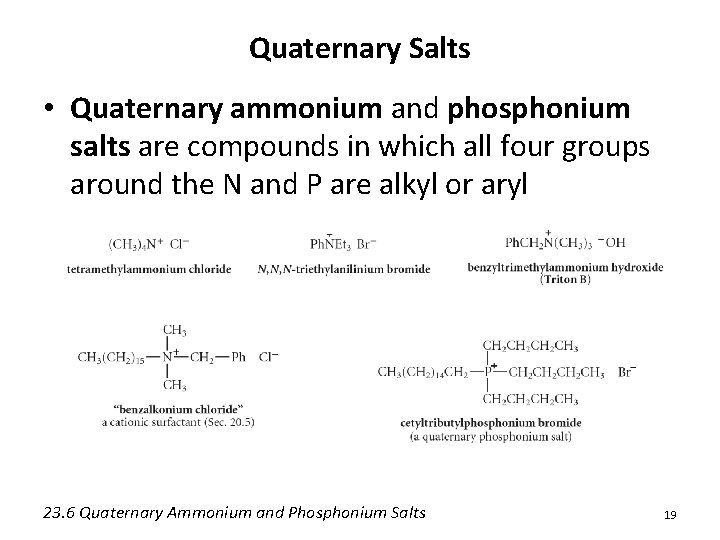
Quaternary Salts • Quaternary ammonium and phosphonium salts are compounds in which all four groups around the N and P are alkyl or aryl 23. 6 Quaternary Ammonium and Phosphonium Salts 19

Phase-Transfer Catalysis 20

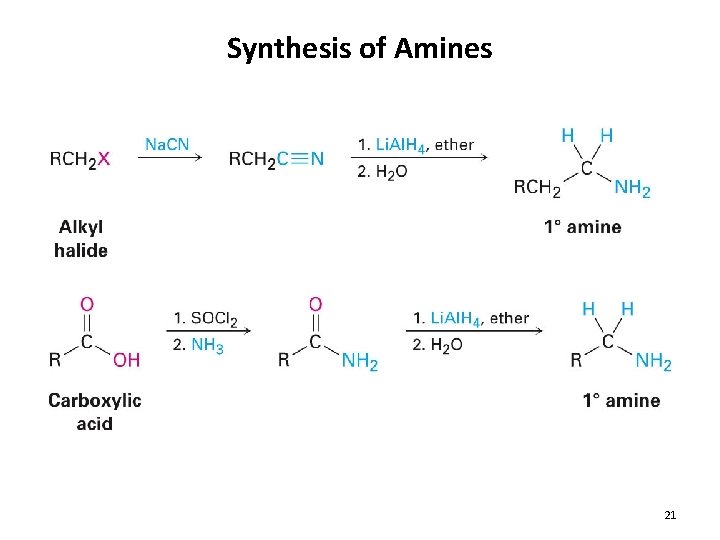
Synthesis of Amines 21

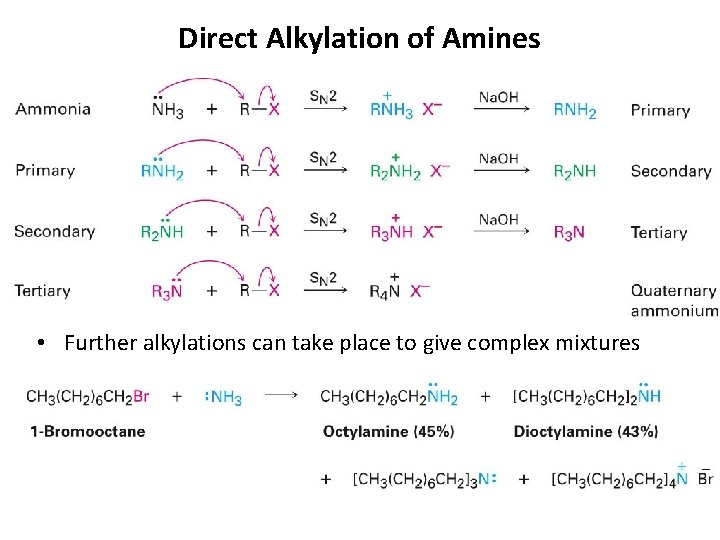
Direct Alkylation of Amines • Further alkylations can take place to give complex mixtures

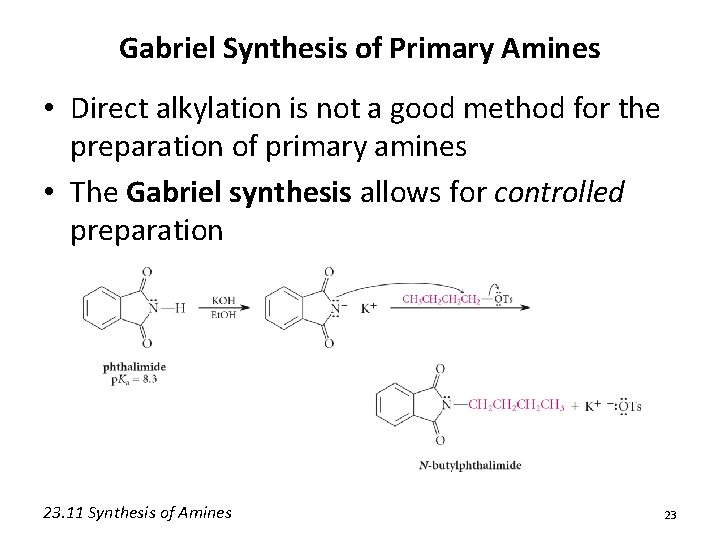
Gabriel Synthesis of Primary Amines • Direct alkylation is not a good method for the preparation of primary amines • The Gabriel synthesis allows for controlled preparation 23. 11 Synthesis of Amines 23

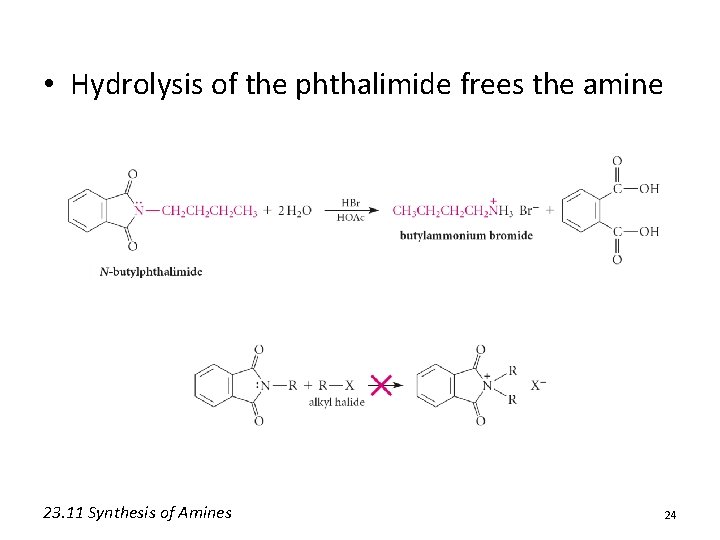
• Hydrolysis of the phthalimide frees the amine 23. 11 Synthesis of Amines 24

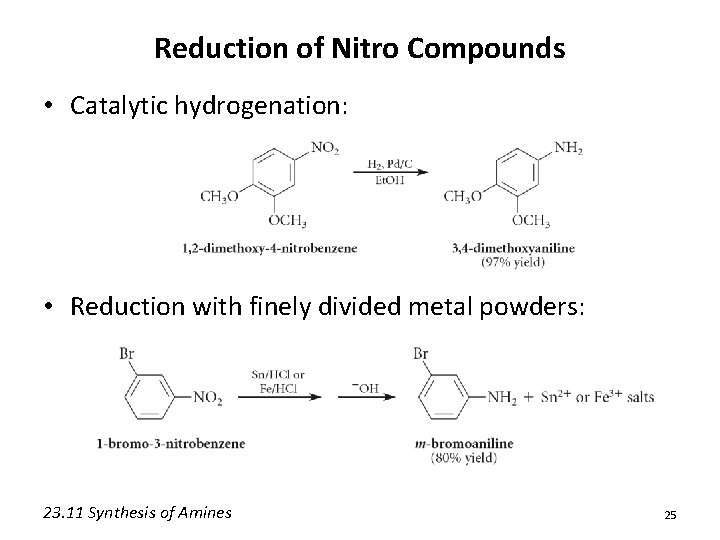
Reduction of Nitro Compounds • Catalytic hydrogenation: • Reduction with finely divided metal powders: 23. 11 Synthesis of Amines 25

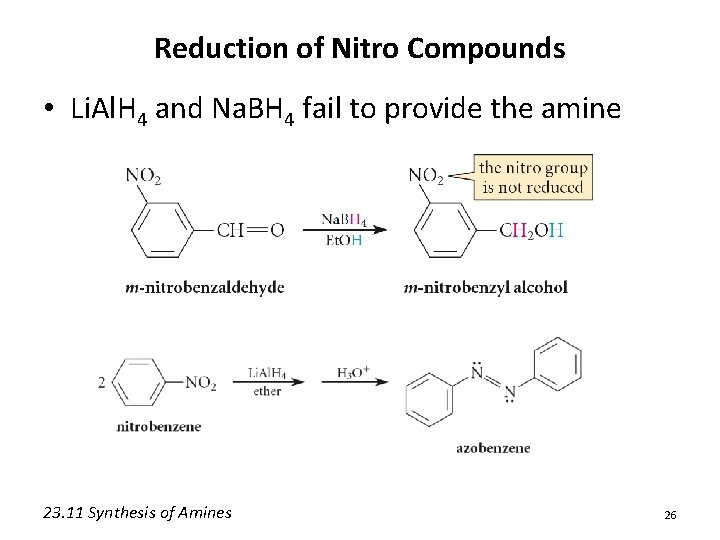
Reduction of Nitro Compounds • Li. Al. H 4 and Na. BH 4 fail to provide the amine 23. 11 Synthesis of Amines 26

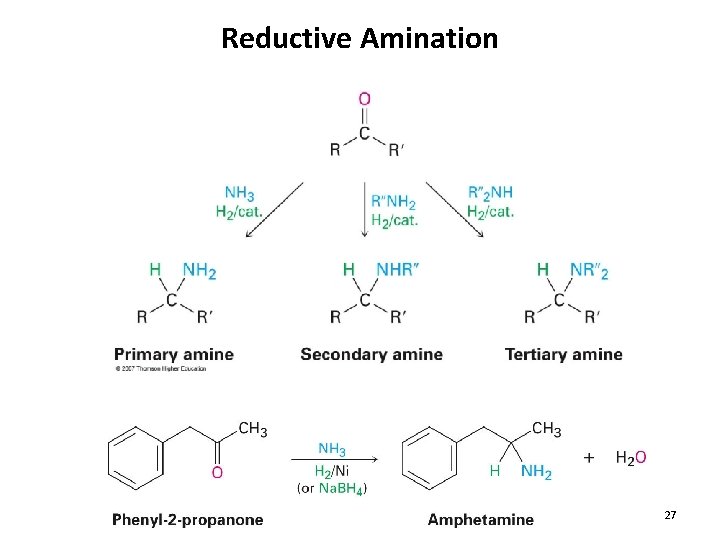
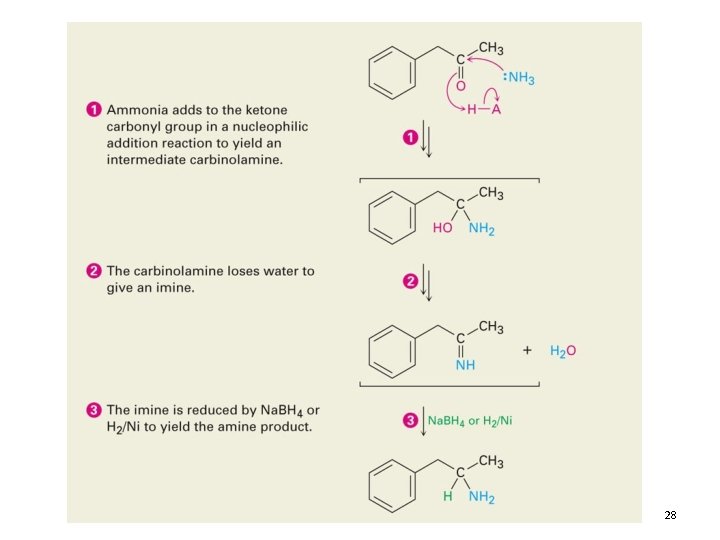
Reductive Amination 27

28

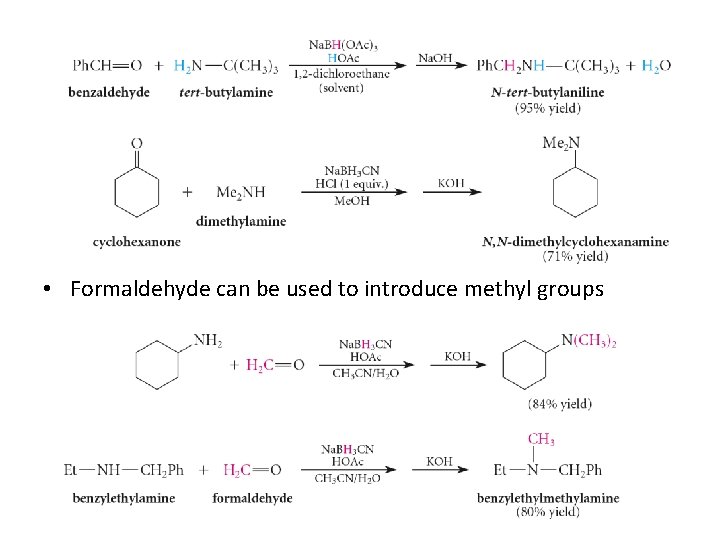
• Formaldehyde can be used to introduce methyl groups

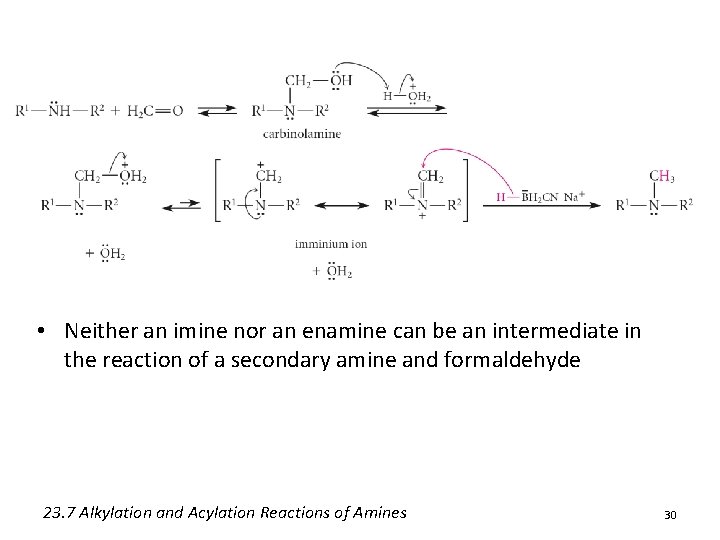
• Neither an imine nor an enamine can be an intermediate in the reaction of a secondary amine and formaldehyde 23. 7 Alkylation and Acylation Reactions of Amines 30
 Amines chemsheets
Amines chemsheets Aromatic amines structure
Aromatic amines structure Guanidine basicity
Guanidine basicity Vital+ amine
Vital+ amine Physical properties of amines
Physical properties of amines Naming ammonium salts
Naming ammonium salts Base conjuguée
Base conjuguée Les drogues vasoactives
Les drogues vasoactives Naming amine
Naming amine Amines geometry
Amines geometry Amines hydrogen bonding
Amines hydrogen bonding Carboxylic acid + amine
Carboxylic acid + amine Chromotagraphie
Chromotagraphie Tertiary amine structure
Tertiary amine structure O ch
O ch Amine soluble in water
Amine soluble in water Solubility of amines
Solubility of amines Basicity amines
Basicity amines Do aromatic amines give hinsberg test
Do aromatic amines give hinsberg test Aniline reacts with bromine water at room temperature
Aniline reacts with bromine water at room temperature Basic character of amines
Basic character of amines Chronotropic effect
Chronotropic effect Ib organic chemistry functional groups
Ib organic chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Thang điểm glasgow
Thang điểm glasgow Hát lên người ơi
Hát lên người ơi