Chapter 23 Enteral and Parenteral Nutrition Support Enteral






















![Adjusted Body Weight Adjusted IBW for obesity Female: ([actual weight – IBW] x 0. Adjusted Body Weight Adjusted IBW for obesity Female: ([actual weight – IBW] x 0.](https://slidetodoc.com/presentation_image_h2/7d6185ea81302ba73b8dddf0173d5436/image-46.jpg)







- Slides: 67

Chapter 23 Enteral and Parenteral Nutrition Support

Enteral & parenteral nutrition © 2004, 2002 Elsevier Inc. All rights reserved.

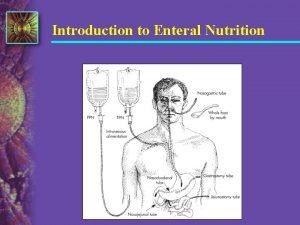
Enteral Nutrition Definition n Nutritional support via placement through the nose, esophagus, stomach, or intestines (duodenum or jejunum) —Tube feedings —Must have functioning GI tract —IF THE GUT WORKS, USE IT! —Exhaust all oral diet methods first. © 2004, 2002 Elsevier Inc. All rights reserved.

Oral Supplements n Between meals n Added to foods n Added into liquids for medication pass by nursing n Enhances otherwise poor intake n May be needed by children or teens to support growth © 2004, 2002 Elsevier Inc. All rights reserved.

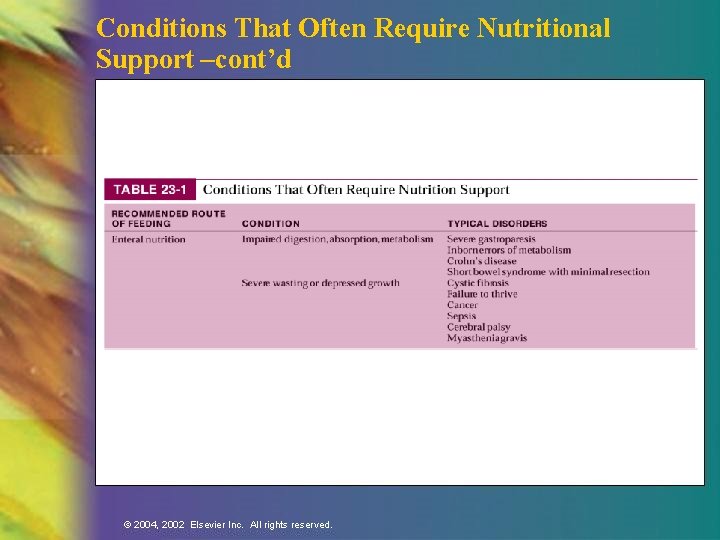
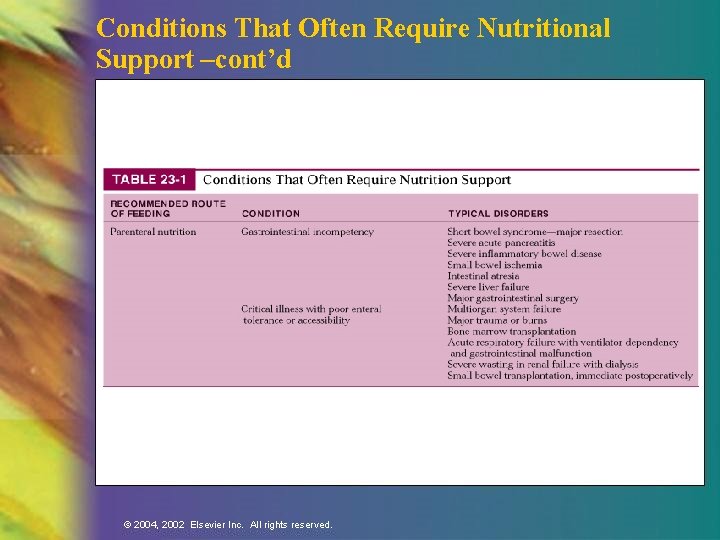
Conditions That Require Other Nutrition Support n Enteral —Impaired ingestion n —Inability to consume adequate nutrition orally —Impaired digestion, absorption, metabolism —Severe wasting or depressed growth Parenteral —Gastrointestinal incompetency —Hypermetabolic state with poor enteral tolerance or accessibility © 2004, 2002 Elsevier Inc. All rights reserved.

Conditions That Often Require Nutritional Support © 2004, 2002 Elsevier Inc. All rights reserved.

Conditions That Often Require Nutritional Support –cont’d © 2004, 2002 Elsevier Inc. All rights reserved.

Conditions That Often Require Nutritional Support –cont’d © 2004, 2002 Elsevier Inc. All rights reserved.

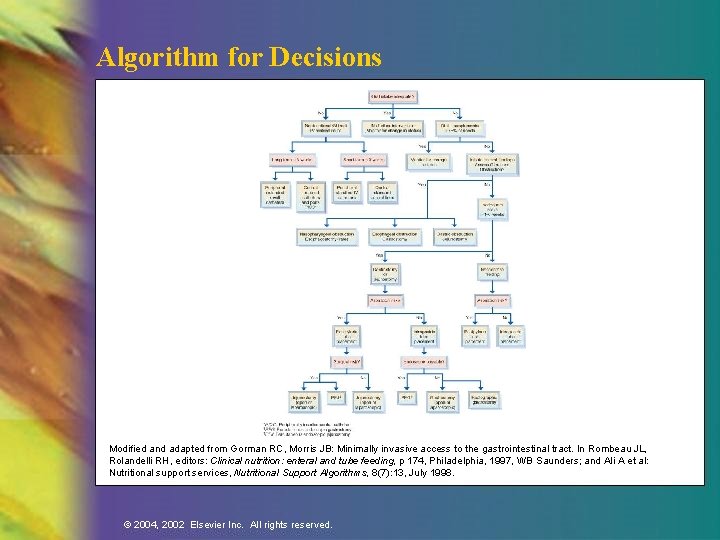
Algorithm for Decisions Modified and adapted from Gorman RC, Morris JB: Minimally invasive access to the gastrointestinal tract. In Rombeau JL, Rolandelli RH, editors: Clinical nutrition: enteral and tube feeding, p 174, Philadelphia, 1997, WB Saunders; and Ali A et al: Nutritional support services, Nutritional Support Algorithms, 8(7): 13, July 1998. © 2004, 2002 Elsevier Inc. All rights reserved.

Considerations in Enteral Nutrition 1. Applicable 2. Site placement 3. Formula selection 4. Nutritional/medical requirements 5. Rate and method of delivery 6. Tolerance © 2004, 2002 Elsevier Inc. All rights reserved.

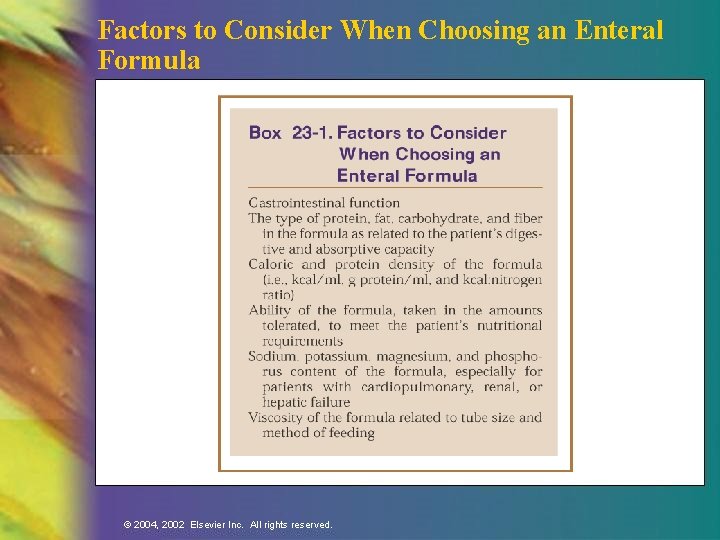
Formula Selection The suitability of a feeding formula should be evaluated based on n Functional status of GI tract n Physical characteristics of formula (osmolality, fiber content, caloric density, viscosity) n Macronutrient ratios n Digestion and absorption capability of patient n Specific metabolic needs n Contribution of the feeding to fluid and electrolyte needs or restriction n Cost effectiveness © 2004, 2002 Elsevier Inc. All rights reserved.

Enteral Formula Categories © 2004, 2002 Elsevier Inc. All rights reserved.

Factors to Consider When Choosing an Enteral Formula © 2004, 2002 Elsevier Inc. All rights reserved.

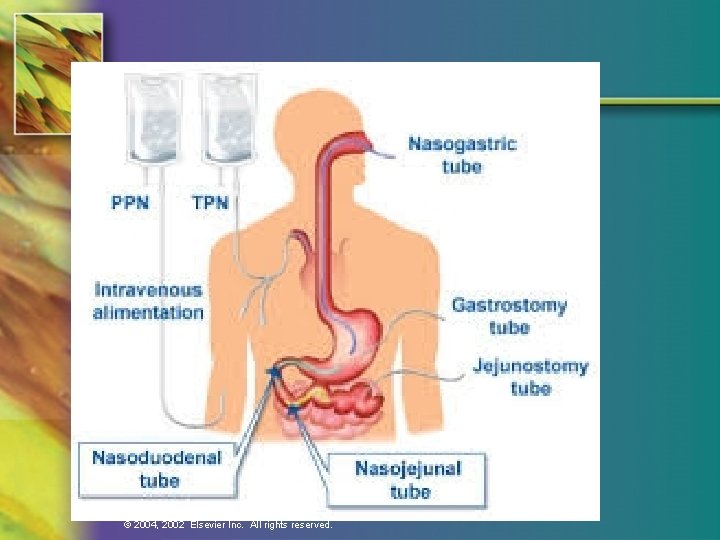
Enteral Access: Clinical Considerations n Duration of tube feeding —Nasogastric or nasoenteric tube for short term —Gastrostomy and jejunostomy tubes for long term n Placement of tube —Gastric —Small bowel © 2004, 2002 Elsevier Inc. All rights reserved.

Placement Site n Access (medical status) n Location (radiographic confirmation) n Duration n Tube measurements and durability n Adequacy of GI functioning © 2004, 2002 Elsevier Inc. All rights reserved.

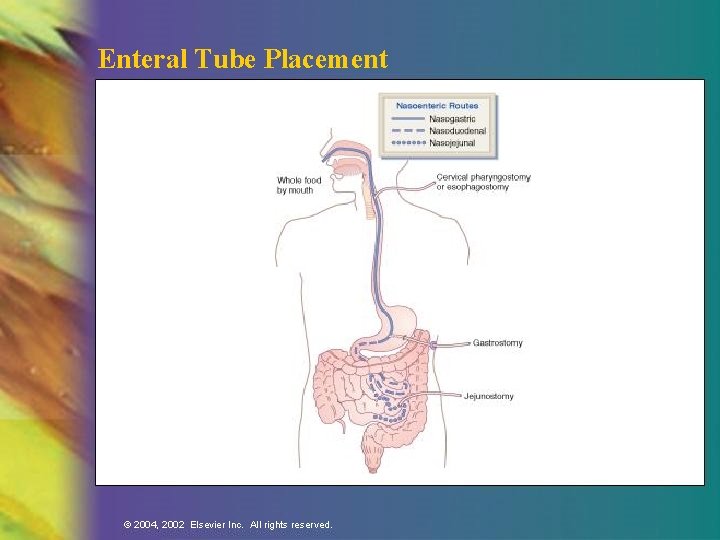
Enteral Tube Placement © 2004, 2002 Elsevier Inc. All rights reserved.

Advantages—Enteral Nutrition n Intake easily/accurately monitored n Provides nutrition when oral is not possible or adequate n Costs less than parenteral nutrition n Supplies readily available n Reduces risks associated with disease state © 2004, 2002 Elsevier Inc. All rights reserved.

More Advantages— Enteral Nutrition n Preserves gut integrity n Decreases likelihood of bacterial translocation n Preserves immunologic function of gut n Increased compliance with intake © 2004, 2002 Elsevier Inc. All rights reserved.

Disadvantages—Enteral Nutrition n GI, metabolic, and mechanical complications —tube migration; increased risk of bacterial contamination; tube obstruction; pneumothorax n Costs more than oral diets n Less “palatable/normal” n Labor-intensive assessment, administration, tube patency and site care, monitoring © 2004, 2002 Elsevier Inc. All rights reserved.

Complications of Enteral Feeding n Access problems (tube obstruction) n Administration problems (aspiration) n Gastrointestinal complications (diarrhea) n Metabolic complications (overhydration) © 2004, 2002 Elsevier Inc. All rights reserved.

Aspiration Pneumonia n Can result from enteral feeds n High-risk patients —Poor gag reflex —Depressed mental status © 2004, 2002 Elsevier Inc. All rights reserved.

Reducing Risk of Aspiration n Check gastric residuals if receiving gastric feeds n Elevate head of the bed >30 degrees during feedings n Postpyloric feeding —Nasoenteric tube placement may require fluoroscopic visualization or endoscopic guidance —Transgastric jejunostomy tube © 2004, 2002 Elsevier Inc. All rights reserved.

Rate and Method of Delivery* n Bolus— 300 to 400 ml rapid delivery via syringe several times daily n Intermittent─300 to 400 ml, 20 to 30 minutes, several times/day via gravity drip or syringe n Cyclic—via pump usually at night n Continuous—via gravity drip or infusion pump *Determined by medical status, feeding route and volume, and nutritional goals © 2004, 2002 Elsevier Inc. All rights reserved.

Consideration of Physical Properties of Enteral Formulas n Residue n Viscosity —Size of tube is important n Osmolality: consider protein source —Intact (do not affect osmolality)—soy isolates; sodium or calcium casein; lactalbumin —Hydrolyzed (more particles)—peptides or free amino acids © 2004, 2002 Elsevier Inc. All rights reserved.

Renal Solute Load n Normal adult tolerance is 1200 to 1400 m. Osm/L n Infants and renal patients may tolerate less © 2004, 2002 Elsevier Inc. All rights reserved.

Lower Osmolality n Large (intact) proteins n Large starch molecules © 2004, 2002 Elsevier Inc. All rights reserved.

Higher Osmolality n Hydrolyzed protein or amino acids n Disaccharides © 2004, 2002 Elsevier Inc. All rights reserved.

Tolerance n Signs and symptoms: —Consciousness —Respiratory distress —Nausea, vomiting, diarrhea —Constipation, cramps —Aspiration —Abdominal distention © 2004, 2002 Elsevier Inc. All rights reserved.

Tolerance—cont’d n Other signs and symptoms —Hydration —Labs —Weight change —Esophageal reflux —Lactose/gluten intolerances —Glucose fluctuations © 2004, 2002 Elsevier Inc. All rights reserved.

How to Determine Energy and Protein kcal/ml x ml given = kcal % protein x kcal = kcal as protein x 1 g/4 kcal = g protein Example: Patient drinks 200 cc of a 15. 3% protein product that has 1 kcal/ml x 200 ml = 200 kcal 0. 153 % protein x 200 kcal = 30. 6 kcal x 1 g protein/4 kcal = 7. 65 g protein © 2004, 2002 Elsevier Inc. All rights reserved.

Energy in Formulas 1 to 1. 2 kcal/ml = usual concentration 2 kcal/ml = highest concentration © 2004, 2002 Elsevier Inc. All rights reserved.

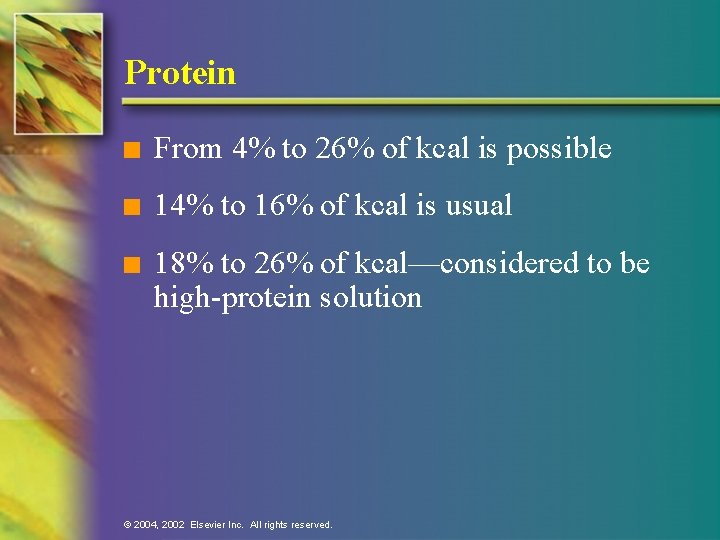
Protein n From 4% to 26% of kcal is possible n 14% to 16% of kcal is usual n 18% to 26% of kcal—considered to be high-protein solution © 2004, 2002 Elsevier Inc. All rights reserved.

Recommended Water n Healthy adult: 1 ml/kcal or 35 ml/kg n Healthy infant: 1. 5 ml/kcal or 150 ml/kg n Normal tube feeding: 1 kcal/ml; 80% to 85% water n Elderly: consider 25 ml/kg with renal, liver, or cardiac failure; or consider 35 ml/kg if history of dehydration © 2004, 2002 Elsevier Inc. All rights reserved.

Sources of Fluid (“Free Water”) n Liquids n Water in food n Water from metabolism n With tube feeding, nurse will flush tube with water about 3 times daily—include this amount in estimated needs —Example: “flush with 200 cc tid” © 2004, 2002 Elsevier Inc. All rights reserved.

Administration: Feeding Rate n Continuous method = slow rate of 50 to 150 ml/hr for 12 to 24 hours n Intermittent method = 250 to 400 ml of feeding given in 5 to 8 feedings per 24 hours n Bolus method = may give 300 to 400 ml several time a day (“push” is not desired) © 2004, 2002 Elsevier Inc. All rights reserved.

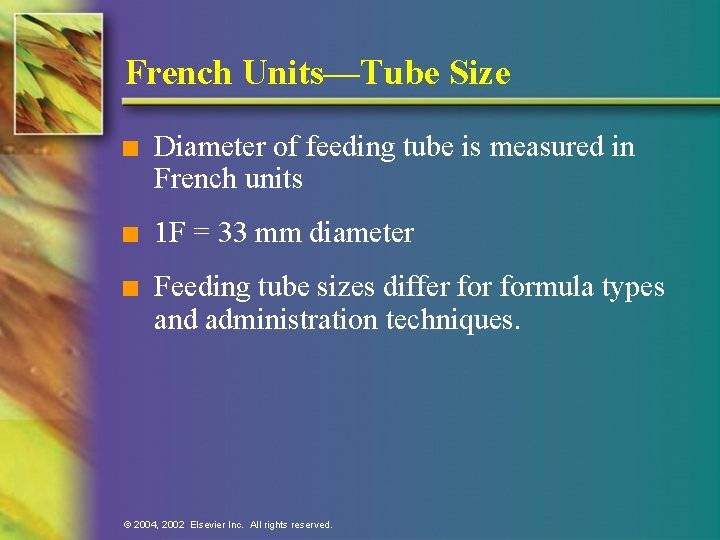
French Units—Tube Size n Diameter of feeding tube is measured in French units n 1 F = 33 mm diameter n Feeding tube sizes differ formula types and administration techniques. © 2004, 2002 Elsevier Inc. All rights reserved.

Examples of Special Formulas n Pediatrics n Low residue n High protein n Volume restriction n Diabetic n Pulmonary/COPD © 2004, 2002 Elsevier Inc. All rights reserved.

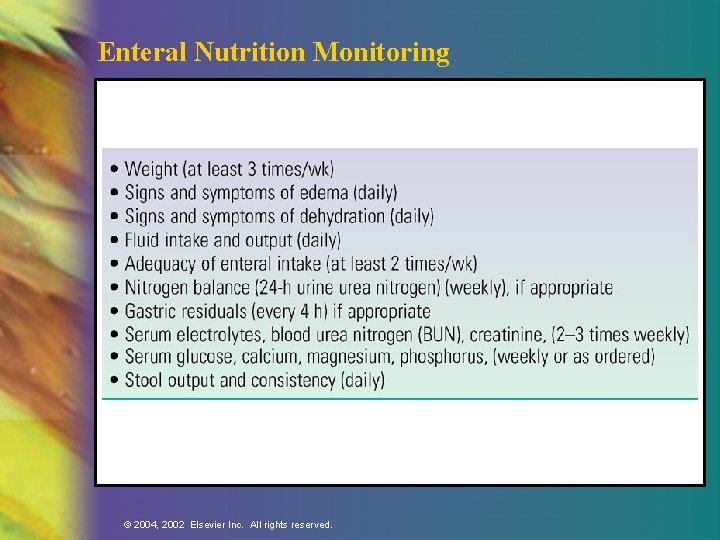
Enteral Nutrition Monitoring © 2004, 2002 Elsevier Inc. All rights reserved.

Routes of Parenteral Nutrition n Central access —TPN both long- and short-term placement n Peripheral or PPN —New catheters allow longer support via this method limited to 800 to 900 m. Osm/kg due to thrombophlebitis <2000 kcal required or <10 days © 2004, 2002 Elsevier Inc. All rights reserved.

PPN vs. TPN n Kcal required (10% dextrose max. PPN conc. ) n Fluid tolerance n Osmolarity n Duration n Central line contraindicated © 2004, 2002 Elsevier Inc. All rights reserved.

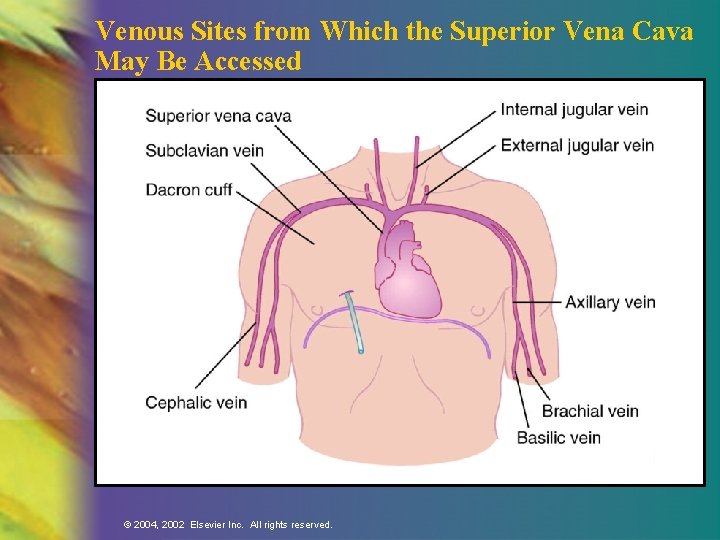
Venous Sites from Which the Superior Vena Cava May Be Accessed © 2004, 2002 Elsevier Inc. All rights reserved.

Advantages—Parenteral Nutrition n Provides nutrients when less than 2 to 3 feet of small intestine remains n Allows nutrition support when GI intolerance prevents oral or enteral support © 2004, 2002 Elsevier Inc. All rights reserved.

Indications for Total Parenteral Nutrition n GI non functioning n NPO >5 days n GI fistula n Acute pancreatitis n Short bowel syndrome n Malnutrition with >10% to 15 % weight loss n Nutritional needs not met; patient refuses food © 2004, 2002 Elsevier Inc. All rights reserved.

Contraindications n GI tract works n Terminally ill n Only needed briefly (<14 days) © 2004, 2002 Elsevier Inc. All rights reserved.

Calculating Nutrient Needs n Avoid excess kcal (> 40 kcal/kg) n Adults kcal/kg BW Obese—use desired BMI range or an adjusted factor © 2004, 2002 Elsevier Inc. All rights reserved.
![Adjusted Body Weight Adjusted IBW for obesity Female actual weight IBW x 0 Adjusted Body Weight Adjusted IBW for obesity Female: ([actual weight – IBW] x 0.](https://slidetodoc.com/presentation_image_h2/7d6185ea81302ba73b8dddf0173d5436/image-46.jpg)
Adjusted Body Weight Adjusted IBW for obesity Female: ([actual weight – IBW] x 0. 32) + IBW Male: ([actual weight – IBW] x 0. 38) + IBW © 2004, 2002 Elsevier Inc. All rights reserved.

Parenteral Components n Carbohydrate glucose or dextrose monohydrate 3. 4 kcal/g n Amino acids 3, 3. 5, 5, 7, 8. 5, 10% solutions n Fat 10% emulsions = 1. 1 kcal/ml 20% emulsions = 2 kcal/ml © 2004, 2002 Elsevier Inc. All rights reserved.

© 2004, 2002 Elsevier Inc. All rights reserved.

Protein Requirements n 1. 2 to 1. 5 g protein/kg IBW mild or moderate stress n 2. 5 g protein/kg IBW burns or severe trauma © 2004, 2002 Elsevier Inc. All rights reserved.

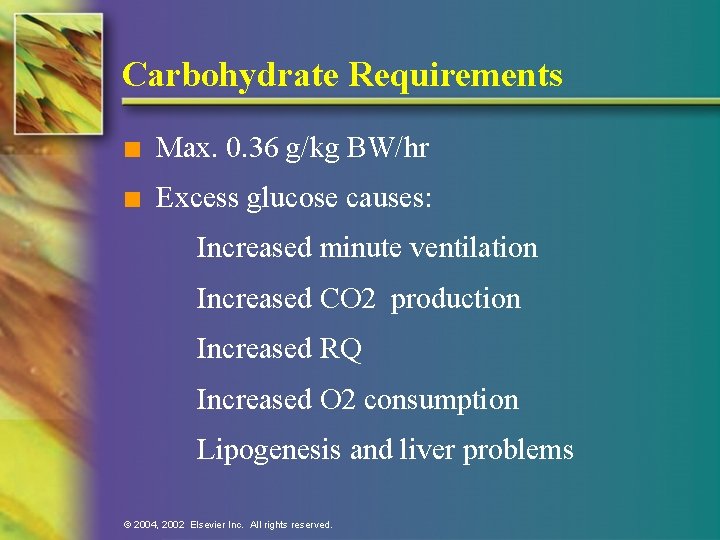
Carbohydrate Requirements n Max. 0. 36 g/kg BW/hr n Excess glucose causes: Increased minute ventilation Increased CO 2 production Increased RQ Increased O 2 consumption Lipogenesis and liver problems © 2004, 2002 Elsevier Inc. All rights reserved.

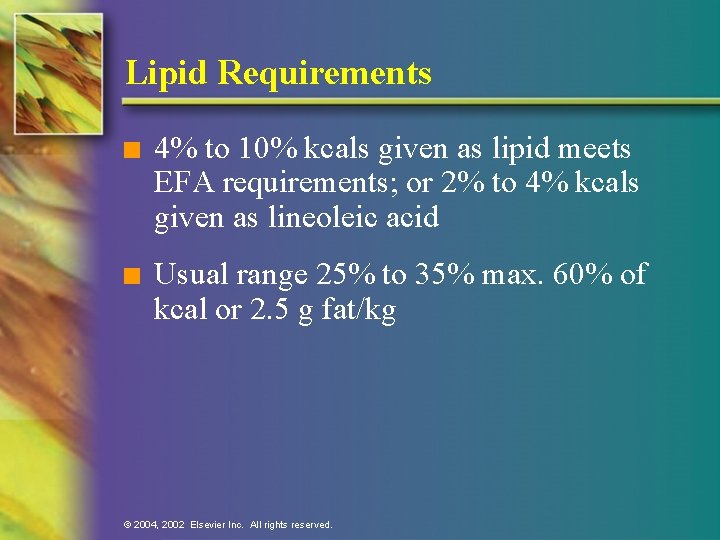
Lipid Requirements n 4% to 10% kcals given as lipid meets EFA requirements; or 2% to 4% kcals given as lineoleic acid n Usual range 25% to 35% max. 60% of kcal or 2. 5 g fat/kg © 2004, 2002 Elsevier Inc. All rights reserved.

Other Requirements n Fluid— 30 to 50 ml/kg n Electrolytes Use acetate or chloride forms to manage acidosis or alkalosis n Vitamins n Trace elements © 2004, 2002 Elsevier Inc. All rights reserved.

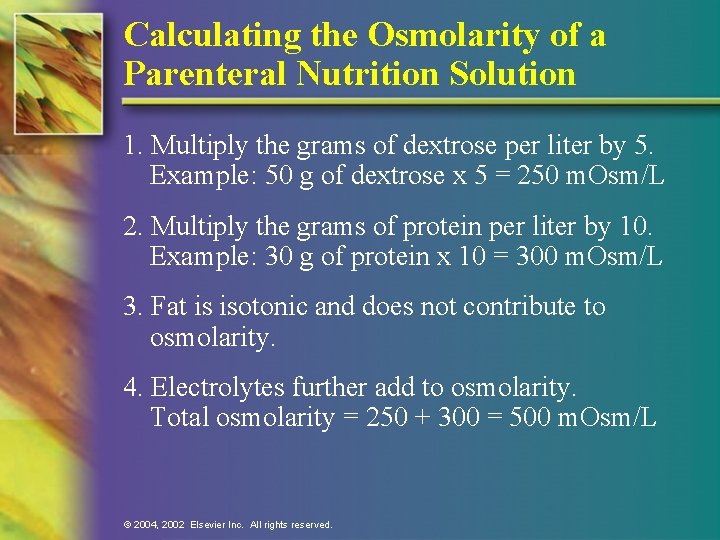
Calculating the Osmolarity of a Parenteral Nutrition Solution 1. Multiply the grams of dextrose per liter by 5. Example: 50 g of dextrose x 5 = 250 m. Osm/L 2. Multiply the grams of protein per liter by 10. Example: 30 g of protein x 10 = 300 m. Osm/L 3. Fat is isotonic and does not contribute to osmolarity. 4. Electrolytes further add to osmolarity. Total osmolarity = 250 + 300 = 500 m. Osm/L © 2004, 2002 Elsevier Inc. All rights reserved.

Compounding Methods n Total nutrient admixture of amino acids, glucose, additives n 3 -in-1 solution of lipid, amino acids, glucose, additives © 2004, 2002 Elsevier Inc. All rights reserved.

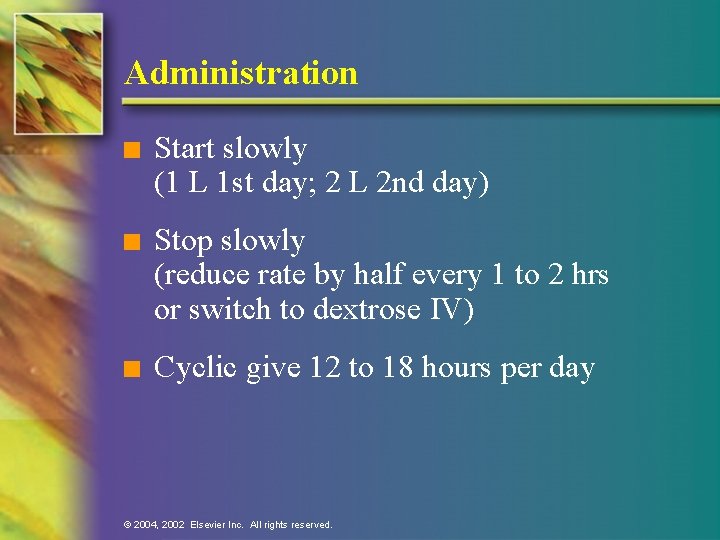
Administration n Start slowly (1 L 1 st day; 2 L 2 nd day) n Stop slowly (reduce rate by half every 1 to 2 hrs or switch to dextrose IV) n Cyclic give 12 to 18 hours per day © 2004, 2002 Elsevier Inc. All rights reserved.

Monitoring and Complications n Infection n Hemodynamic stability n Catheter care n Refeeding syndrome © 2004, 2002 Elsevier Inc. All rights reserved.

Refeeding Syndrome n Hypophosphatemia n Hyperglycemia n Fluid retention n Cardiac arrest © 2004, 2002 Elsevier Inc. All rights reserved.

Monitor n Weight (daily) n Blood Daily Electrolytes (Na+, K+, Cl-) Glucose Acid-base status 3 times/week BUN Ca+, P Plasma transaminases © 2004, 2002 Elsevier Inc. All rights reserved.

Monitor—cont’d n Blood Twice/week Ammonia Mg Plasma transaminases Weekly Hgb Prothrombin time Zn Cu Triglycerides © 2004, 2002 Elsevier Inc. All rights reserved.

Monitor—cont’d n Urine: Glucose and ketones (4 -6/day) Specific gravity or osmolarity (2 -4/day) Urinary urea nitrogen (weekly) n Other: Volume infusate (daily) Oral intake (daily) if applicable Urinary output (daily) Activity, temperature, respiration (daily) WBC and differential (as needed) Cultures (as needed) © 2004, 2002 Elsevier Inc. All rights reserved.

Problems n PPN Site irritation n TPN 1. Catheter sepsis 2. Placement problems 3. Metabolic © 2004, 2002 Elsevier Inc. All rights reserved.

Pediatric n Energy Infant 50 to 60 kcal/kg/day maintenance 70 to 120 kcal/kg/day growth n Child >1 yr BEE 1 to 8 yrs 70 to 100 kcal/kg/day 8 to 12 yrs 60 to 75 kcal/kg/day 12 to 18 yrs 45 to 60 kcal/kg/day Injury factors 1. 25 mild stress 1. 50 nutritional depletion 2. 00 high stress © 2004, 2002 Elsevier Inc. All rights reserved.

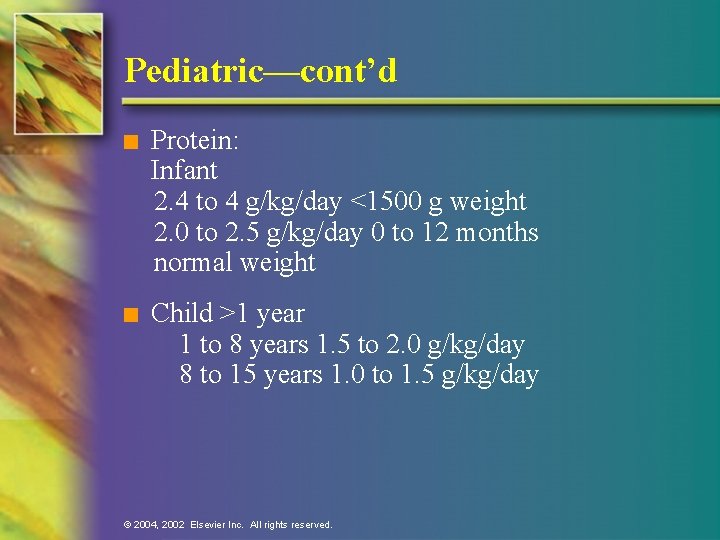
Pediatric—cont’d n Protein: Infant 2. 4 to 4 g/kg/day <1500 g weight 2. 0 to 2. 5 g/kg/day 0 to 12 months normal weight n Child >1 year 1 to 8 years 1. 5 to 2. 0 g/kg/day 8 to 15 years 1. 0 to 1. 5 g/kg/day © 2004, 2002 Elsevier Inc. All rights reserved.

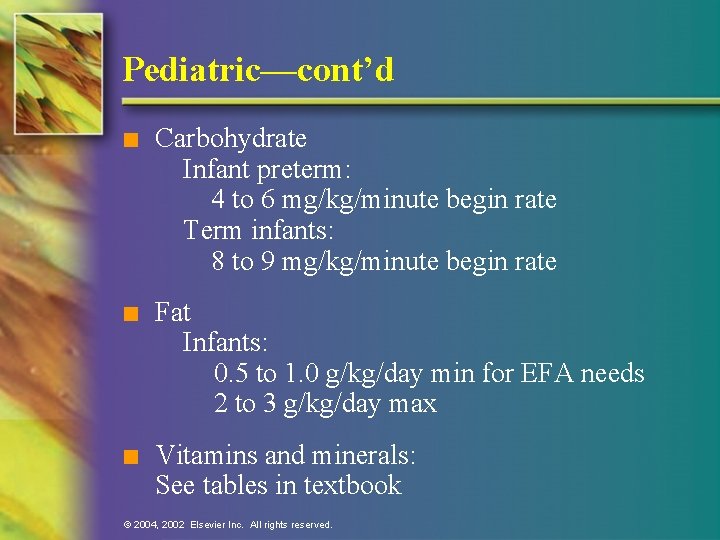
Pediatric—cont’d n Carbohydrate Infant preterm: 4 to 6 mg/kg/minute begin rate Term infants: 8 to 9 mg/kg/minute begin rate n Fat Infants: 0. 5 to 1. 0 g/kg/day min for EFA needs 2 to 3 g/kg/day max n Vitamins and minerals: See tables in textbook © 2004, 2002 Elsevier Inc. All rights reserved.

Pediatric—cont’d n Fluid and electrolytes Infant: LBW 125 to 150 ml/kg/day 2 to 4 mmol/kg/day for electrolytes n Other infants and children © 2004, 2002 Elsevier Inc. All rights reserved.

Document in Chart n Type of feeding formula and tube n Method (bolus, drip, pump) n Rate and water flush n Intake energy and protein n Tolerance, complications, and corrective actions n Patient education © 2004, 2002 Elsevier Inc. All rights reserved.

n For more information see chapter 7 n Walker and Roger © 2004, 2002 Elsevier Inc. All rights reserved.
 Dieta enteral e parenteral
Dieta enteral e parenteral Via parenteral
Via parenteral Formula enteral rumah sakit
Formula enteral rumah sakit Enteral beslenme ürün saklama koşulları
Enteral beslenme ürün saklama koşulları Enteral parenteral beslenme
Enteral parenteral beslenme Specialized nutrition support
Specialized nutrition support Tpn calculation example
Tpn calculation example Tpn tapering guidelines
Tpn tapering guidelines Complication of parenteral nutrition
Complication of parenteral nutrition Ppn vs tpn
Ppn vs tpn Rxkinetics tpn
Rxkinetics tpn Complication of parenteral nutrition
Complication of parenteral nutrition Cost of tpn
Cost of tpn Small bowel obstruction
Small bowel obstruction Glucerna cpt code
Glucerna cpt code Enteral nutrition
Enteral nutrition What are the signal words
What are the signal words Disadvantages of subcutaneous route
Disadvantages of subcutaneous route Mydriatics and miotics drugs
Mydriatics and miotics drugs Pharmacological and parenteral therapies
Pharmacological and parenteral therapies Kelsey carbonetta
Kelsey carbonetta Fitness chapter 7
Fitness chapter 7 A saturated fatty acid holds all the hydrogen atoms it can.
A saturated fatty acid holds all the hydrogen atoms it can. Chapter 4 nutrition and your personal fitness
Chapter 4 nutrition and your personal fitness Test chapter 11 nutrition and diets
Test chapter 11 nutrition and diets Test chapter 11 nutrition and diets
Test chapter 11 nutrition and diets Chapter 10 lesson 4 nutrition labels and food safety
Chapter 10 lesson 4 nutrition labels and food safety Chapter 10 lesson 4 nutrition labels and food safety
Chapter 10 lesson 4 nutrition labels and food safety Nutrition and hydration chapter 15
Nutrition and hydration chapter 15 Chapter 6 microbial nutrition and growth
Chapter 6 microbial nutrition and growth Chapter 7 skin structure growth and nutrition
Chapter 7 skin structure growth and nutrition Chapter 6 microbial nutrition and growth
Chapter 6 microbial nutrition and growth Chapter 11 nutrition and diet
Chapter 11 nutrition and diet Seven nutrition and fitness
Seven nutrition and fitness Pour plate vs streak plate
Pour plate vs streak plate Chapter 55 nutrition and health
Chapter 55 nutrition and health Chapter 15 maternal and fetal nutrition
Chapter 15 maternal and fetal nutrition Chapter 8 nutrition and hydration
Chapter 8 nutrition and hydration Chapter 8 food and nutrition
Chapter 8 food and nutrition Chapter 11 nutrition and diets
Chapter 11 nutrition and diets Diet care logo
Diet care logo Chapter 15 digestion and nutrition
Chapter 15 digestion and nutrition Parenteral dosage calculator
Parenteral dosage calculator Apakah yang dimaksud dengan injeksi volume kecil
Apakah yang dimaksud dengan injeksi volume kecil Pemberian obat secara parenteral disebut
Pemberian obat secara parenteral disebut Quality control test for parenterals
Quality control test for parenterals Parenteral beslenme komplikasyonları
Parenteral beslenme komplikasyonları Parenteral routes
Parenteral routes Parenteral emulsion
Parenteral emulsion Diselectrolitemia
Diselectrolitemia Hidratación parenteral
Hidratación parenteral Iv admixture
Iv admixture Dosage forms and drug delivery systems
Dosage forms and drug delivery systems Ostoma
Ostoma Parenteral dosage examples
Parenteral dosage examples Apendicitis
Apendicitis Non parenteral
Non parenteral Large volume parentrals
Large volume parentrals Rumus tetesan infus
Rumus tetesan infus Aminovel
Aminovel Parenteral calculations
Parenteral calculations Outpatient parenteral antimicrobial therapy (opat)
Outpatient parenteral antimicrobial therapy (opat) Postpilorik beslenme nedir
Postpilorik beslenme nedir Parenteral nutriton
Parenteral nutriton Gastrostomia
Gastrostomia La vía parenteral
La vía parenteral Local route of drug administration
Local route of drug administration Parenteral equipment
Parenteral equipment