Chapter 23 Carbonyl Condensation Reactions Based on Mc

Chapter 23. Carbonyl Condensation Reactions Based on Mc. Murry’s Organic Chemistry, 6 th edition © 2003 Ronald Kluger Department of Chemistry University of Toronto Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003
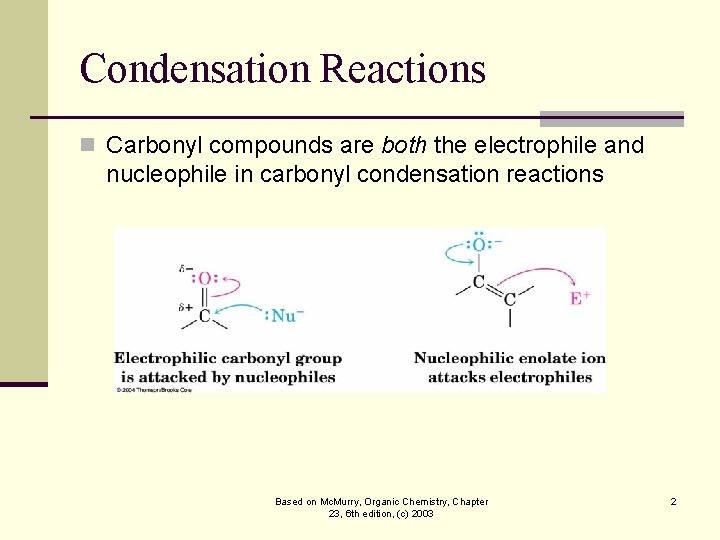
Condensation Reactions n Carbonyl compounds are both the electrophile and nucleophile in carbonyl condensation reactions Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 2
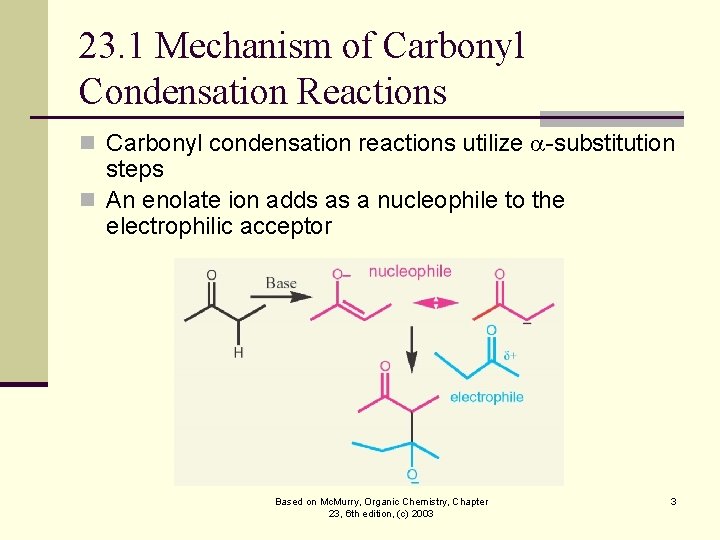
23. 1 Mechanism of Carbonyl Condensation Reactions n Carbonyl condensation reactions utilize -substitution steps n An enolate ion adds as a nucleophile to the electrophilic acceptor Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 3
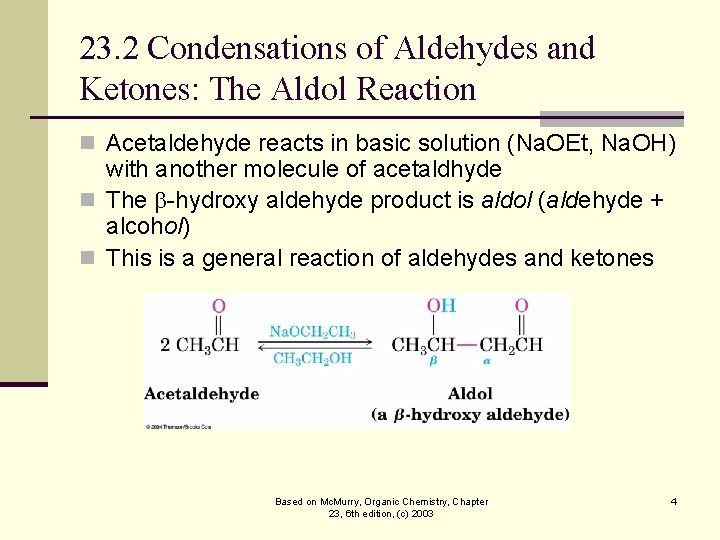
23. 2 Condensations of Aldehydes and Ketones: The Aldol Reaction n Acetaldehyde reacts in basic solution (Na. OEt, Na. OH) with another molecule of acetaldhyde n The -hydroxy aldehyde product is aldol (aldehyde + alcohol) n This is a general reaction of aldehydes and ketones Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 4
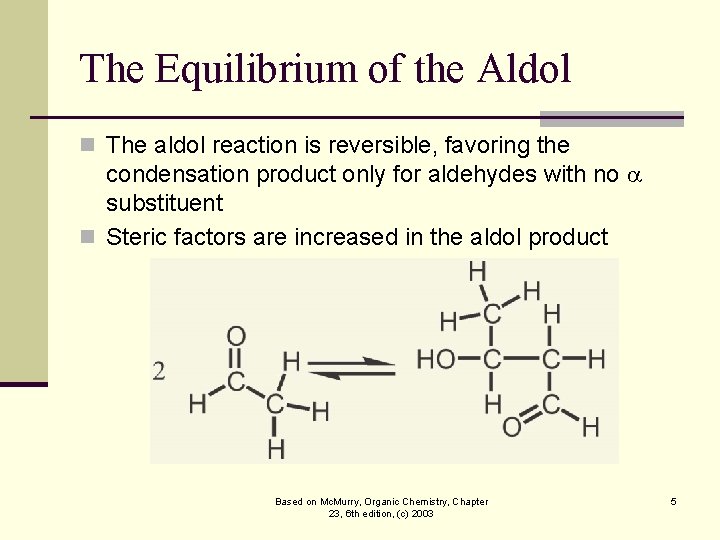
The Equilibrium of the Aldol n The aldol reaction is reversible, favoring the condensation product only for aldehydes with no substituent n Steric factors are increased in the aldol product Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 5
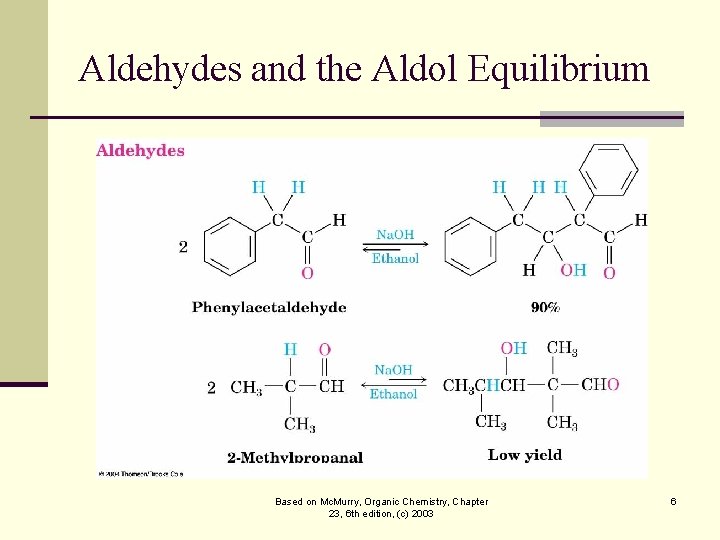
Aldehydes and the Aldol Equilibrium Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 6
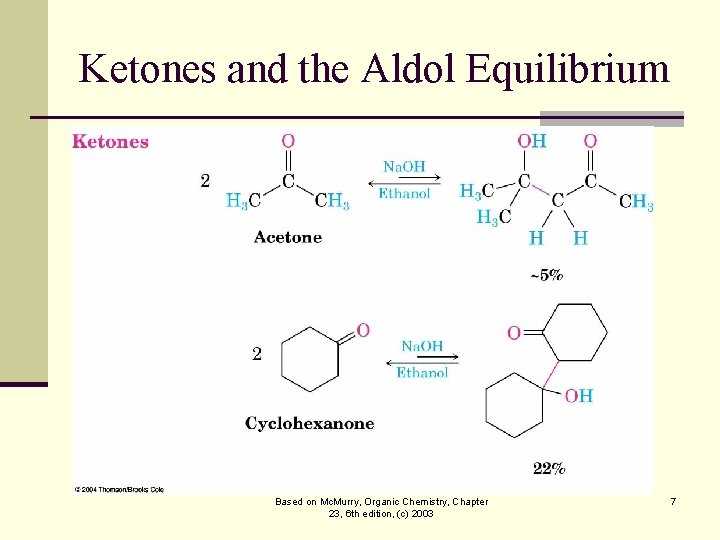
Ketones and the Aldol Equilibrium Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 7
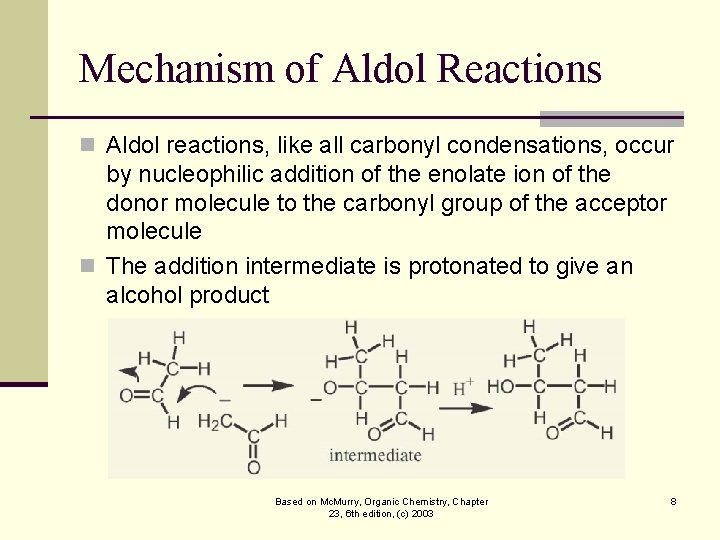
Mechanism of Aldol Reactions n Aldol reactions, like all carbonyl condensations, occur by nucleophilic addition of the enolate ion of the donor molecule to the carbonyl group of the acceptor molecule n The addition intermediate is protonated to give an alcohol product Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 8
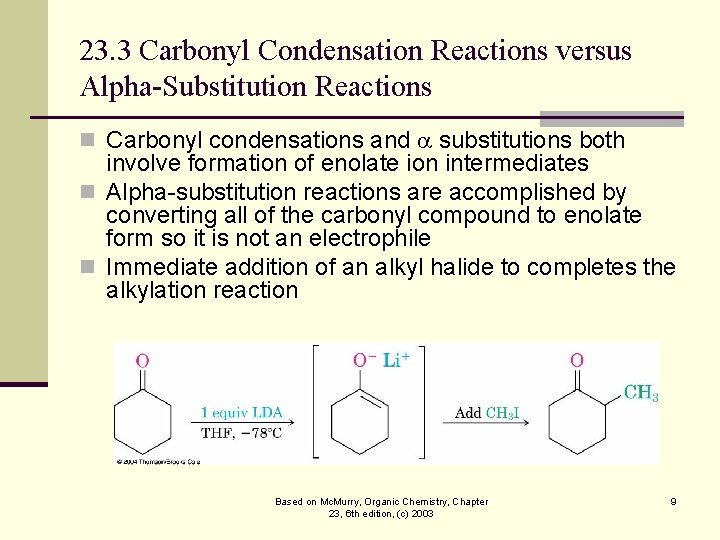
23. 3 Carbonyl Condensation Reactions versus Alpha-Substitution Reactions n Carbonyl condensations and substitutions both involve formation of enolate ion intermediates n Alpha-substitution reactions are accomplished by converting all of the carbonyl compound to enolate form so it is not an electrophile n Immediate addition of an alkyl halide to completes the alkylation reaction Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 9
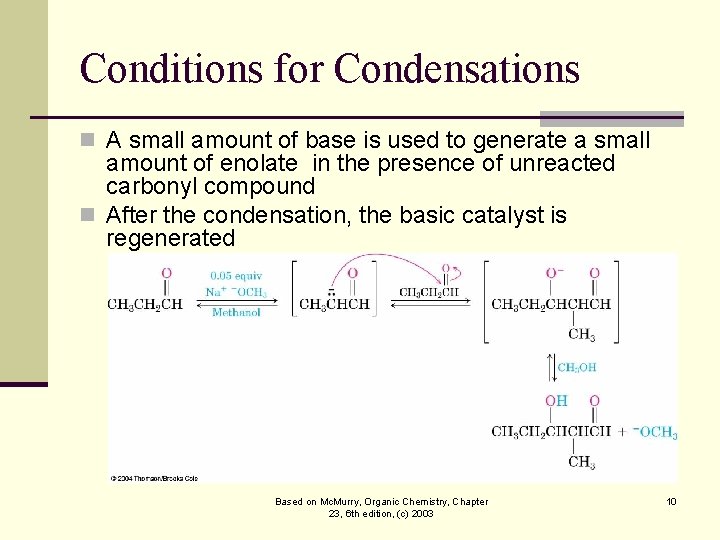
Conditions for Condensations n A small amount of base is used to generate a small amount of enolate in the presence of unreacted carbonyl compound n After the condensation, the basic catalyst is regenerated Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 10
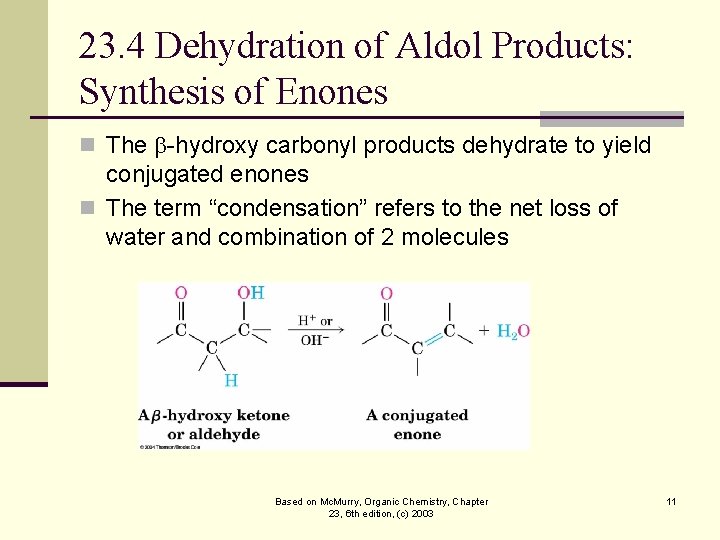
23. 4 Dehydration of Aldol Products: Synthesis of Enones n The -hydroxy carbonyl products dehydrate to yield conjugated enones n The term “condensation” refers to the net loss of water and combination of 2 molecules Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 11
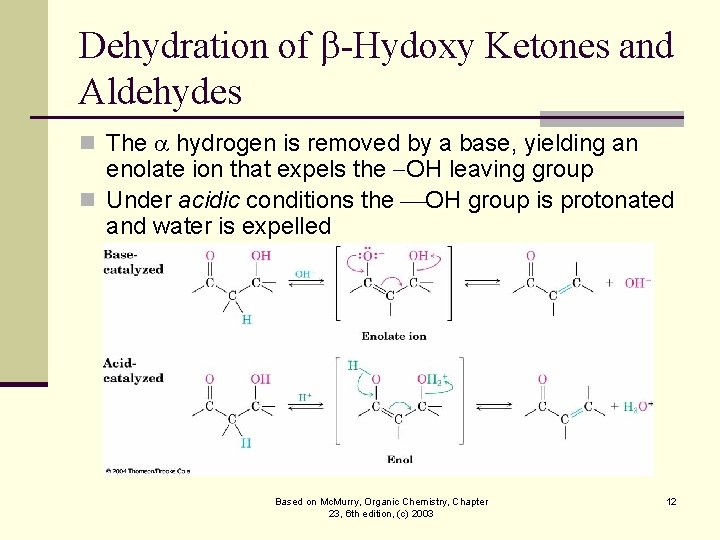
Dehydration of -Hydoxy Ketones and Aldehydes n The hydrogen is removed by a base, yielding an enolate ion that expels the OH leaving group n Under acidic conditions the OH group is protonated and water is expelled Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 12
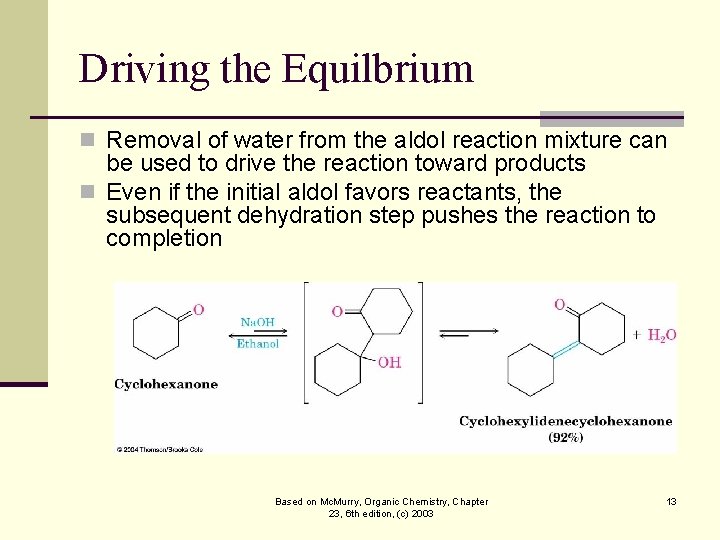
Driving the Equilbrium n Removal of water from the aldol reaction mixture can be used to drive the reaction toward products n Even if the initial aldol favors reactants, the subsequent dehydration step pushes the reaction to completion Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 13
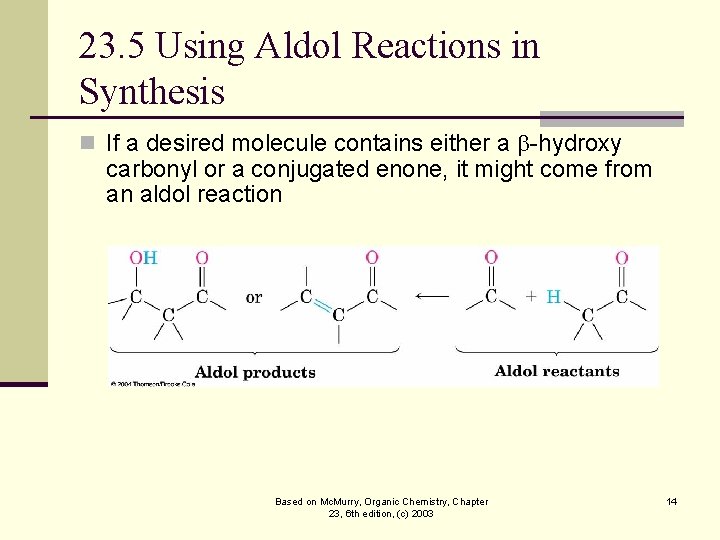
23. 5 Using Aldol Reactions in Synthesis n If a desired molecule contains either a -hydroxy carbonyl or a conjugated enone, it might come from an aldol reaction Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 14
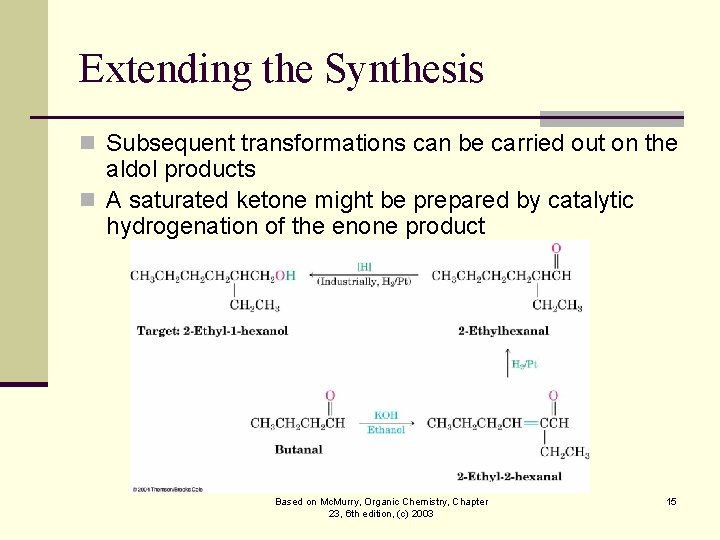
Extending the Synthesis n Subsequent transformations can be carried out on the aldol products n A saturated ketone might be prepared by catalytic hydrogenation of the enone product Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 15
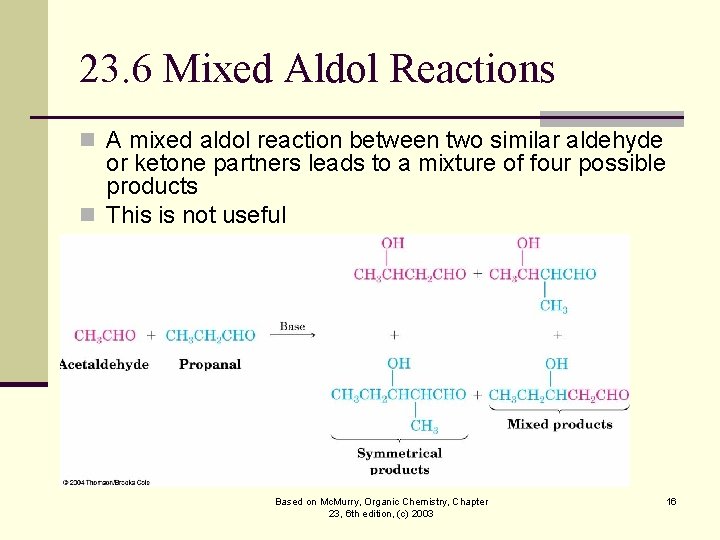
23. 6 Mixed Aldol Reactions n A mixed aldol reaction between two similar aldehyde or ketone partners leads to a mixture of four possible products n This is not useful Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 16
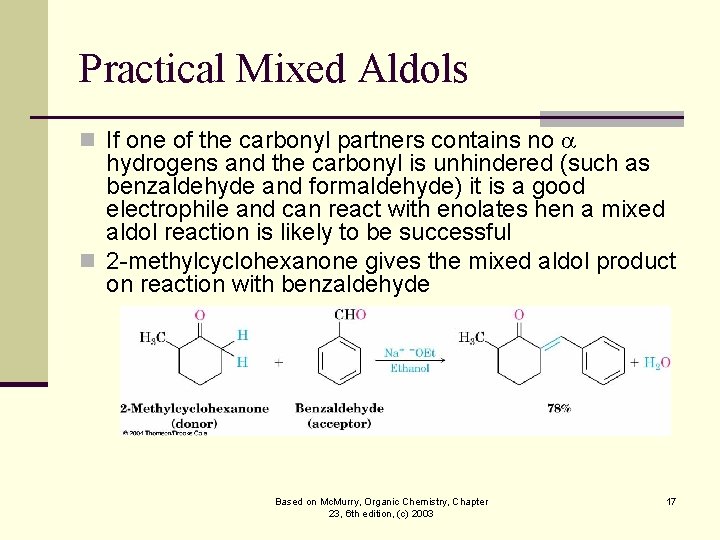
Practical Mixed Aldols n If one of the carbonyl partners contains no hydrogens and the carbonyl is unhindered (such as benzaldehyde and formaldehyde) it is a good electrophile and can react with enolates hen a mixed aldol reaction is likely to be successful n 2 -methylcyclohexanone gives the mixed aldol product on reaction with benzaldehyde Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 17
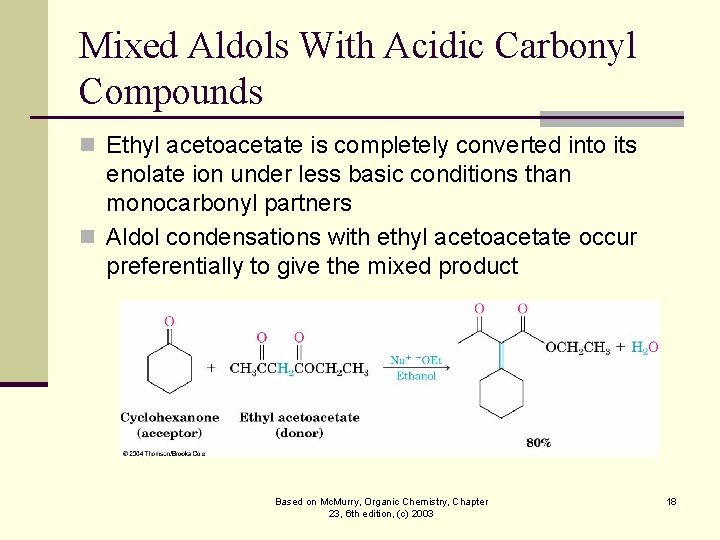
Mixed Aldols With Acidic Carbonyl Compounds n Ethyl acetoacetate is completely converted into its enolate ion under less basic conditions than monocarbonyl partners n Aldol condensations with ethyl acetoacetate occur preferentially to give the mixed product Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 18
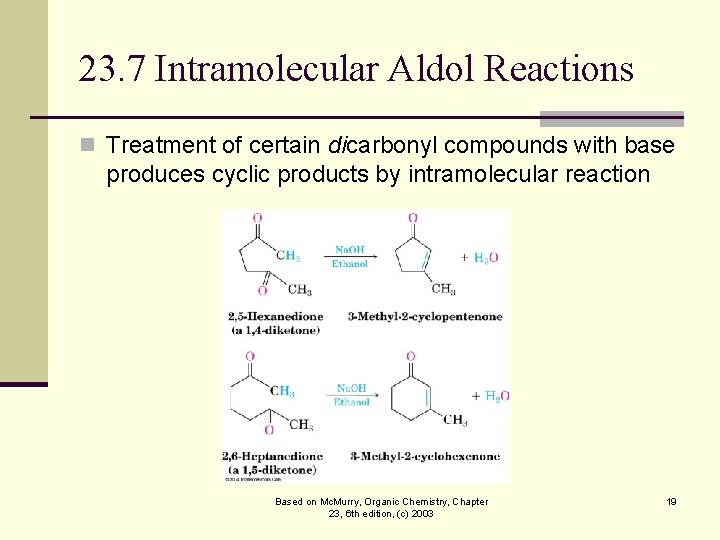
23. 7 Intramolecular Aldol Reactions n Treatment of certain dicarbonyl compounds with base produces cyclic products by intramolecular reaction Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 19
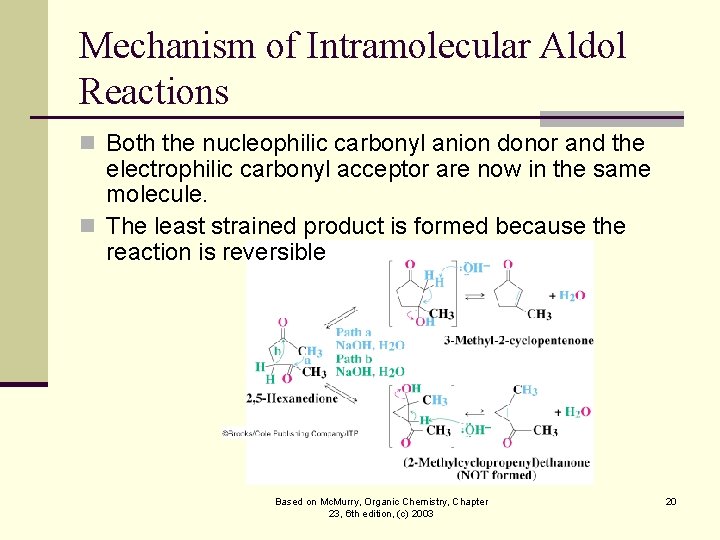
Mechanism of Intramolecular Aldol Reactions n Both the nucleophilic carbonyl anion donor and the electrophilic carbonyl acceptor are now in the same molecule. n The least strained product is formed because the reaction is reversible Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 20
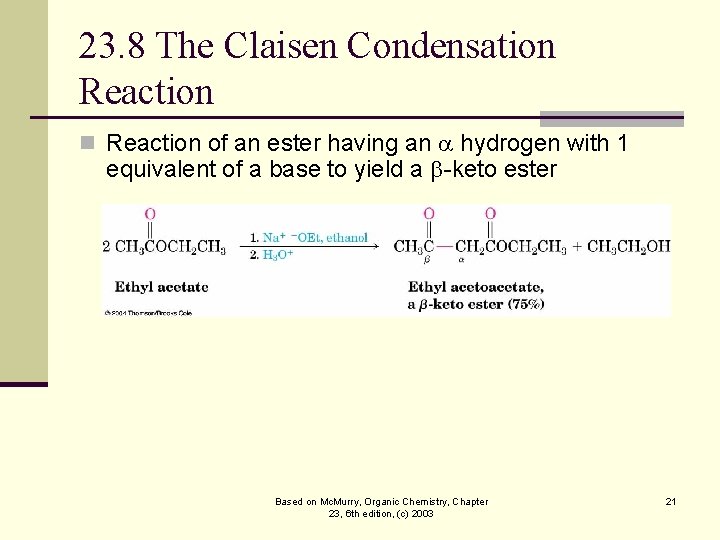
23. 8 The Claisen Condensation Reaction of an ester having an hydrogen with 1 equivalent of a base to yield a -keto ester Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 21
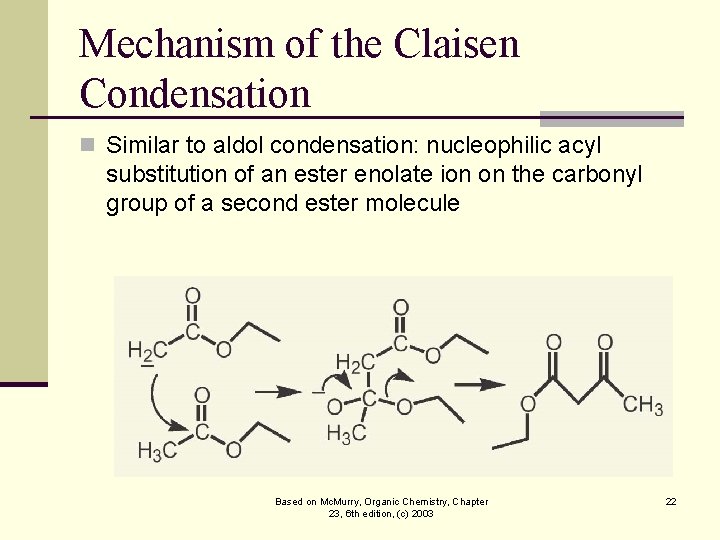
Mechanism of the Claisen Condensation n Similar to aldol condensation: nucleophilic acyl substitution of an ester enolate ion on the carbonyl group of a second ester molecule Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 22
Features of the Claisen Condensation n If the starting ester has more than one acidic a hydrogen, the product -keto ester has a doubly activated proton that can be abstracted by base n Requires a full equivalent of base rather than a catalytic amount n The deprotonation drives the reaction to the product Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 23
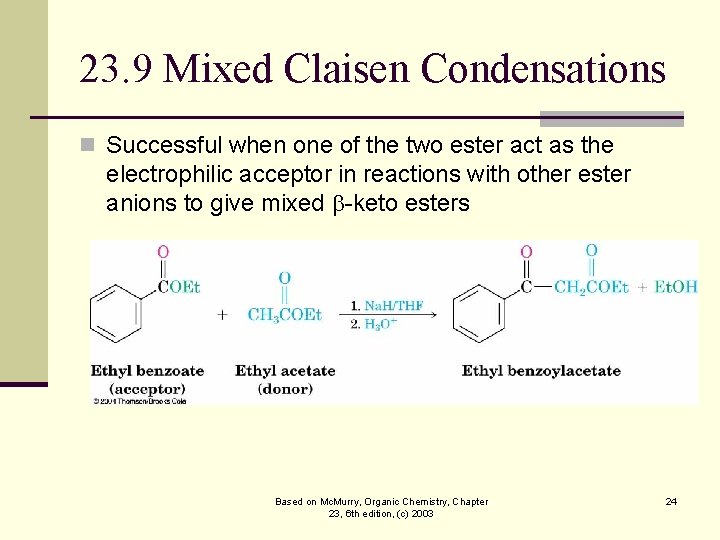
23. 9 Mixed Claisen Condensations n Successful when one of the two ester act as the electrophilic acceptor in reactions with other ester anions to give mixed -keto esters Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 24
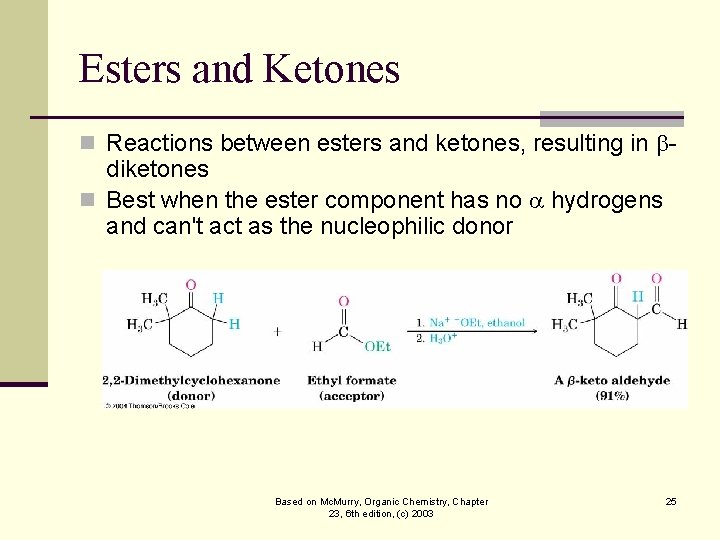
Esters and Ketones n Reactions between esters and ketones, resulting in - diketones n Best when the ester component has no hydrogens and can't act as the nucleophilic donor Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 25
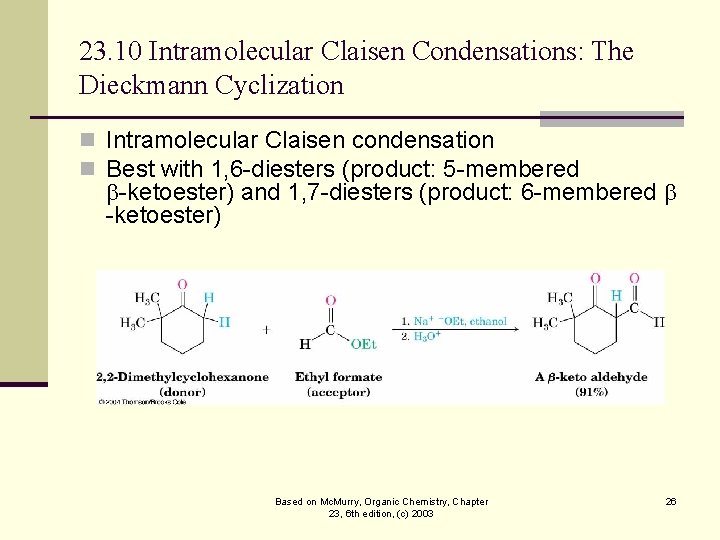
23. 10 Intramolecular Claisen Condensations: The Dieckmann Cyclization n Intramolecular Claisen condensation n Best with 1, 6 -diesters (product: 5 -membered -ketoester) and 1, 7 -diesters (product: 6 -membered -ketoester) Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 26
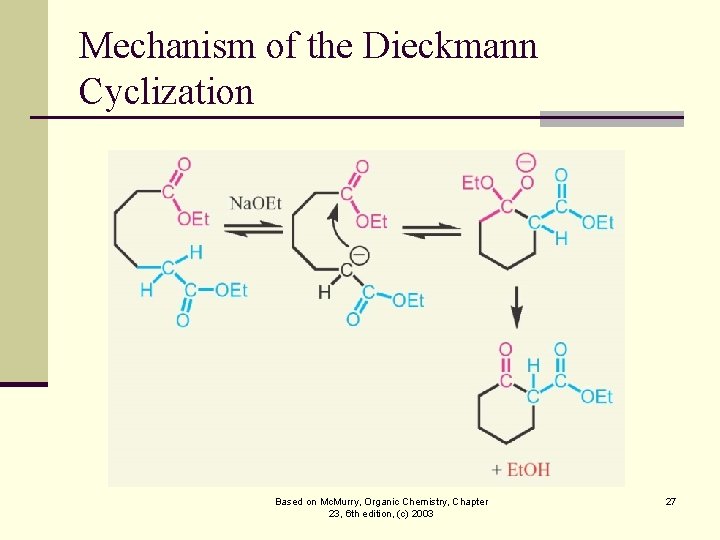
Mechanism of the Dieckmann Cyclization Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 27
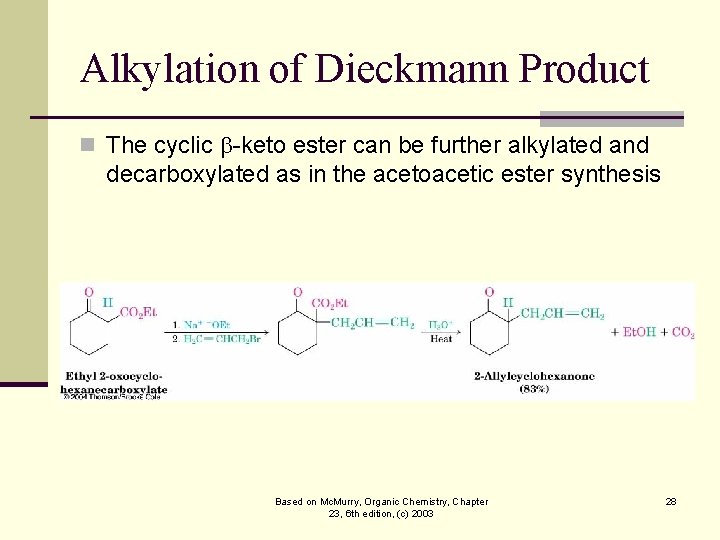
Alkylation of Dieckmann Product n The cyclic -keto ester can be further alkylated and decarboxylated as in the acetoacetic ester synthesis Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 28
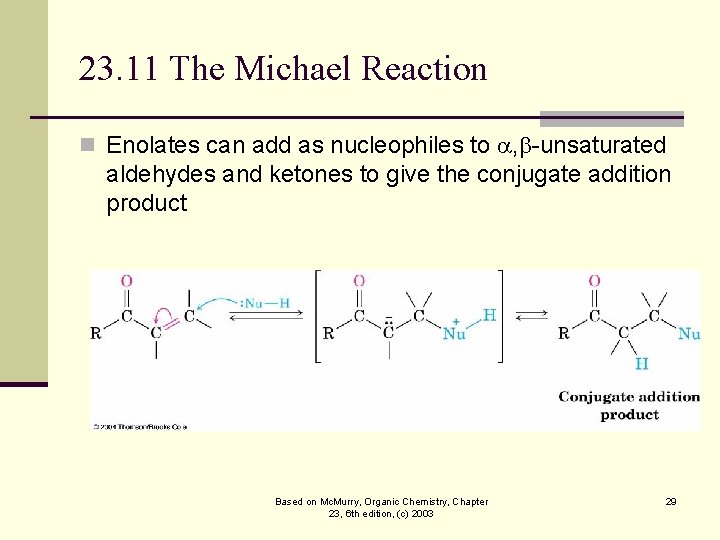
23. 11 The Michael Reaction n Enolates can add as nucleophiles to , -unsaturated aldehydes and ketones to give the conjugate addition product Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 29
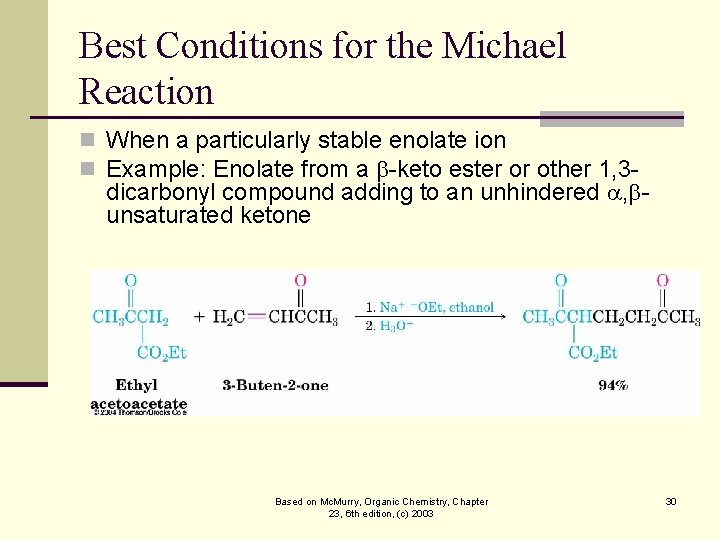
Best Conditions for the Michael Reaction n When a particularly stable enolate ion n Example: Enolate from a -keto ester or other 1, 3 - dicarbonyl compound adding to an unhindered , unsaturated ketone Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 30
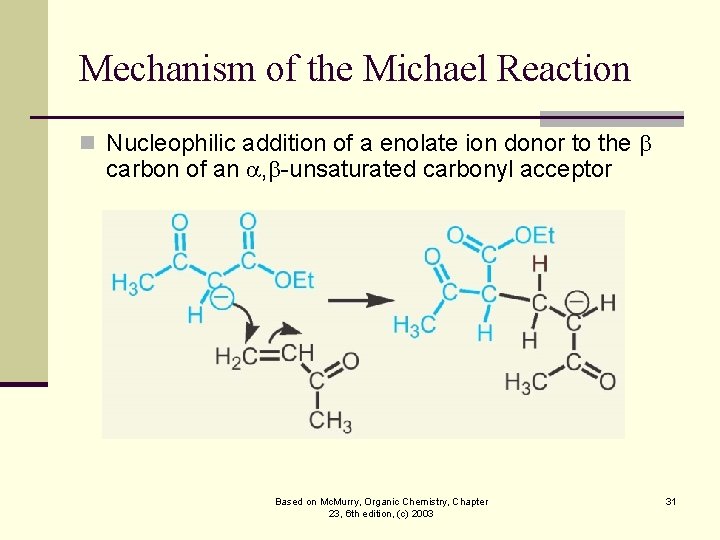
Mechanism of the Michael Reaction n Nucleophilic addition of a enolate ion donor to the carbon of an , -unsaturated carbonyl acceptor Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 31

Generality of the Michael Reaction n Occurs with a variety of , -unsaturated carbonyl compounds (aldehydes, esters, nitriles, amides, and nitro compounds) n Donors include -diketones, -keto esters, malonic esters, -keto nitriles, and nitro compounds n See Table 23. 1 Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 32
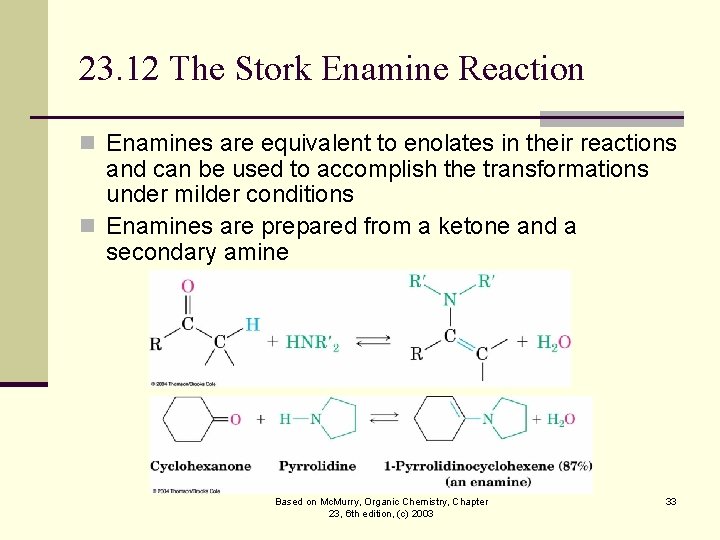
23. 12 The Stork Enamine Reaction n Enamines are equivalent to enolates in their reactions and can be used to accomplish the transformations under milder conditions n Enamines are prepared from a ketone and a secondary amine Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 33
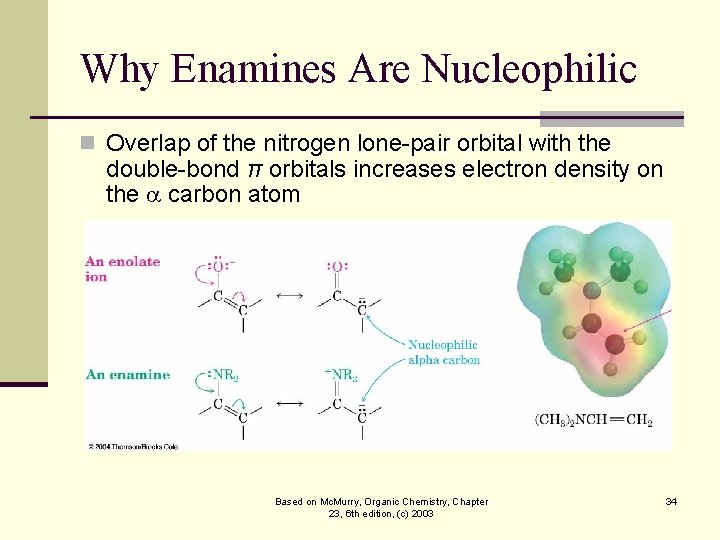
Why Enamines Are Nucleophilic n Overlap of the nitrogen lone-pair orbital with the double-bond π orbitals increases electron density on the carbon atom Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 34
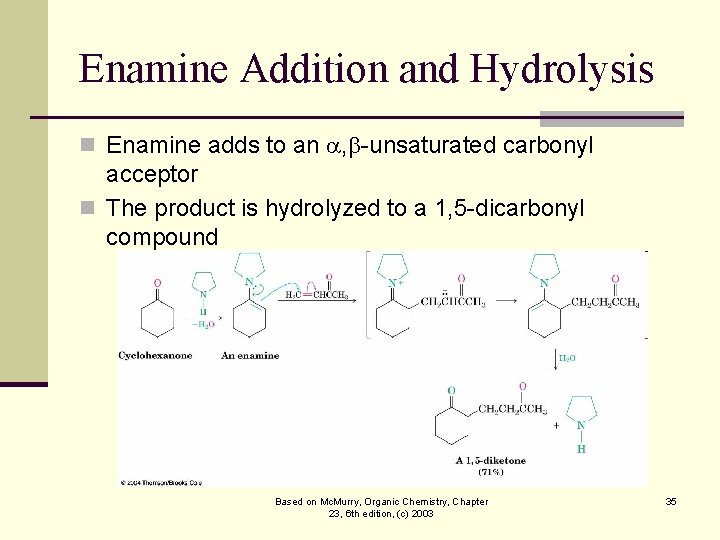
Enamine Addition and Hydrolysis n Enamine adds to an , -unsaturated carbonyl acceptor n The product is hydrolyzed to a 1, 5 -dicarbonyl compound Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 35
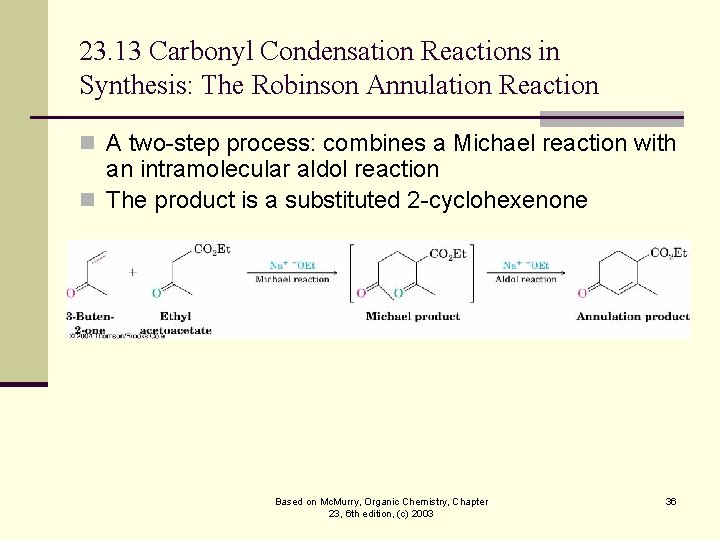
23. 13 Carbonyl Condensation Reactions in Synthesis: The Robinson Annulation Reaction n A two-step process: combines a Michael reaction with an intramolecular aldol reaction n The product is a substituted 2 -cyclohexenone Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 36
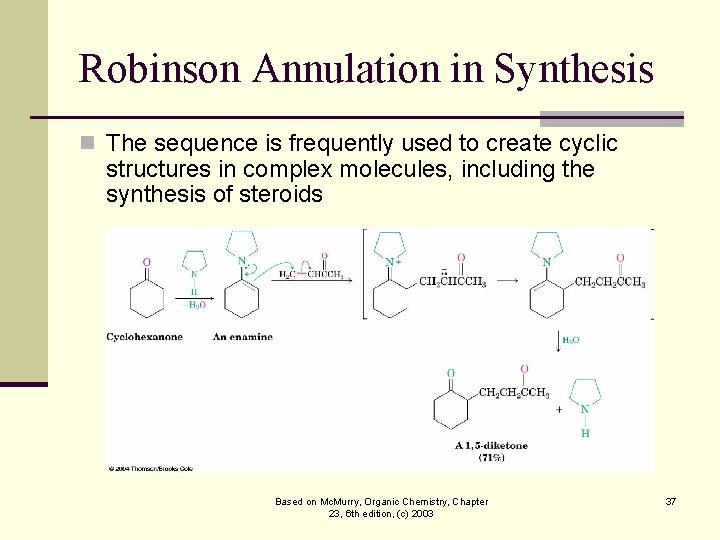
Robinson Annulation in Synthesis n The sequence is frequently used to create cyclic structures in complex molecules, including the synthesis of steroids Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 37
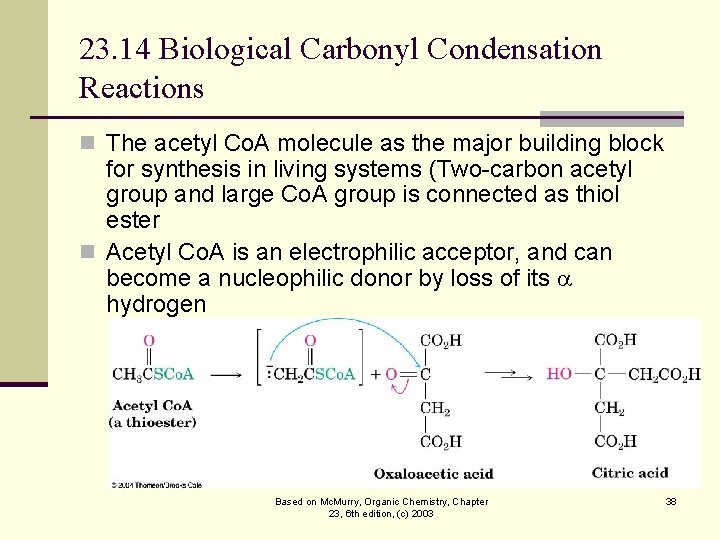
23. 14 Biological Carbonyl Condensation Reactions n The acetyl Co. A molecule as the major building block for synthesis in living systems (Two-carbon acetyl group and large Co. A group is connected as thiol ester n Acetyl Co. A is an electrophilic acceptor, and can become a nucleophilic donor by loss of its hydrogen Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 38
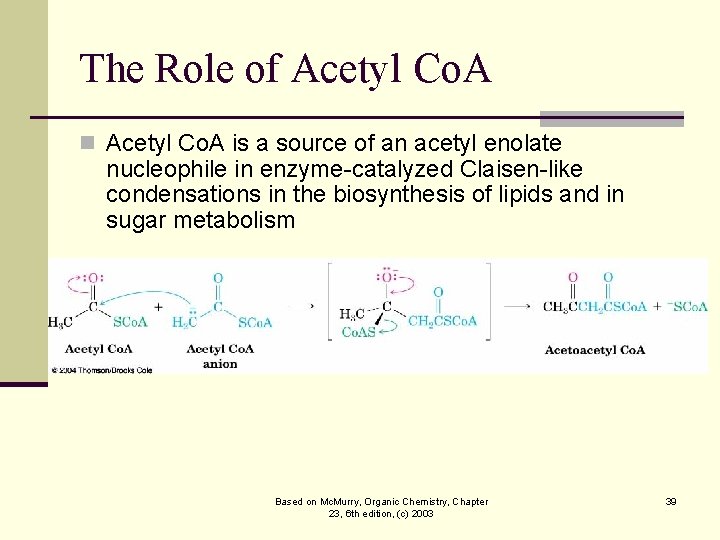
The Role of Acetyl Co. A n Acetyl Co. A is a source of an acetyl enolate nucleophile in enzyme-catalyzed Claisen-like condensations in the biosynthesis of lipids and in sugar metabolism Based on Mc. Murry, Organic Chemistry, Chapter 23, 6 th edition, (c) 2003 39
- Slides: 39