Chapter 22 Reaction Rate Chemical Equilibrium Stability of

Chapter 22 Reaction Rate & Chemical Equilibrium
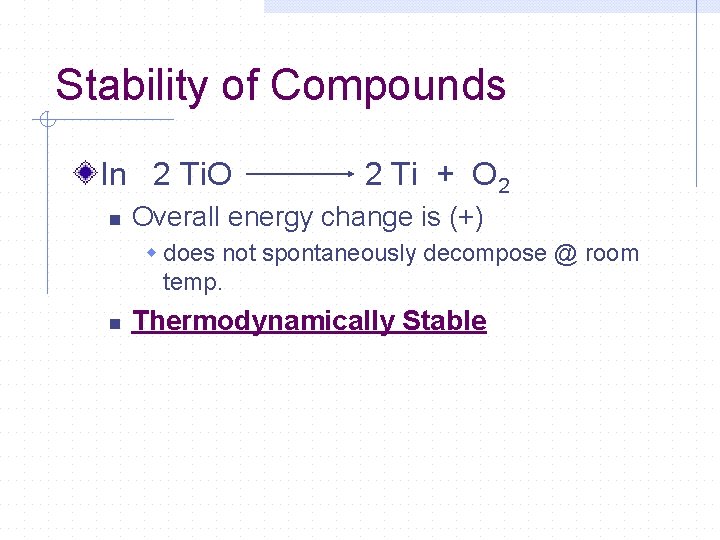
Stability of Compounds In 2 Ti. O n 2 Ti + O 2 Overall energy change is (+) w does not spontaneously decompose @ room temp. n Thermodynamically Stable
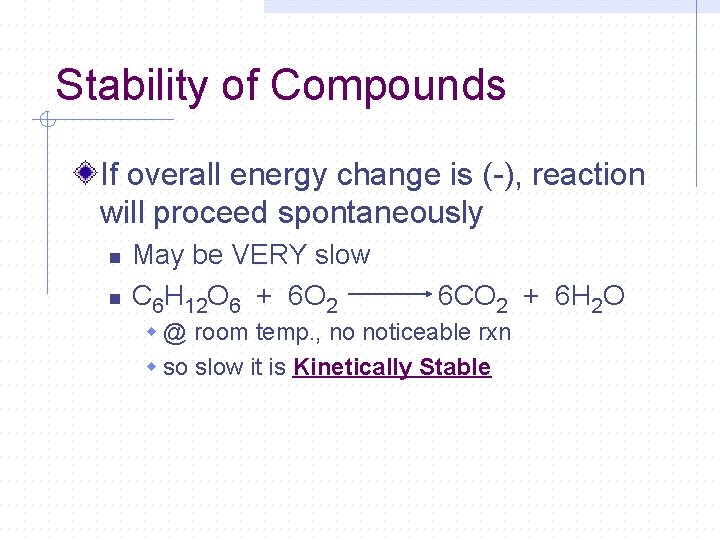
Stability of Compounds If overall energy change is (-), reaction will proceed spontaneously n n May be VERY slow C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O w @ room temp. , no noticeable rxn w so slow it is Kinetically Stable

Stability of Compounds to predict whether a spont. rxn. will be useful, must know the rate @ which rxn. occurs and @ what pt. equilibrium is established.

Reversible Rxns. & Equilibrium Many rxns. result in an equilibrium mixture A rxn. goes to completion when all of one of the reactants is used up & rxn. stops n Completion Rxn.

Reversible Rxns. & Equilibrium Completion Rxn. n 1 or more product is removed from rxn. environment w gas is formed w PPT is formed w Water or undissociated, unionized subst. is formed.

Reversible Rxns. & Equilibrium Not all rxns. go to completion H 2(g) + I 2(g) n 2 HI(g) H 2 & I 2 make HI w bond betw. HI is weak & easily decomposes to H 2 & I 2.

Reversible Rxns. & Equilibrium 1 st rxn. goes from left to rt n H 2 + I 2 2 HI 2 nd rxn. goes from rt. to left n H 2 + I 2 2 HI combined eqn. represents a reversible rxn. n H 2 + I 2 2 HI Eventually reaches equilibrium

Reaction Rate If the product of a reversible rxn. decomposes faster than reactants form products, there will always be more reactant than product. Reaction Rate - the rate of appearance of a product or rate of disappearance of a reactant

Reaction Rate n n usually units are (moles/ L) / s or M/s actually measures rate of change of concentration If the 2 rxn. rates are known, we can predict whether the product or reactant will be in higher concentration @ equilibrium.
Factors Affecting Reaction Rate Nature of reactants Concentration Temperature Catalysis Surface Area Pressure n gases only
Nature of Reactants Determines kind of rxn. that occurs n Rxns. w/ bond rearrangement or e- transfer take longer w neutral molec. n n Ionic rxns. involve no e- transfer - faster Active metals & nonmetals react faster than less active ones atomic structure affects rxn. rate
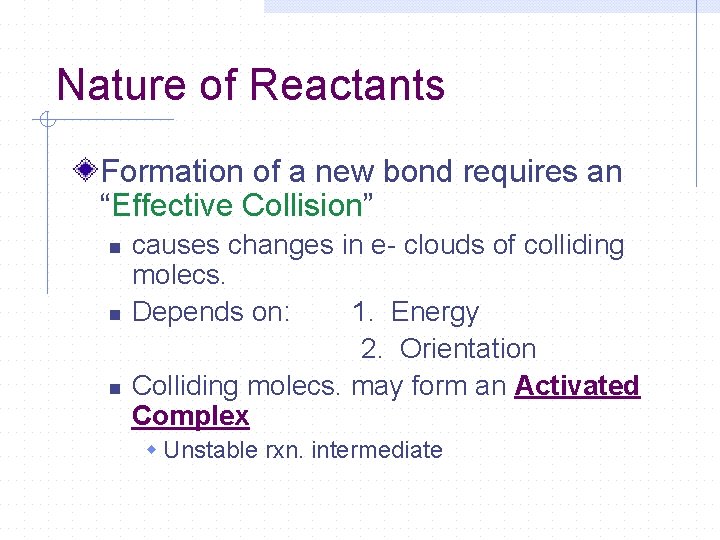
Nature of Reactants Formation of a new bond requires an “Effective Collision” n n n causes changes in e- clouds of colliding molecs. Depends on: 1. Energy 2. Orientation Colliding molecs. may form an Activated Complex w Unstable rxn. intermediate

Nature of Reactants Activation Energy - energy that must be attained in order for a collision betw. reactants to result in the formation of an activated complex n n energy to weaken or destroy original bonds If act. energy is high, few collisions have enough energy to form activated complex w Very slow rxn w Kinetically stable
![Concentration [ ] = mol / L - quantity of matter that exists in Concentration [ ] = mol / L - quantity of matter that exists in](http://slidetodoc.com/presentation_image_h2/a76db29d7664aaeb6ec5fee628467b6d/image-15.jpg)
Concentration [ ] = mol / L - quantity of matter that exists in a unit vol. - molarity (M) For a rxn. to take place, particles must collide n n If # of particles per unit vol. (conc. ) is incr. , the chance of effective collisions is incr. If conc. of 1 reactant doubles, the rate may double bec. twice as many collisions
![Concentration Ex) A + B + C n n If [A] is doubled, rate Concentration Ex) A + B + C n n If [A] is doubled, rate](http://slidetodoc.com/presentation_image_h2/a76db29d7664aaeb6ec5fee628467b6d/image-16.jpg)
Concentration Ex) A + B + C n n If [A] is doubled, rate doubles If [A] & [B] are doubled, rate incr. 4 X Ex) N 2 + 3 H 2 n D 2 NH 3 Rate 1 = k 1 [N 2] - rate varies directly w/ [N 2] Rate 2 = k 2 [H 2]3 - rate varies directly w/ [H 2] Rate 3 = k 3 [NH 3]2
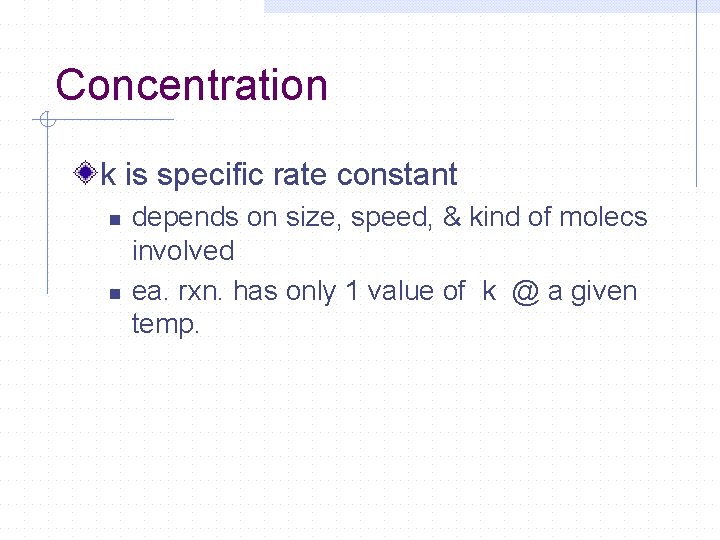
Concentration k is specific rate constant n n depends on size, speed, & kind of molecs involved ea. rxn. has only 1 value of k @ a given temp.
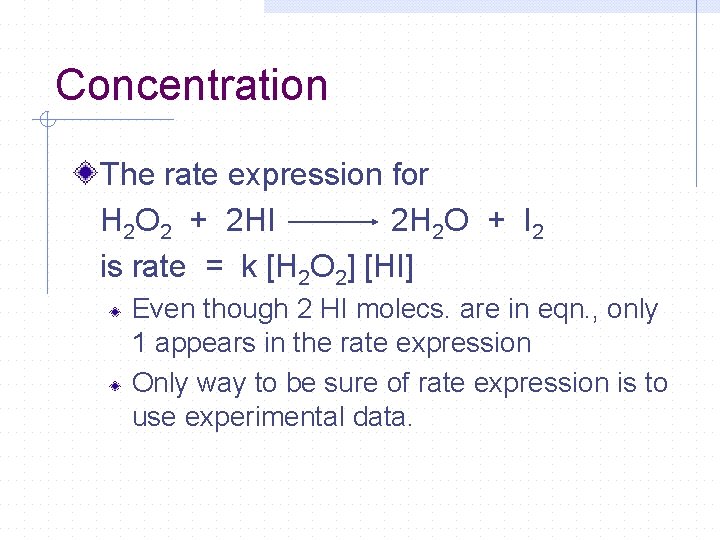
Concentration The rate expression for H 2 O 2 + 2 HI 2 H 2 O + I 2 is rate = k [H 2 O 2] [HI] Even though 2 HI molecs. are in eqn. , only 1 appears in the rate expression Only way to be sure of rate expression is to use experimental data.
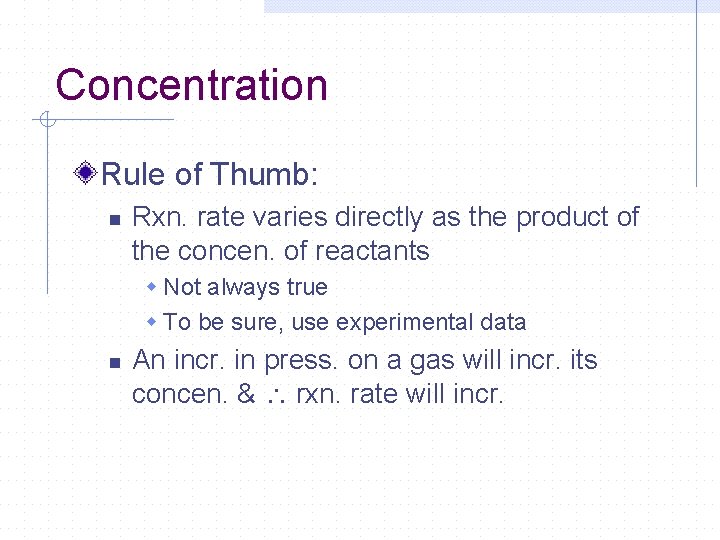
Concentration Rule of Thumb: n Rxn. rate varies directly as the product of the concen. of reactants w Not always true w To be sure, use experimental data n An incr. in press. on a gas will incr. its concen. & rxn. rate will incr.
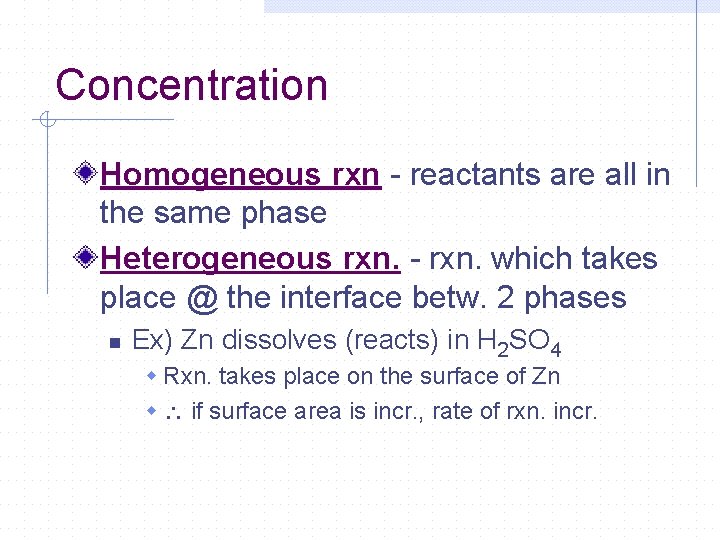
Concentration Homogeneous rxn - reactants are all in the same phase Heterogeneous rxn. - rxn. which takes place @ the interface betw. 2 phases n Ex) Zn dissolves (reacts) in H 2 SO 4 w Rxn. takes place on the surface of Zn w if surface area is incr. , rate of rxn. incr.
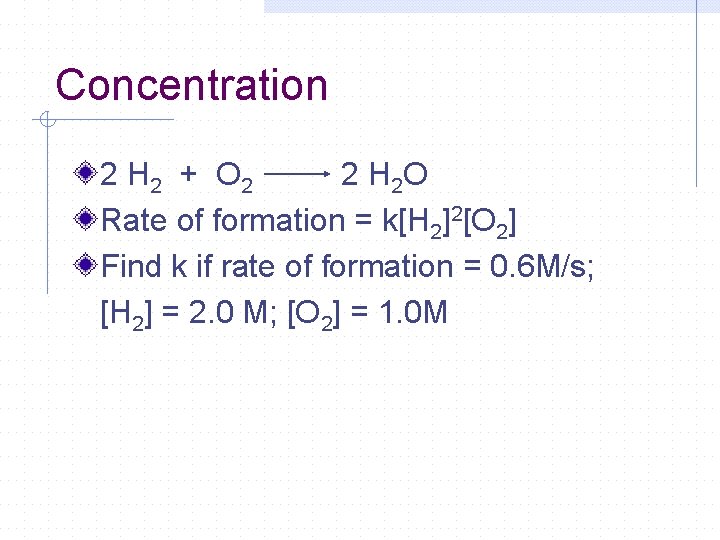
Concentration 2 H 2 + O 2 2 H 2 O Rate of formation = k[H 2]2[O 2] Find k if rate of formation = 0. 6 M/s; [H 2] = 2. 0 M; [O 2] = 1. 0 M
![Concentration In General for m. A + n. B n C rate = k[A]m[B]n Concentration In General for m. A + n. B n C rate = k[A]m[B]n](http://slidetodoc.com/presentation_image_h2/a76db29d7664aaeb6ec5fee628467b6d/image-22.jpg)
Concentration In General for m. A + n. B n C rate = k[A]m[B]n w exponents are “order of the expression n Rate Laws are determined experimentally
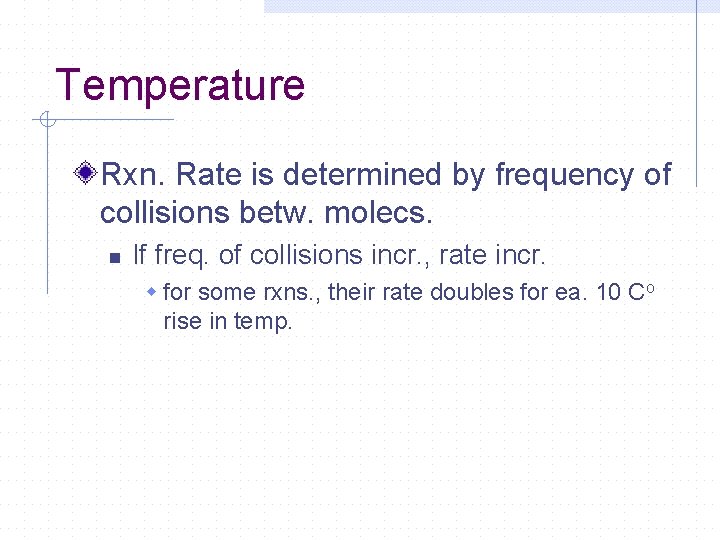
Temperature Rxn. Rate is determined by frequency of collisions betw. molecs. n If freq. of collisions incr. , rate incr. w for some rxns. , their rate doubles for ea. 10 Co rise in temp.
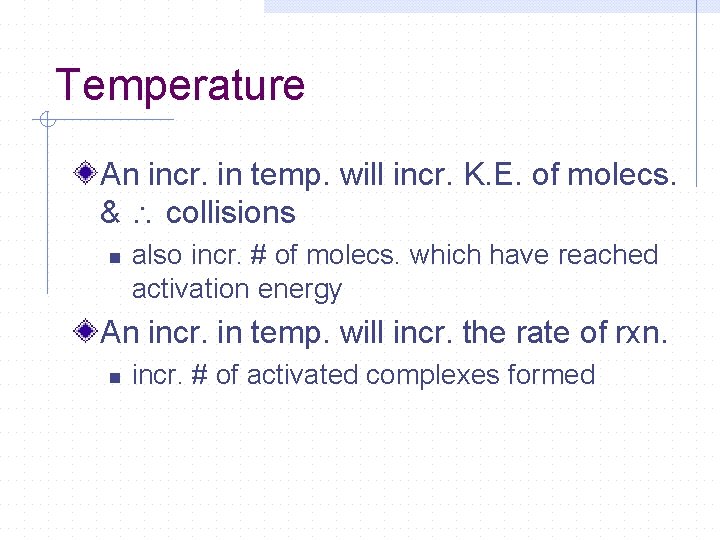
Temperature An incr. in temp. will incr. K. E. of molecs. & collisions n also incr. # of molecs. which have reached activation energy An incr. in temp. will incr. the rate of rxn. n incr. # of activated complexes formed
Catalysis The process of increasing rxn. rates by the presence of a catalyst Catalyst - subst. which incr. a rxn. rate w/out being permanently changed n decreases required activation energy
Catalysis Heterogeneous Catalyst - reactants & catalyst are not in the same state n has a surface on which the substs. can react. w adsorbs one of the reactants w Adsorbtion - the adherence of 1 subst. to the surface of another n ex) catalytic converters

Catalysis Homogeneous Catalyst - exists in same phase as reactants n enters into the rxn. - forms rxn. intermediate or activated complex w requires less activation energy n returns unchanged in final step of rxn.

Catalysis Inhibitors - “tie up” a reactant or catalyst in a complex so it will not react. n does not slow down rxn. - stops it

Reaction Mechanism Most rxns. occur in a series of steps. n usually involves collision of only 2 particles w rarely involve 3 or more particles

Reaction Mechanism If a rxn. consists of several steps: A B; B C; C final product One of the steps will be slower than all the others n n Rate Determining Step Faster steps will not affect the rate
Reaction Mechanism - The series of steps that must occur for a rxn. to go to completion n @ a given temp. , the rate of a rxn. varies directly w/ the product of the concentrations of the reactants in the slowest step.
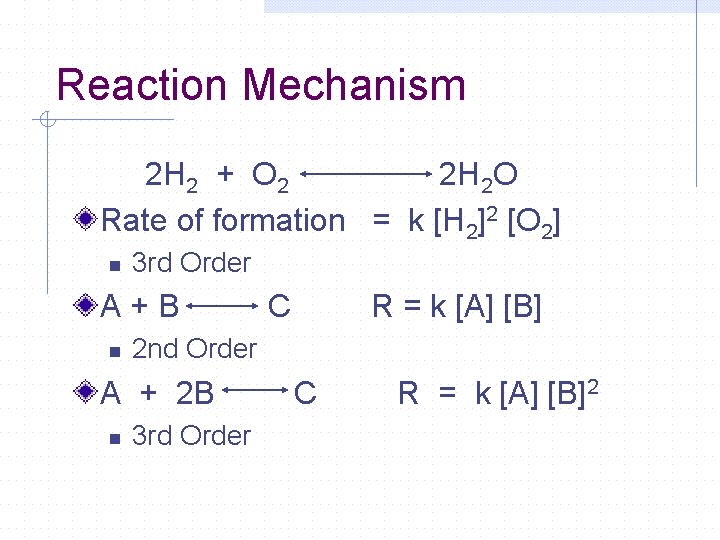
Reaction Mechanism 2 H 2 + O 2 2 H 2 O Rate of formation = k [H 2]2 [O 2] n 3 rd Order A+B n R = k [A] [B] 2 nd Order A + 2 B n C 3 rd Order C R = k [A] [B] 2
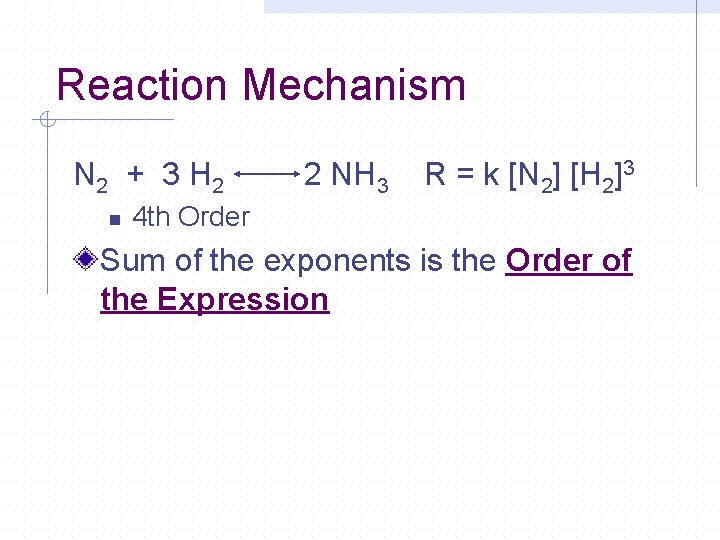
Reaction Mechanism N 2 + 3 H 2 n 2 NH 3 R = k [N 2] [H 2]3 4 th Order Sum of the exponents is the Order of the Expression
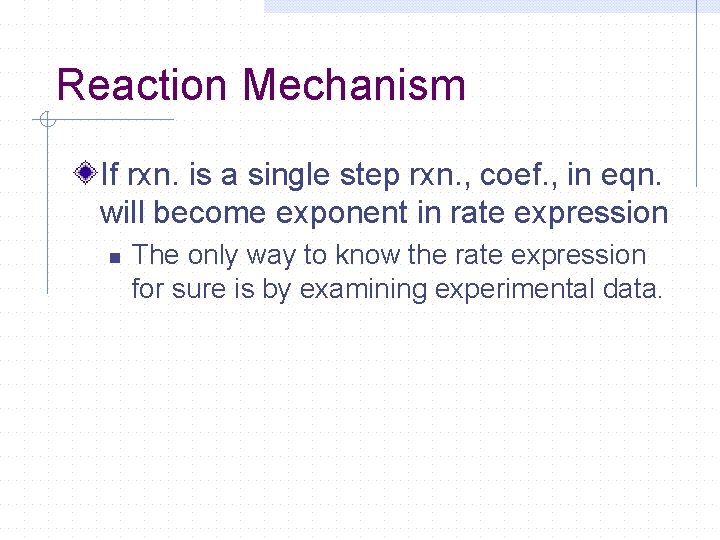
Reaction Mechanism If rxn. is a single step rxn. , coef. , in eqn. will become exponent in rate expression n The only way to know the rate expression for sure is by examining experimental data.

Equilibrium Constant H 2 + I 2 2 HI (Forward rxn. ) As rxn starts, lots of H 2 & I 2, no HI n as rxn. proceeds, there’s less & less H 2 &I 2 w fewer molecs. mean fewer collisions n There’s more & more HI w rxn. of 2 HI H 2 + I 2 is incr. (reverse rxn. )
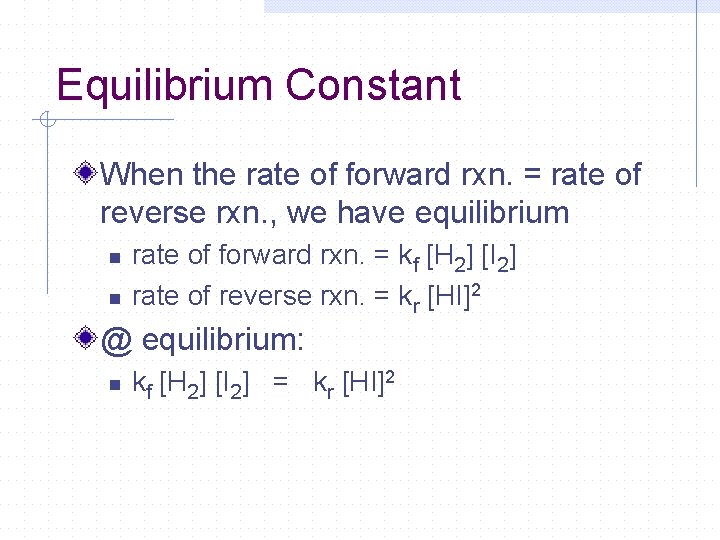
Equilibrium Constant When the rate of forward rxn. = rate of reverse rxn. , we have equilibrium n n rate of forward rxn. = kf [H 2] [I 2] rate of reverse rxn. = kr [HI]2 @ equilibrium: n kf [H 2] [I 2] = kr [HI]2
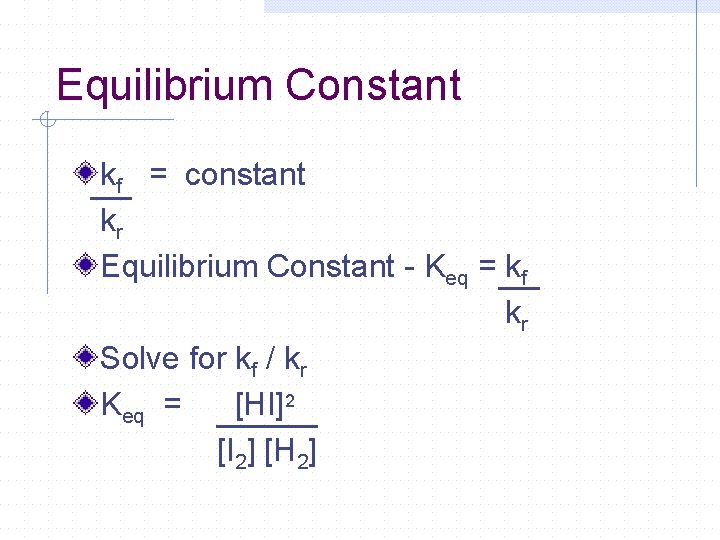
Equilibrium Constant kf = constant kr Equilibrium Constant - Keq = kf kr Solve for kf / kr Keq = [HI]2 [I 2] [H 2]
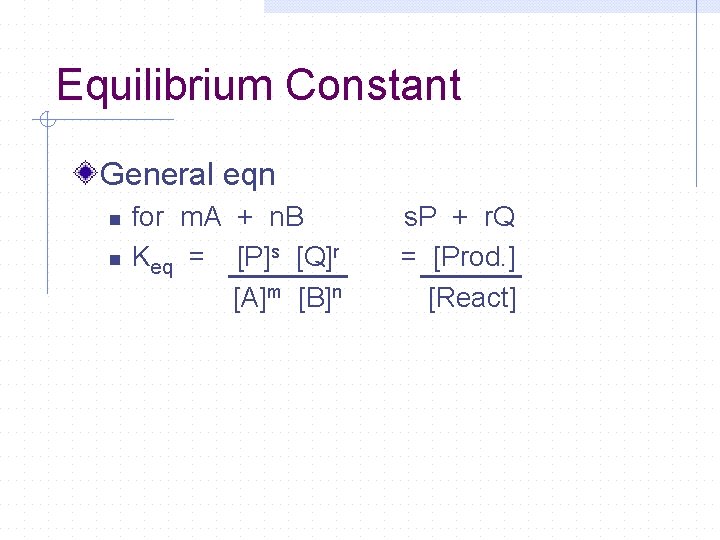
Equilibrium Constant General eqn n n for m. A + n. B Keq = [P]s [Q]r [A]m [B]n s. P + r. Q = [Prod. ] [React]

Equilibrium Constant If Keq is small (<1), very little product is formed. n Reactant is favored. If Keq is lg. (>1), rxn. is nearly complete n n much product is formed product is favored.

Equilibrium Constant What is the equilibrium constant for the following rxn. if the final concentrations are CH 3 COOH = 0. 302 M, CH 3 CH 2 OH = 0. 428 M, H 2 O = 0. 654 M, and CH 3 CH 2 OOCCH 3 = 0. 655 M? CH 3 COOH + CH 3 CH 2 OH H 2 O + CH 3 CH 2 OOCCH 3

Equilibrium Constant What is the equilibrium concentration of SO 3 in the following rxn. if the concentrations of SO 2 and O 2 are each 0. 0500 M and Keq = 85. 0? 2 SO 2 + O 2 2 SO 3

Le Chatelier’s Principle Conditions affecting equilibrium: 1. Temp. 2. Press. 3. Concentration (of prods. & reacts. ) If a condition is changed (stress) on a syst. in equilib. , then the equilib. will shift to restore the original conditions (relieve the stress).
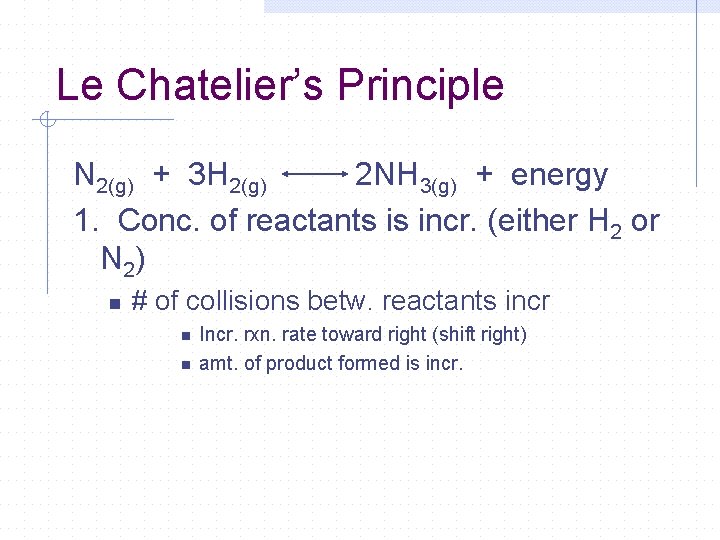
Le Chatelier’s Principle N 2(g) + 3 H 2(g) 2 NH 3(g) + energy 1. Conc. of reactants is incr. (either H 2 or N 2) n # of collisions betw. reactants incr n n Incr. rxn. rate toward right (shift right) amt. of product formed is incr.
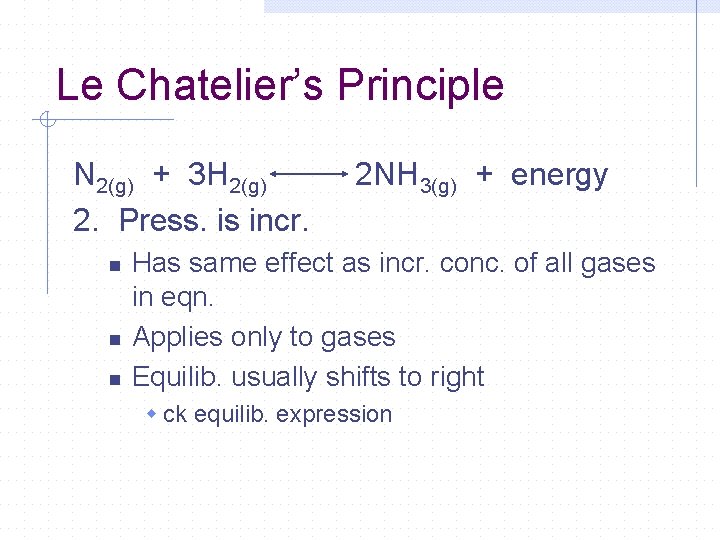
Le Chatelier’s Principle N 2(g) + 3 H 2(g) 2. Press. is incr. n n n 2 NH 3(g) + energy Has same effect as incr. conc. of all gases in eqn. Applies only to gases Equilib. usually shifts to right w ck equilib. expression
![Le Chatelier’s Principle Keq = n n [NH 3]2 [N 2] [H 2]3 If Le Chatelier’s Principle Keq = n n [NH 3]2 [N 2] [H 2]3 If](http://slidetodoc.com/presentation_image_h2/a76db29d7664aaeb6ec5fee628467b6d/image-45.jpg)
Le Chatelier’s Principle Keq = n n [NH 3]2 [N 2] [H 2]3 If press. doubles, reverse rxn. must speed up by a factor of 4 since [H 2] is cubed doubling press. (which doubles conc. ) speeds up forward rxn. by a factor of 16

Le Chatelier’s Principle In H 2(g) + Cl 2(g) n 2 HCl(g) Doubling press. will not shift equilib. w Why? w Rate in ea. direction is affected the same way. An incr. is press. will always drive a rxn. in the direction of the smaller # of moles of gas. n Press. affects only gases
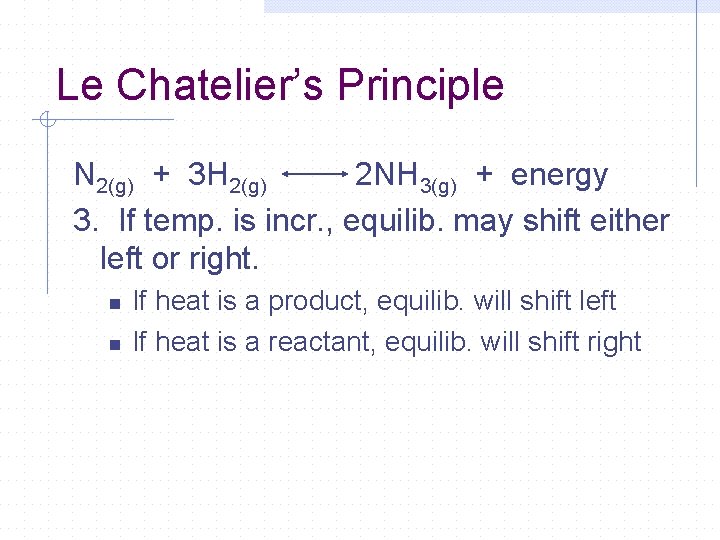
Le Chatelier’s Principle N 2(g) + 3 H 2(g) 2 NH 3(g) + energy 3. If temp. is incr. , equilib. may shift either left or right. n n If heat is a product, equilib. will shift left If heat is a reactant, equilib. will shift right

Optimum Conditions which produce hightest yield. In Haber process: 1. High conc. of H 2 & N 2 should be maintained. 2. NH 3 should be removed as it’s formed. 3. Temp. should be high enough to maintain a reasonable rate, but low enough not to favor reverse rxn.
Optimum Conditions 4. Catalyst should be used to lower activation energy 5. High press. should be maintained.
- Slides: 49