CHAPTER 22 OxidationReduction Reactions LEO SAYS GER or















- Slides: 18

CHAPTER 22 “Oxidation-Reduction Reactions” LEO SAYS GER or OIL REG

Oxidation and Reduction (Redox) Early chemists saw “_oxidation_” reactions only as the _combination of a material with oxygen to produce an oxide. • For example, when methane burns in air, it oxidizes and forms oxides of _carbon____ and _hydrogen___.

Oxidation and Reduction (Redox) But, not all _oxidation processes_ that use oxygen involve burning___: • Elemental iron slowly oxidizes to compounds such as iron (III) oxide, commonly called “_rust__” • Bleaching stains in fabrics • Hydrogen peroxide also releases __oxygen__ when it decomposes.

Oxidation and Reduction (Redox) A process called “_reduction__” is the opposite of oxidation, and originally meant the _loss of oxygen_ from a compound Oxidation and reduction _always occur simultaneously. The substance gaining oxygen (or losing electrons) is _oxidized_, while the substance losing oxygen (or gaining electrons) is _reduced_.

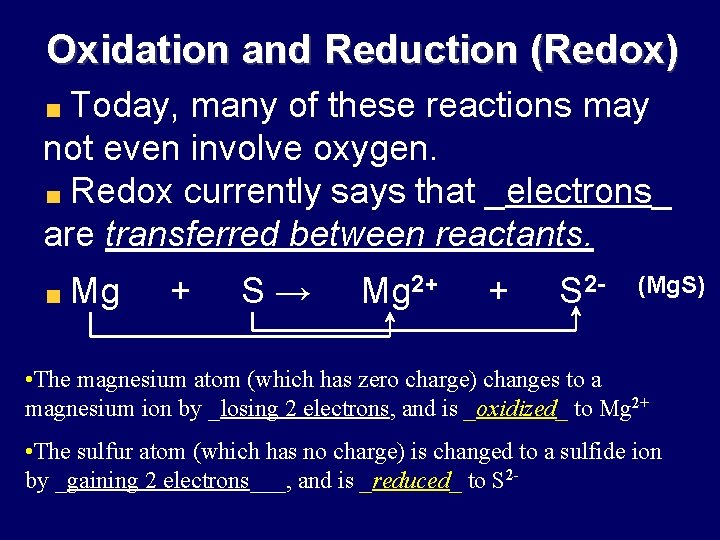
Oxidation and Reduction (Redox) Today, many of these reactions may not even involve oxygen. Redox currently says that _electrons_ are transferred between reactants. Mg + S→ Mg 2+ + S 2 - (Mg. S) • The magnesium atom (which has zero charge) changes to a magnesium ion by _losing 2 electrons, and is _oxidized_ to Mg 2+ • The sulfur atom (which has no charge) is changed to a sulfide ion by _gaining 2 electrons___, and is _reduced_ to S 2 -

Oxidation and Reduction (Redox) Each sodium atom _loses_ one electron: Each chlorine atom _gains_ one electron:

LEO says GER : Lose Electrons = Oxidation Sodium is oxidized Gain Electrons = Reduction Chlorine is reduced

LEO says GER : - Losing electrons is oxidation, and the substance that loses the electrons is called the _reducing agent_. - Gaining electrons is reduction, and the substance that gains the electrons is called the _oxidizing agent_. Mg is the reducing agent Mg is oxidized: loses e-, becomes a Mg 2+ ion Mg(s) + S(s) → Mg. S(s) S is the oxidizing agent S is reduced: gains e- = S 2 - ion

Not All Reactions are Redox Reactions - Reactions in which there has been no change in oxidation number are NOT redox reactions. Examples:

Corrosion • Damage done to metal is costly to prevent and repair • Iron, a common construction metal often used in forming steel alloys, corrodes by being oxidized to ions of iron by oxygen. • This corrosion is even faster in the presence of salts and acids, because these materials make _electrically conductive solutions_ that make electron transfer easy

Corrosion • Luckily, not all metals corrode easily • Gold and platinum are called _noble metals_ because they are _resistant_ to losing their electrons by corrosion • Other metals may lose their electrons easily, but are protected from corrosion by the oxide coating on their surface, such as __aluminum_. • Iron has an oxide coating, but it is not tightly packed, so water and air can penetrate it easily

Corrosion • Serious problems can result if bridges, storage tanks, or hulls of ships _corrode_. • Can be _prevented_ by a coating of oil, paint, plastic, or another metal • If this surface is scratched or worn away, the protection is lost • Other methods of prevention involve the “sacrifice” of one metal to save the second • Magnesium, chromium, or even zinc (called galvanized) coatings can be applied

Rules for Assigning Oxidation Numbers 1) The oxidation number of any uncombined element is _zero__. 2) The oxidation number of a monatomic ion equals its charge.

Rules for Assigning Oxidation Numbers 3) The oxidation number of _oxygen in compounds_ is -2, except in peroxides, such as H 2 O 2 where it is -1. 4) The oxidation number of hydrogen in compounds is +1, except in metal hydrides, like Na. H, where it is -1.

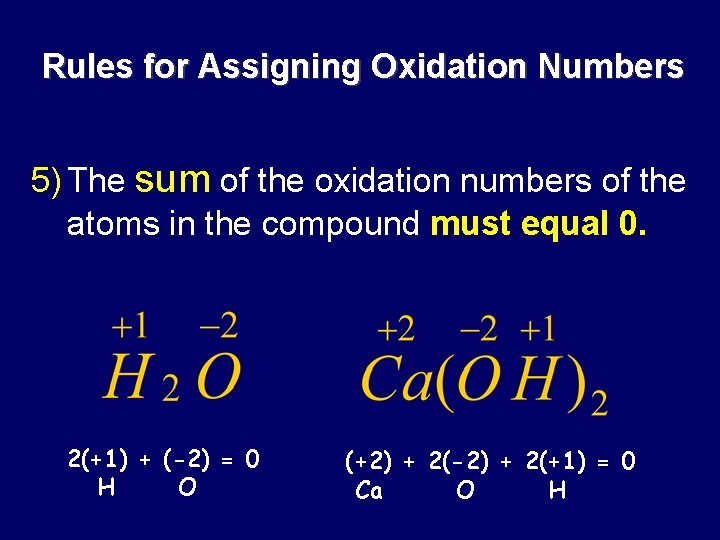
Rules for Assigning Oxidation Numbers 5) The sum of the oxidation numbers of the atoms in the compound must equal 0. 2(+1) + (-2) = 0 H O (+2) + 2(-2) + 2(+1) = 0 Ca O H

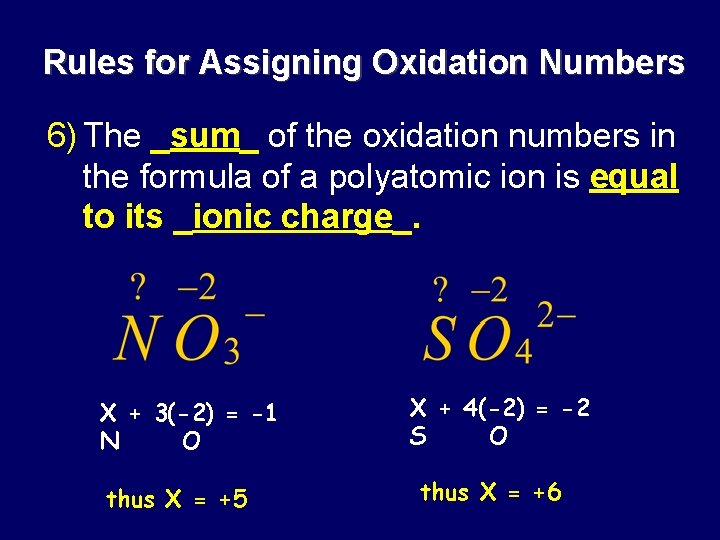
Rules for Assigning Oxidation Numbers 6) The _sum_ of the oxidation numbers in the formula of a polyatomic ion is equal to its _ionic charge_. X + 3(-2) = -1 N O thus X = +5 X + 4(-2) = -2 S O thus X = +6

Reducing Agents and Oxidizing Agents • An increase in oxidation number = oxidation_ • A decrease in oxidation number = _reduction_ Sodium is _oxidized_– it is the reducing agent Chlorine is reduced – it is the oxidizing agent
