Chapter 22 Organic and Biological Molecules Chapter 22

Chapter 22 Organic and Biological Molecules

Chapter 22 Organic Chemistry and Biochemistry § Organic Chemistry § The study of carbon-containing compounds and their properties. The vast majority of organic compounds contain chains or rings of carbon atoms. § Biochemistry § The study of the chemistry of living things. Copyright © Cengage Learning. All rights reserved 2
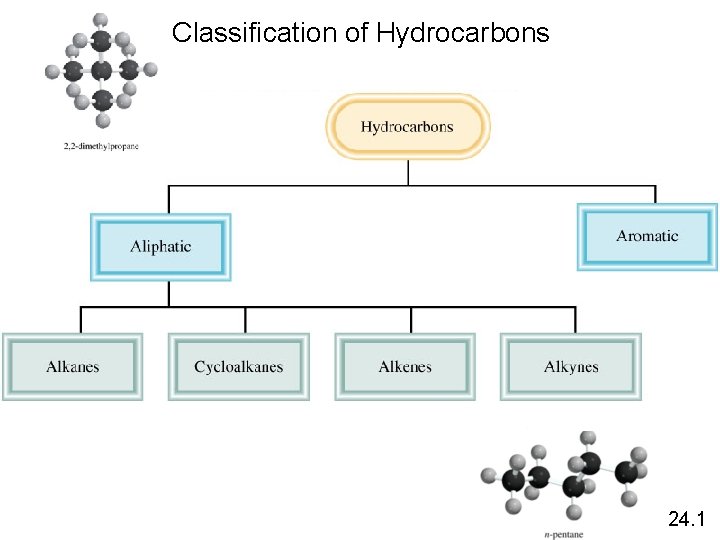
Classification of Hydrocarbons 24. 1
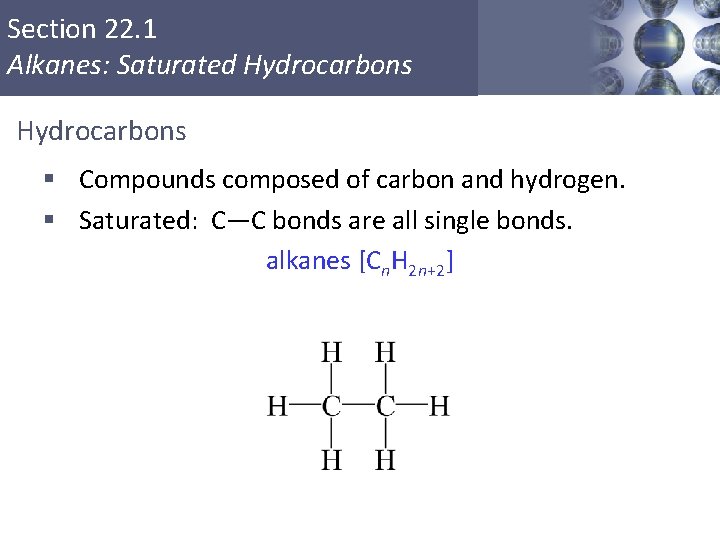
Section 22. 1 Alkanes: Saturated Hydrocarbons § Compounds composed of carbon and hydrogen. § Saturated: C—C bonds are all single bonds. alkanes [Cn. H 2 n+2] Copyright © Cengage Learning. All rights reserved 4
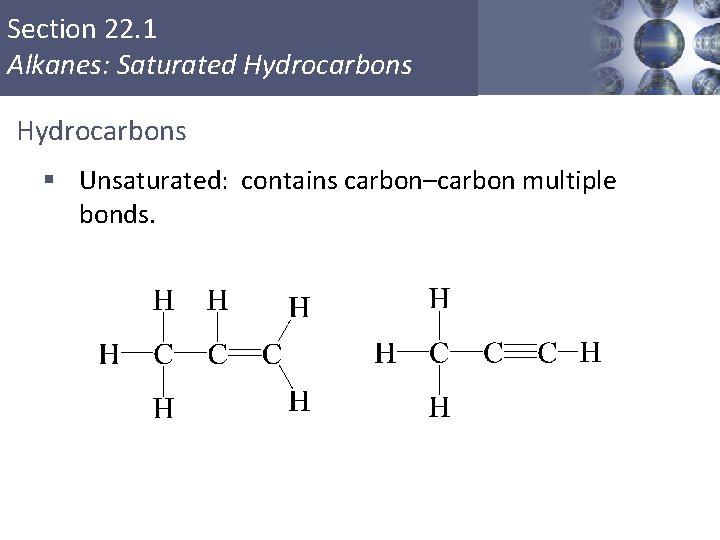
Section 22. 1 Alkanes: Saturated Hydrocarbons § Unsaturated: contains carbon–carbon multiple bonds.

Section 22. 1 Alkanes: Saturated Hydrocarbons Isomerism in Alkanes § Structural isomerism – occurs when two molecules have the same atoms but different bonds. § Butane and all succeeding members of the alkanes exhibit structural isomerism. Copyright © Cengage Learning. All rights reserved 6
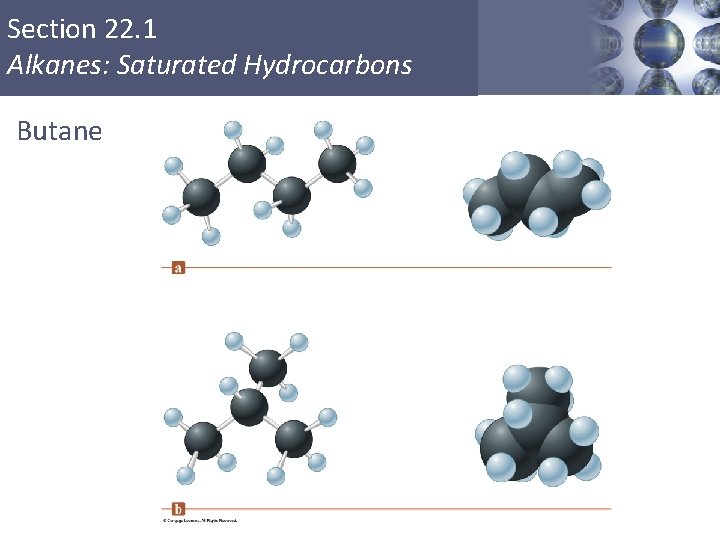
Section 22. 1 Alkanes: Saturated Hydrocarbons Butane Copyright © Cengage Learning. All rights reserved 7

Section 22. 1 Alkanes: Saturated Hydrocarbons Rules for Naming Alkanes 1. For alkanes beyond butane, add –ane to the Greek root for the number of carbons. CH 3–CH 2–CH 3 = hexane 2. Alkyl substituents: drop the –ane and add –yl. C 2 H 6 is ethane C 2 H 5 is ethyl Copyright © Cengage Learning. All rights reserved 8

Section 22. 1 Alkanes: Saturated Hydrocarbons Rules for Naming Alkanes 3. Positions of substituent groups are specified by numbering the longest chain sequentially. The numbering is such that substituents are at lowest possible number along chain. CH 3–CH 2–CH 3 1 2 3 4 5 6 3 -methylhexane Copyright © Cengage Learning. All rights reserved 9
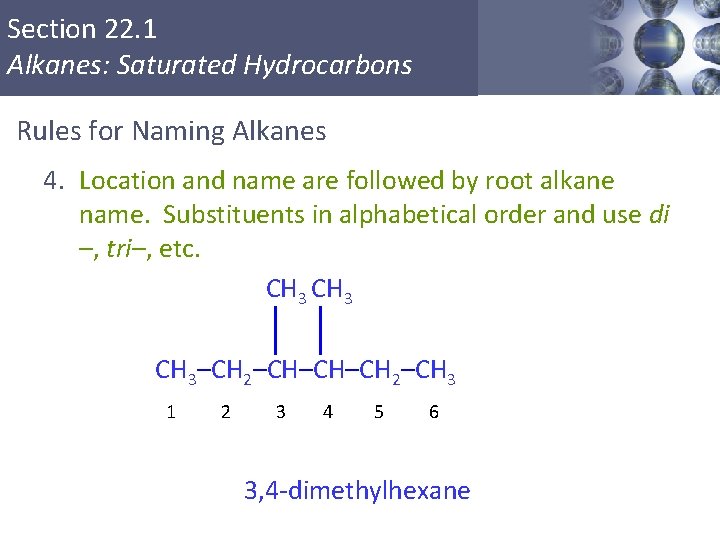
Section 22. 1 Alkanes: Saturated Hydrocarbons Rules for Naming Alkanes 4. Location and name are followed by root alkane name. Substituents in alphabetical order and use di –, tri–, etc. CH 3–CH 2–CH–CH–CH 2–CH 3 1 2 3 4 5 6 3, 4 -dimethylhexane Copyright © Cengage Learning. All rights reserved 10
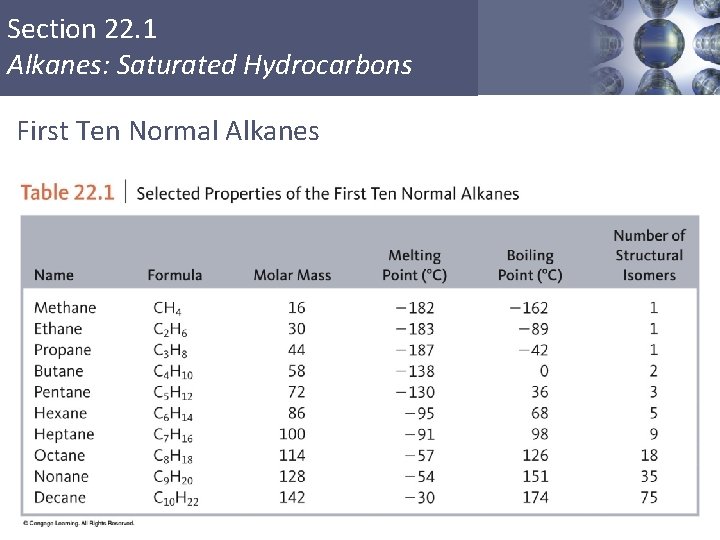
Section 22. 1 Alkanes: Saturated Hydrocarbons First Ten Normal Alkanes Copyright © Cengage Learning. All rights reserved 11
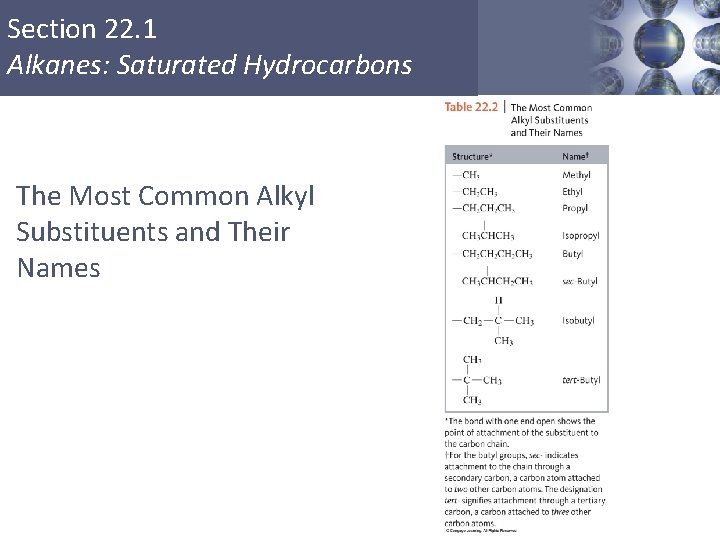
Section 22. 1 Alkanes: Saturated Hydrocarbons The Most Common Alkyl Substituents and Their Names
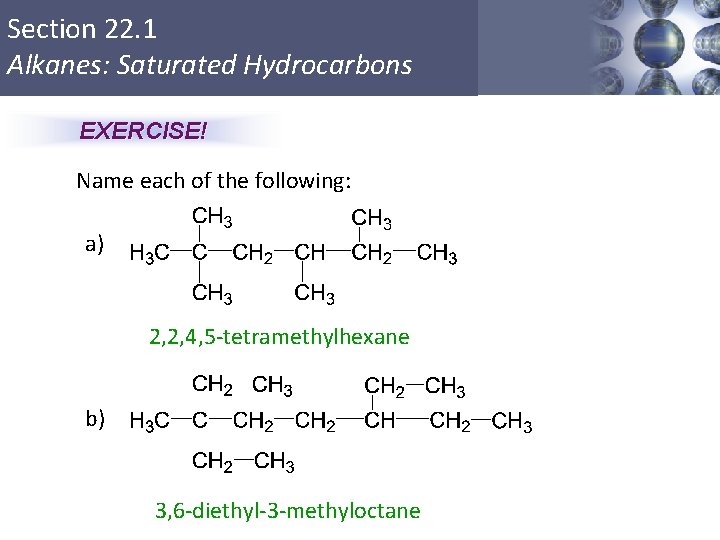
Section 22. 1 Alkanes: Saturated Hydrocarbons EXERCISE! Name each of the following: a) 2, 2, 4, 5 -tetramethylhexane b) 3, 6 -diethyl-3 -methyloctane Copyright © Cengage Learning. All rights reserved 13
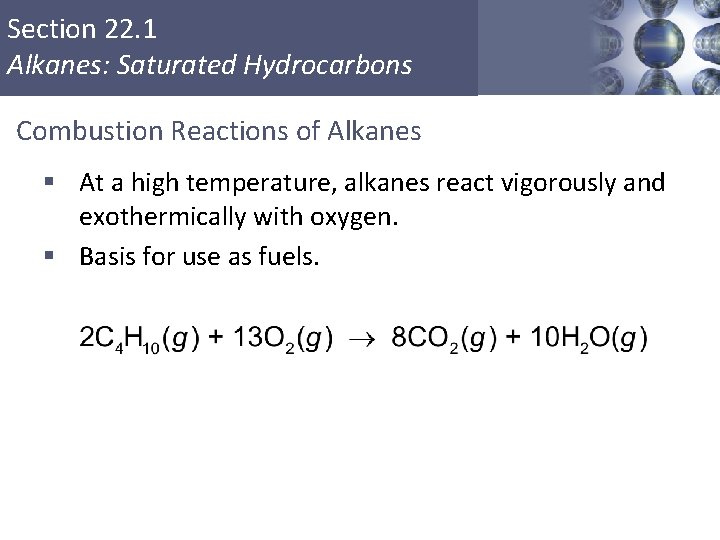
Section 22. 1 Alkanes: Saturated Hydrocarbons Combustion Reactions of Alkanes § At a high temperature, alkanes react vigorously and exothermically with oxygen. § Basis for use as fuels. Copyright © Cengage Learning. All rights reserved 14
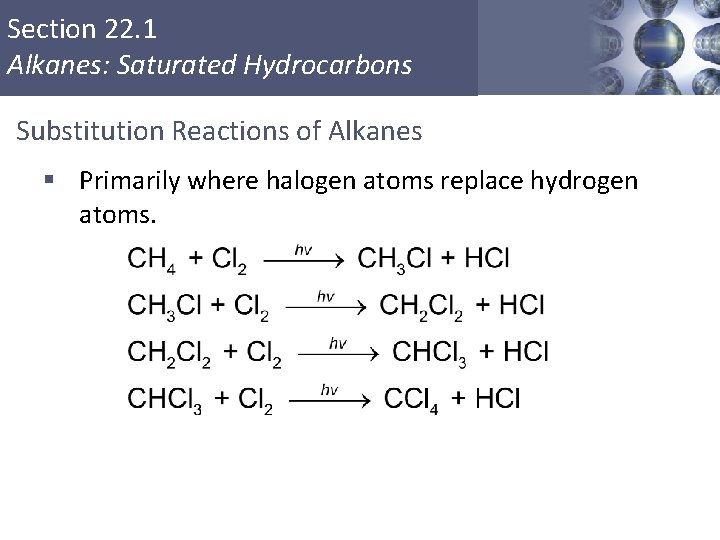
Section 22. 1 Alkanes: Saturated Hydrocarbons Substitution Reactions of Alkanes § Primarily where halogen atoms replace hydrogen atoms. Copyright © Cengage Learning. All rights reserved 15
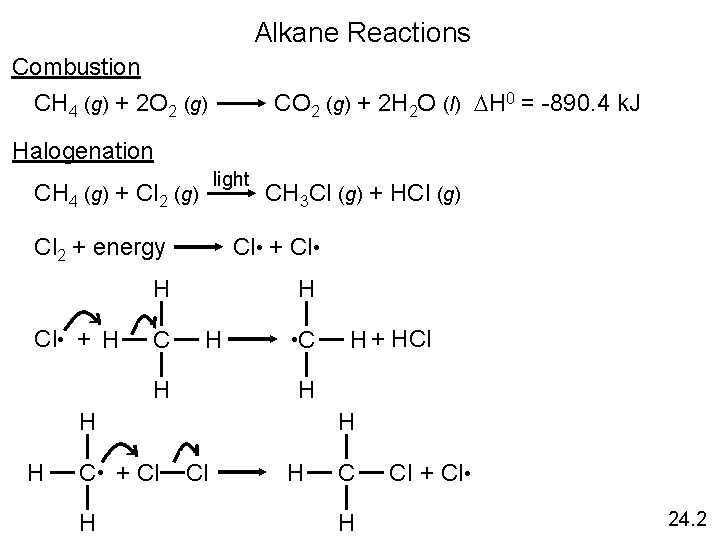
Alkane Reactions Combustion CO 2 (g) + 2 H 2 O (l) DH 0 = -890. 4 k. J CH 4 (g) + 2 O 2 (g) Halogenation light CH 4 (g) + Cl 2 (g) CH 3 Cl (g) + HCl (g) Cl 2 + energy Cl • + Cl • H H Cl • + H C H H • C H H H C • + Cl H H + HCl H C H Cl + Cl • 24. 2

Section 22. 1 Alkanes: Saturated Hydrocarbons Dehydrogenation Reactions of Alkanes § Hydrogen atoms are removed and the product is an unsaturated hydrocarbon. Copyright © Cengage Learning. All rights reserved 17
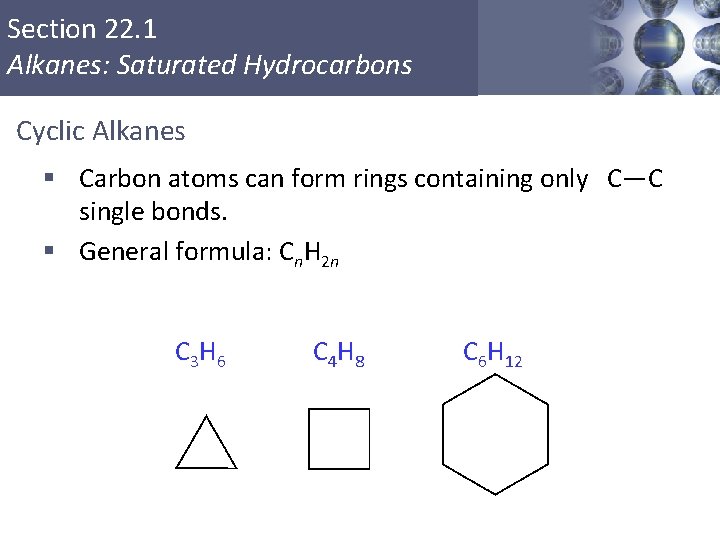
Section 22. 1 Alkanes: Saturated Hydrocarbons Cyclic Alkanes § Carbon atoms can form rings containing only C—C single bonds. § General formula: Cn. H 2 n C 3 H 6 Copyright © Cengage Learning. All rights reserved C 4 H 8 C 6 H 12 18
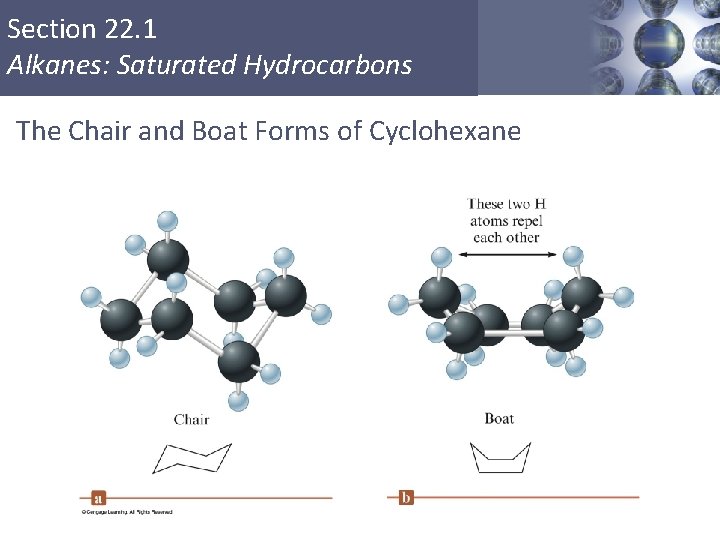
Section 22. 1 Alkanes: Saturated Hydrocarbons The Chair and Boat Forms of Cyclohexane Copyright © Cengage Learning. All rights reserved 19
Sources of Alkanes and Cycloalkanes • Petroleum, or crude oil, is a mixture of alkanes and cycloalkanes with small amount of aromatic hydrocarbons. • Crude oil from different regions differ in their compositions of these compounds. • 1. Oil from the Canadian north slope contain molecules composed of 20 to 40 carbon atoms. • 2. Oil from Saudi Arabia contain molecules composed of 5 to 20 carbon atoms. This oil is more desirable as a source because it is easier to transport crude oil containing low molecular mass molecules. • Hydrocarbon mixtures in crude oil are separated by a process known as fractional distillation. This process separates molecules according to molecular mass. 23 | 20

Section 22. 2 Alkenes and Alkynes Hydrocarbons § Alkenes: hydrocarbons that contain at least one carbon– carbon double bond. [Cn. H 2 n] CH 3–CH=CH 2 propene § Alkynes: hydrocarbons containing at least one carbon– carbon triple bond. [Cn. Hn] CH 3–CH 2–CΞC–CH 3 2–pentyne

Section 22. 2 Alkenes and Alkynes Rules for Naming Alkenes 1. Root hydrocarbon name ends in –ene. C 2 H 4 is ethene 2. With more than 3 carbons, double bond is indicated by the lowest–numbered carbon atom in the bond. CH 2=CH–CH 2–CH 3 1 2 3 4 1–butene Copyright © Cengage Learning. All rights reserved 22

Section 22. 2 Alkenes and Alkynes Rules for Naming Alkynes § Same as for alkenes except use –yne as suffix. CH 3–CH 2–CΞC–CH 2–CH 3 3–octyne Copyright © Cengage Learning. All rights reserved 23
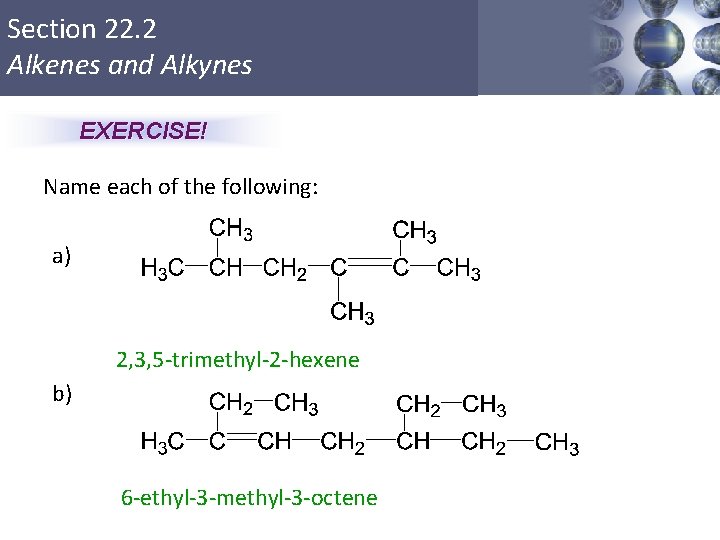
Section 22. 2 Alkenes and Alkynes EXERCISE! Name each of the following: a) 2, 3, 5 -trimethyl-2 -hexene b) 6 -ethyl-3 -methyl-3 -octene Copyright © Cengage Learning. All rights reserved 24
Addition Reactions of Alkenes are more reactive than alkanes owing to the presence of the double bond. Many reactions add to the double bond. In an addition reaction, parts of a reactant are added to each carbon atom of a carbon– carbon double bond, which converts it to a carbon–carbon single bond. 23 | 25
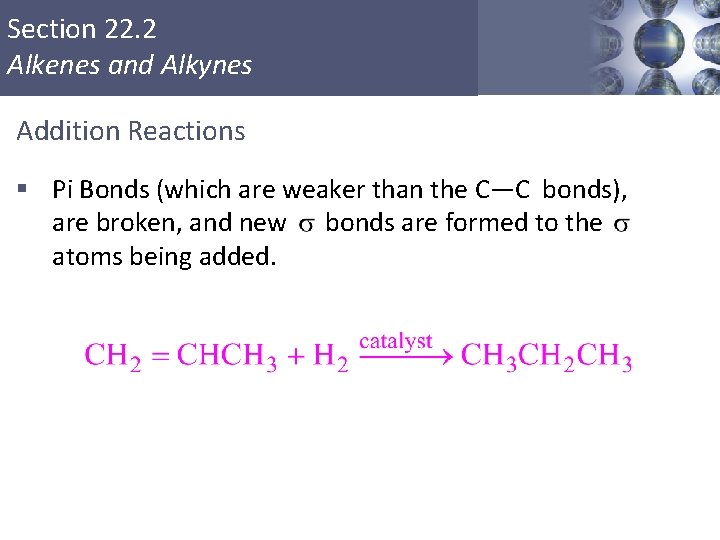
Section 22. 2 Alkenes and Alkynes Addition Reactions § Pi Bonds (which are weaker than the C—C bonds), are broken, and new bonds are formed to the atoms being added. Copyright © Cengage Learning. All rights reserved 26

Section 22. 2 Alkenes and Alkynes Halogenation Reactions § Addition of halogen atoms of alkenes and alkynes. Copyright © Cengage Learning. All rights reserved 27

Section 22. 3 Aromatic Hydrocarbons § A special class of cyclic unsaturated hydrocarbons. § Simplest of these is benzene (C 6 H 6). § The delocalization of the electrons makes the benzene ring behave differently from a typical unsaturated hydrocarbon. Copyright © Cengage Learning. All rights reserved 28
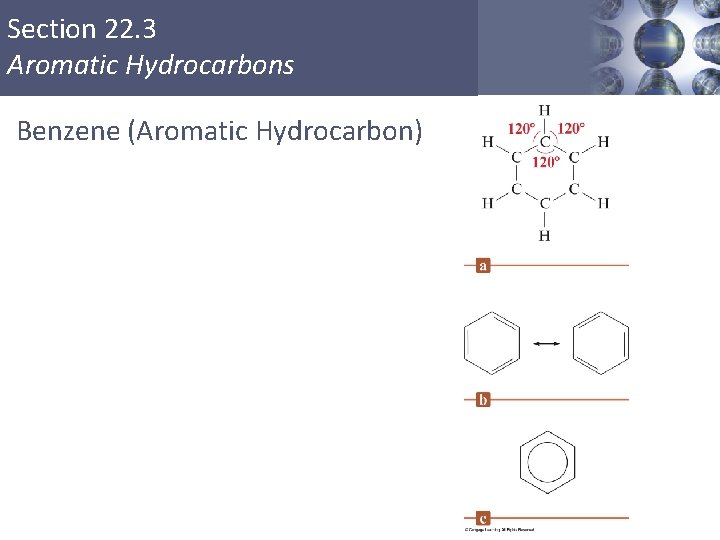
Section 22. 3 Aromatic Hydrocarbons Benzene (Aromatic Hydrocarbon)
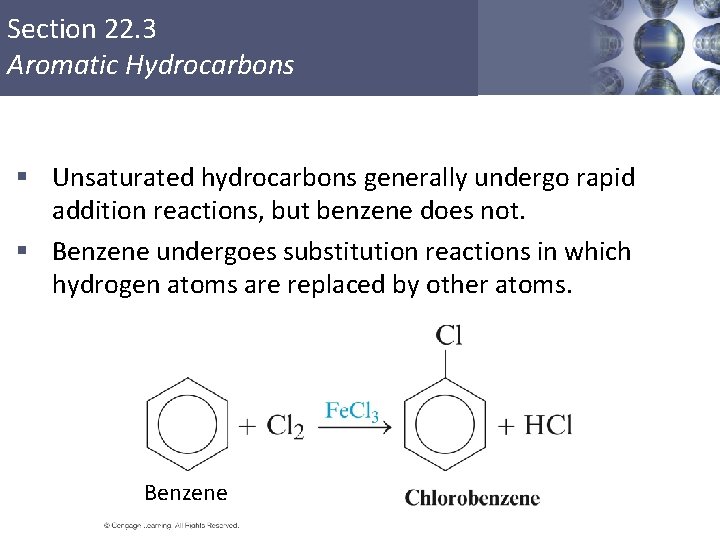
Section 22. 3 Aromatic Hydrocarbons § Unsaturated hydrocarbons generally undergo rapid addition reactions, but benzene does not. § Benzene undergoes substitution reactions in which hydrogen atoms are replaced by other atoms. Benzene Copyright © Cengage Learning. All rights reserved 30
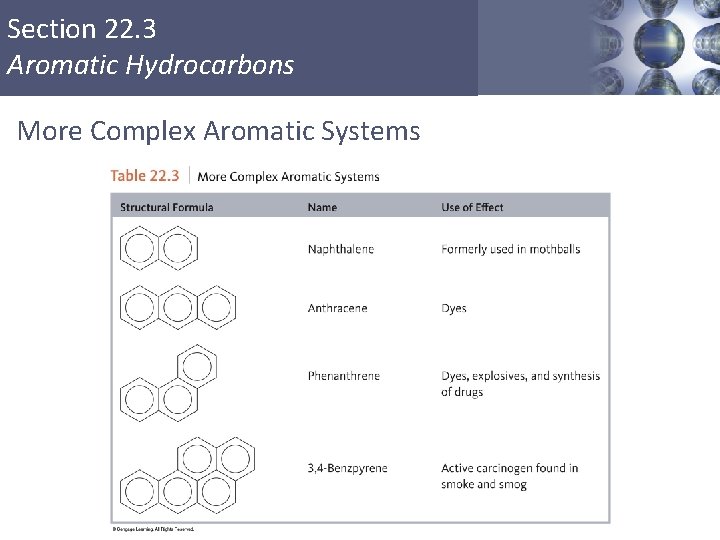
Section 22. 3 Aromatic Hydrocarbons More Complex Aromatic Systems Copyright © Cengage Learning. All rights reserved 31

Section 22. 4 Hydrocarbon Derivatives § Molecules that are fundamentally hydrocarbons but have additional atoms or groups of atoms called functional groups. Copyright © Cengage Learning. All rights reserved 32
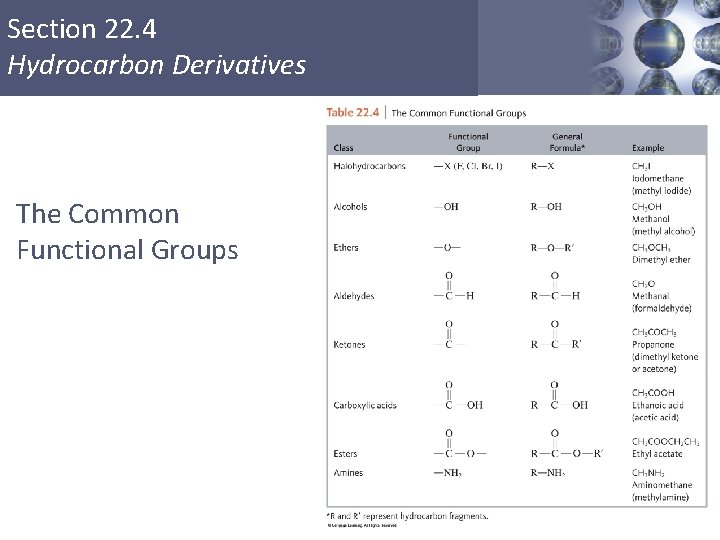
Section 22. 4 Hydrocarbon Derivatives The Common Functional Groups Copyright © Cengage Learning. All rights reserved 33

Section 22. 5 Polymers § Large, usually chainlike molecules that are built from small molecules called monomers. Copyright © Cengage Learning. All rights reserved 34
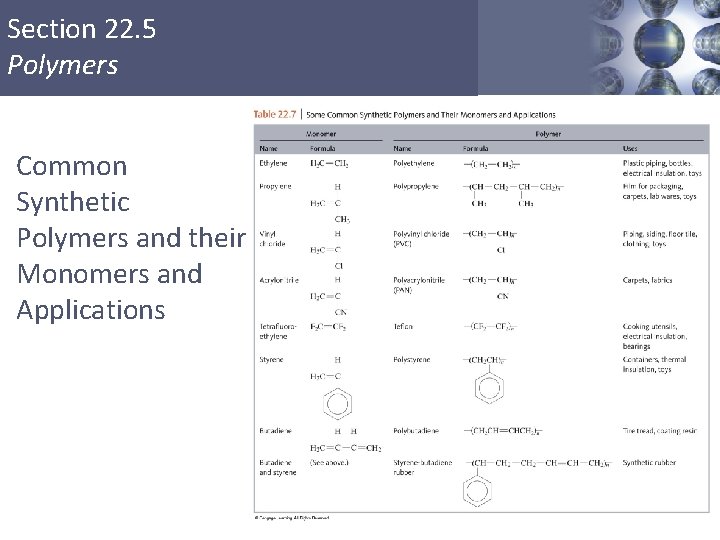
Section 22. 5 Polymers Common Synthetic Polymers and their Monomers and Applications Copyright © Cengage Learning. All rights reserved 35
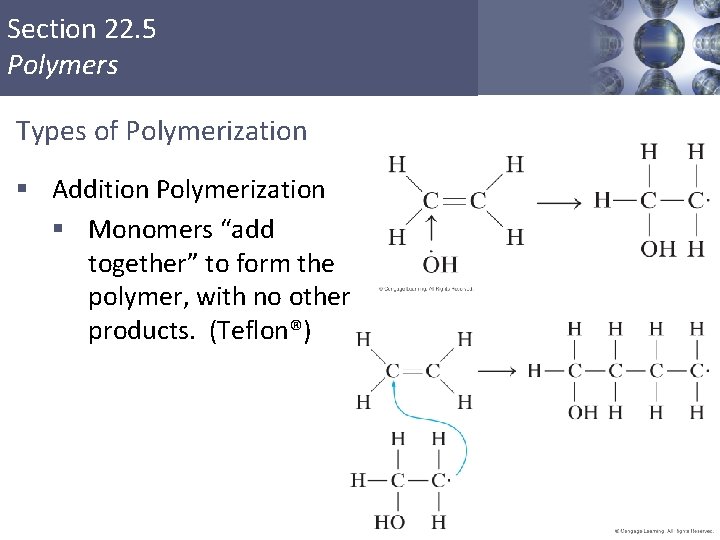
Section 22. 5 Polymers Types of Polymerization § Addition Polymerization § Monomers “add together” to form the polymer, with no other products. (Teflon®)
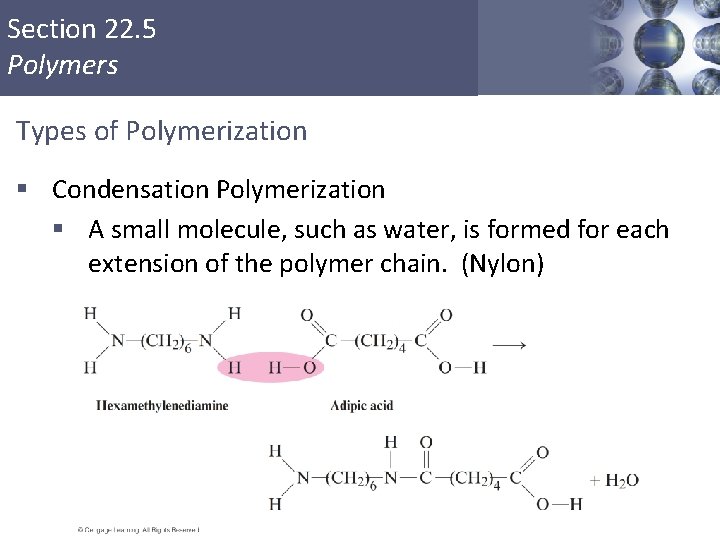
Section 22. 5 Polymers Types of Polymerization § Condensation Polymerization § A small molecule, such as water, is formed for each extension of the polymer chain. (Nylon) Copyright © Cengage Learning. All rights reserved 37

Section 22. 6 Natural Polymers Proteins § Natural polymers made up of �-amino acids with molar masses: ~ 6000 to > 1, 000 g/mol § Fibrous Proteins: provide structural integrity and strength to muscle, hair and cartilage. Copyright © Cengage Learning. All rights reserved 38

Section 22. 6 Natural Polymers Proteins § Globular Proteins: § Roughly spherical shape § Transport and store oxygen and nutrients § Act as catalysts § Fight invasion by foreign objects § Participate in the body’s regulatory system § Transport electrons in metabolism Copyright © Cengage Learning. All rights reserved 39
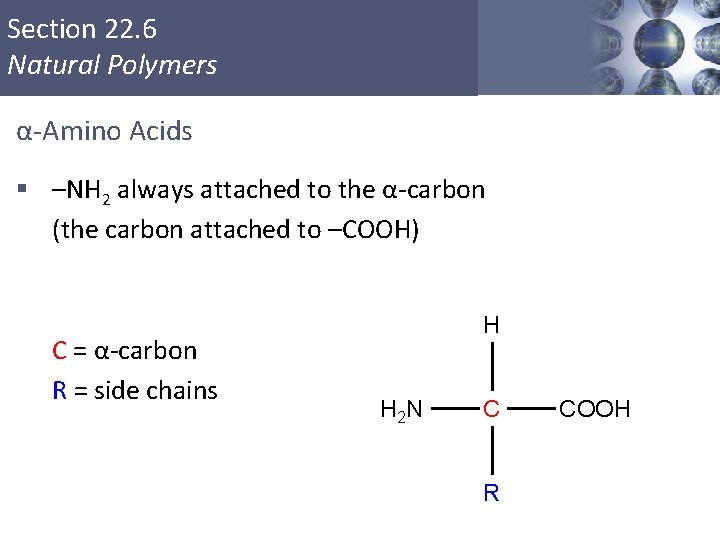
Section 22. 6 Natural Polymers α-Amino Acids § –NH 2 always attached to the α-carbon (the carbon attached to –COOH) C = α-carbon R = side chains H H 2 N C COOH R Copyright © Cengage Learning. All rights reserved 40
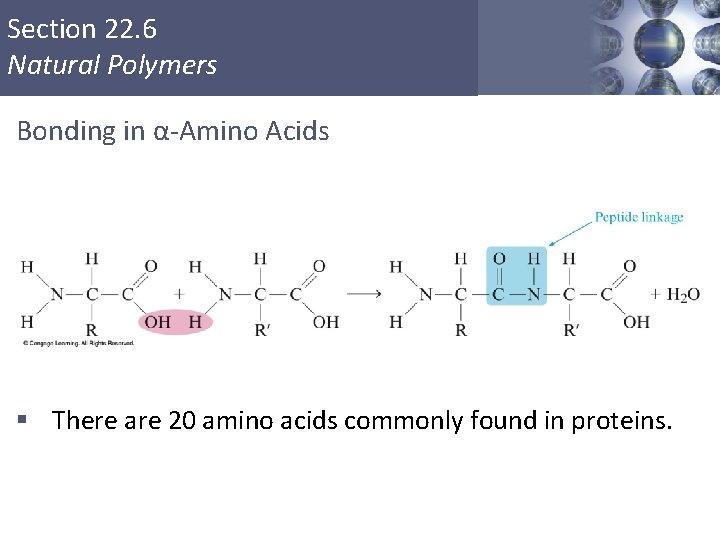
Section 22. 6 Natural Polymers Bonding in α-Amino Acids § There are 20 amino acids commonly found in proteins. Copyright © Cengage Learning. All rights reserved 41
Section 22. 6 Natural Polymers Levels of Structure in Proteins § Primary: Sequence of amino acids in the protein chain. § Secondary: The arrangement of the protein chain in the long molecule (hydrogen bonding determines this). § Tertiary: The overall shape of the protein (determined by hydrogen-bonding, dipole-dipole interactions, ionic bonds, covalent bonds and London forces). Copyright © Cengage Learning. All rights reserved 42
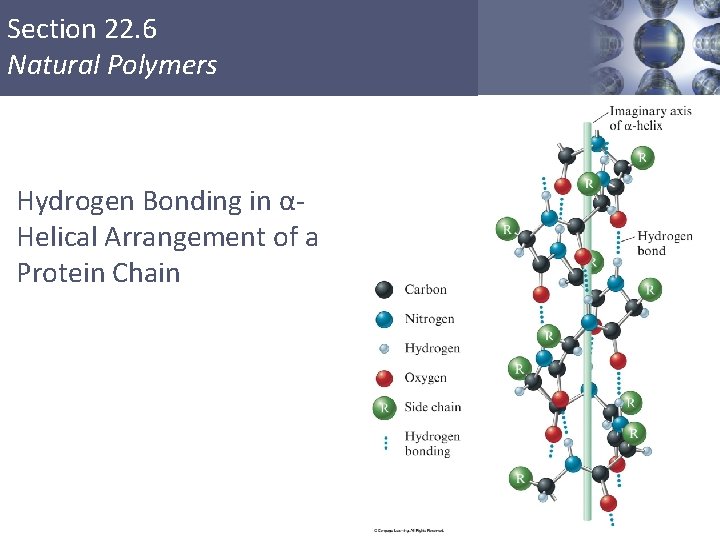
Section 22. 6 Natural Polymers Hydrogen Bonding in αHelical Arrangement of a Protein Chain
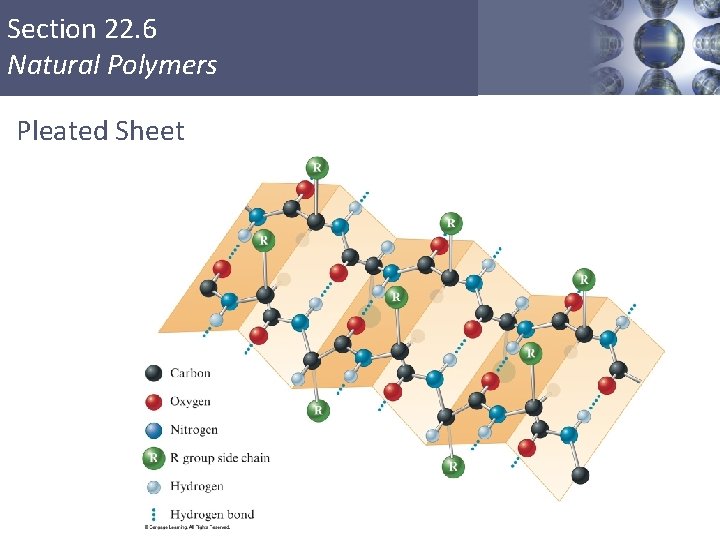
Section 22. 6 Natural Polymers Pleated Sheet Copyright © Cengage Learning. All rights reserved 44

Section 22. 6 Natural Polymers Carbohydrates § Food source for most organisms and structural material for plants. § Empirical formula = CH 2 O § Monosaccharides (simple sugars) pentoses – ribose, arabinose hexoses – fructose, glucose Copyright © Cengage Learning. All rights reserved 45
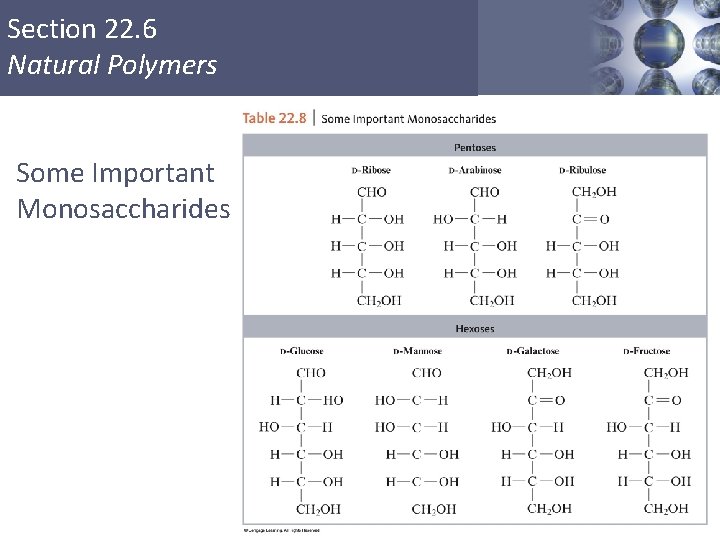
Section 22. 6 Natural Polymers Some Important Monosaccharides Copyright © Cengage Learning. All rights reserved 46

Section 22. 6 Natural Polymers Carbohydrates § Disaccharides (formed from 2 monosaccharides joined by a glycoside linkage, a C—O—C bond between the rings): sucrose (glucose + fructose) § Polysaccharides (many monosaccharide units): starch, cellulose Copyright © Cengage Learning. All rights reserved 47
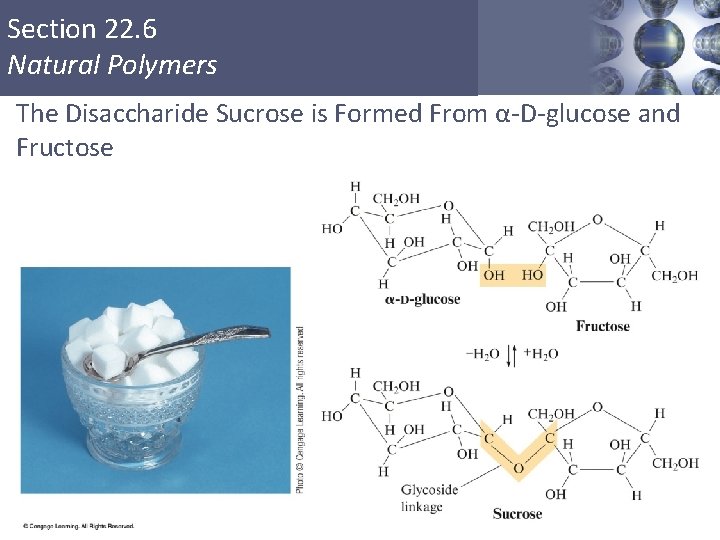
Section 22. 6 Natural Polymers The Disaccharide Sucrose is Formed From α-D-glucose and Fructose Copyright © Cengage Learning. All rights reserved 48

Section 22. 6 Natural Polymers Nucleic Acids § DNA (deoxyribonucleic acid): stores and transmits genetic information, responsible (with RNA) for protein synthesis. (Molar masses = several billion) § RNA (ribonucleic acid): helps in protein synthesis. (Molar masses from 20, 000 to 40, 000 g/mol) Copyright © Cengage Learning. All rights reserved 49

Section 22. 6 Natural Polymers Nucleotides § Monomers of the nucleic acids. § Three distinct parts: § A five–carbon sugar, deoxyribose in DNA and ribose in RNA. § A nitrogen–containing organic base. § A phosphoric acid molecule (H 3 PO 4). Copyright © Cengage Learning. All rights reserved 50
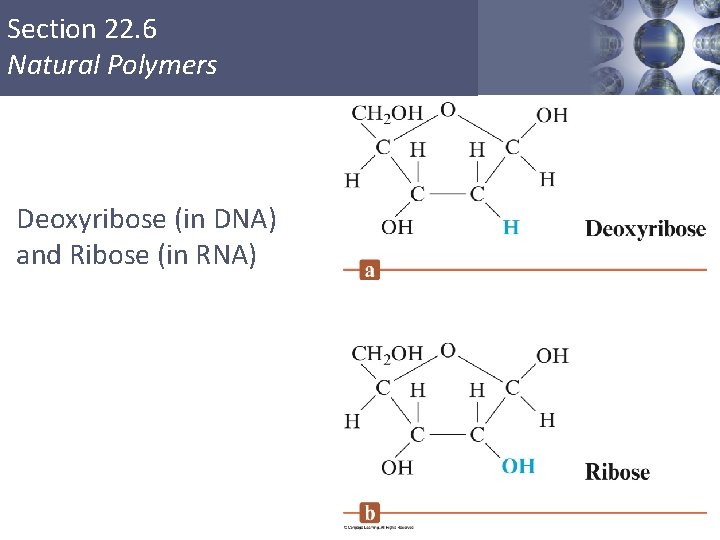
Section 22. 6 Natural Polymers Deoxyribose (in DNA) and Ribose (in RNA) Copyright © Cengage Learning. All rights reserved 51
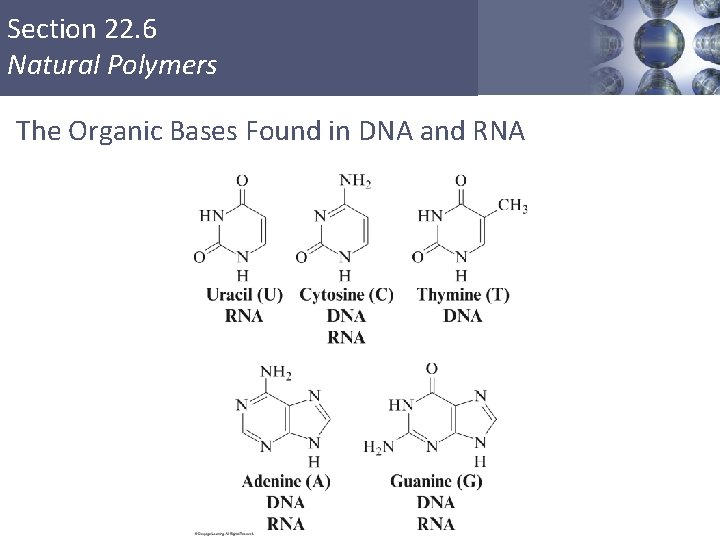
Section 22. 6 Natural Polymers The Organic Bases Found in DNA and RNA Copyright © Cengage Learning. All rights reserved 52

Section 22. 6 Natural Polymers DNA § Key to DNA’s functioning is its double-helical structure with complementary bases on the two strands. § The bases form hydrogen bonds to each other. Copyright © Cengage Learning. All rights reserved 53
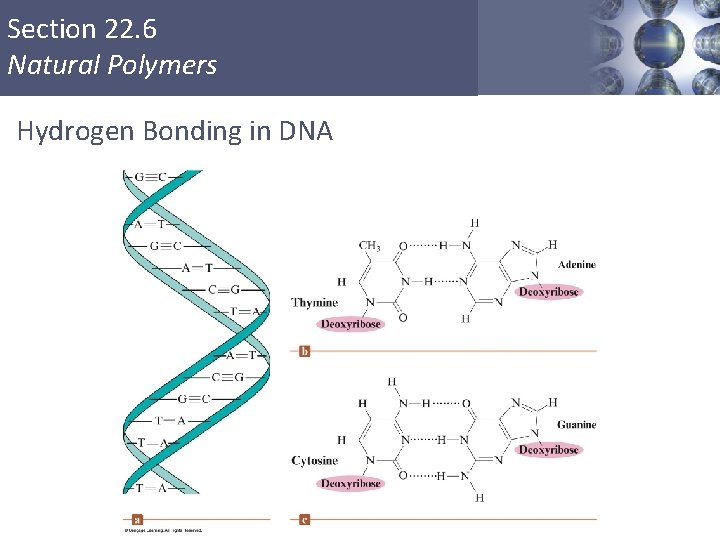
Section 22. 6 Natural Polymers Hydrogen Bonding in DNA Copyright © Cengage Learning. All rights reserved 54
- Slides: 54