Chapter 22 23 Organic Chemistry 1 Organic Chemistry












- Slides: 46

Chapter 22, 23 Organic Chemistry 1

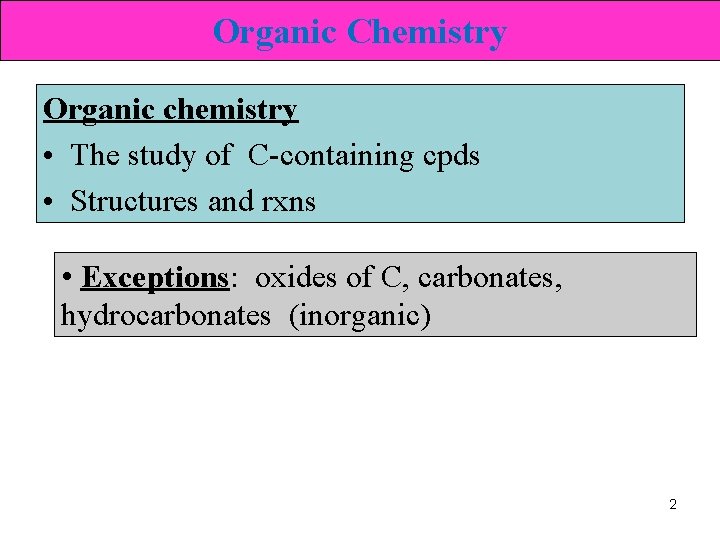
Organic Chemistry Organic chemistry • The study of C-containing cpds • Structures and rxns • Exceptions: oxides of C, carbonates, hydrocarbonates (inorganic) 2

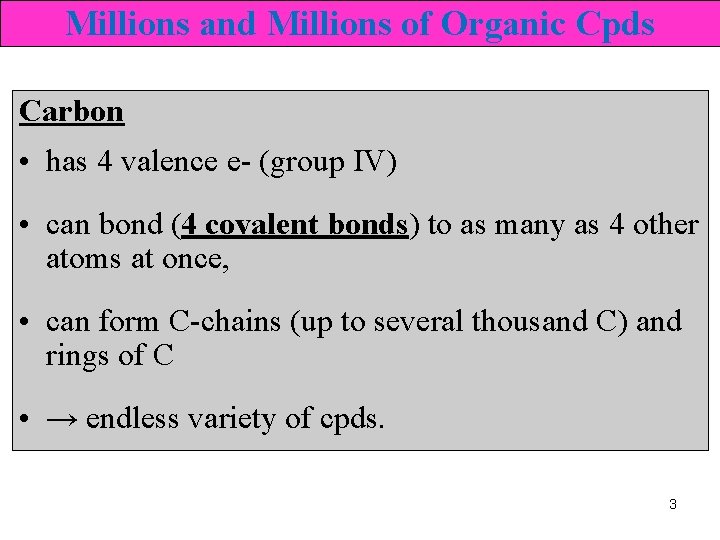
Millions and Millions of Organic Cpds Carbon • has 4 valence e- (group IV) • can bond (4 covalent bonds) to as many as 4 other atoms at once, • can form C-chains (up to several thousand C) and rings of C • → endless variety of cpds. 3

Millions and Millions of Organic Cpds • can bond strongly to elements such as O and N, halogens • form double and triple bonds. • millions of organic cpds • classified into groups of cpds that have similar structures and properties. 4

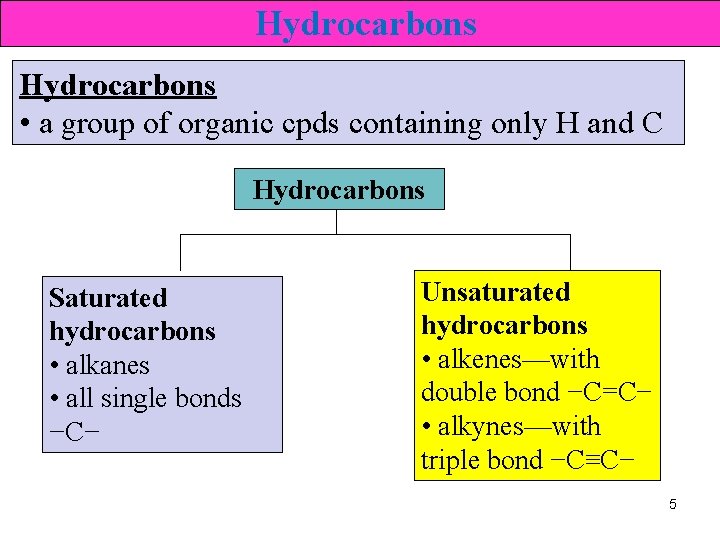
Hydrocarbons • a group of organic cpds containing only H and C Hydrocarbons Saturated hydrocarbons • alkanes • all single bonds −C− Unsaturated hydrocarbons • alkenes—with double bond −C=C− • alkynes—with triple bond −C≡C− 5

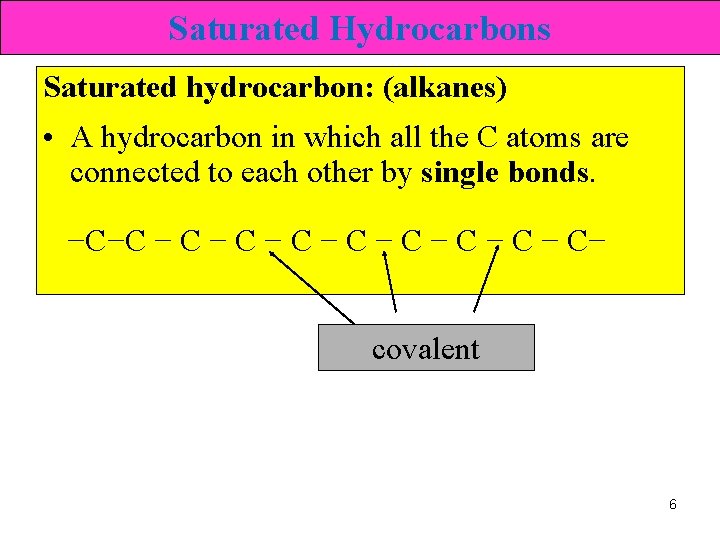
Saturated Hydrocarbons Saturated hydrocarbon: (alkanes) • A hydrocarbon in which all the C atoms are connected to each other by single bonds. −C−C − C − C− covalent 6

Saturated Hydrocarbons Uses of Alkanes • the simplest hydrocarbons. (saturated) • fuels e. g. methane (in town gas), propane (bottled fuel for BBQ), butane (lighter), gasoline • as solvents in paint removers, glues, and other products. • Other uses 7

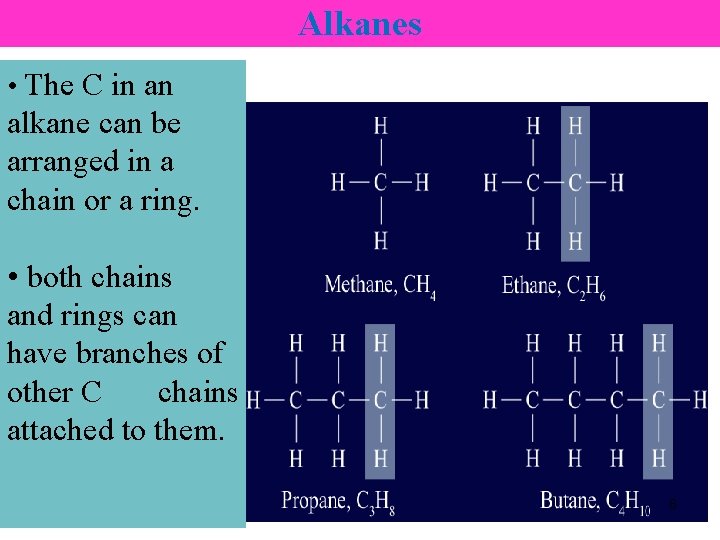
Alkanes • The C in an alkane can be arranged in a chain or a ring. • both chains and rings can have branches of other C chains attached to them. 8

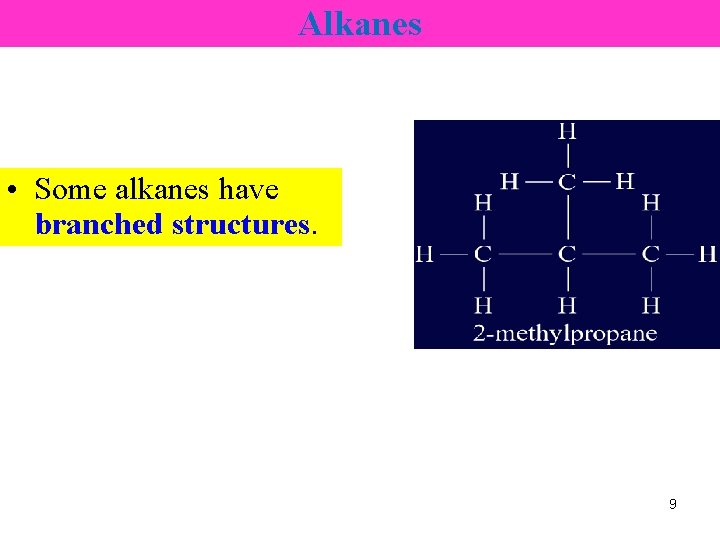
Alkanes • Some alkanes have branched structures. 9

Alkanes • The condensed structural formula for propane can be written as CH 3 CH 2 CH 3. 10

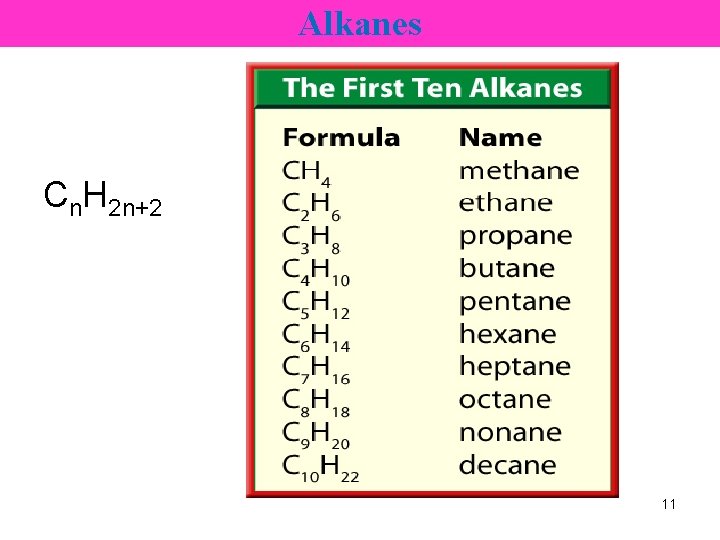
Alkanes Cn. H 2 n+2 11

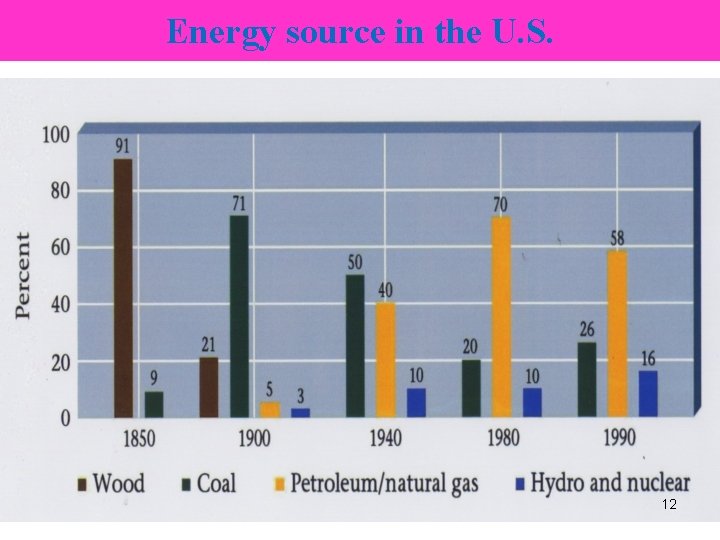
Energy source in the U. S. 12

13

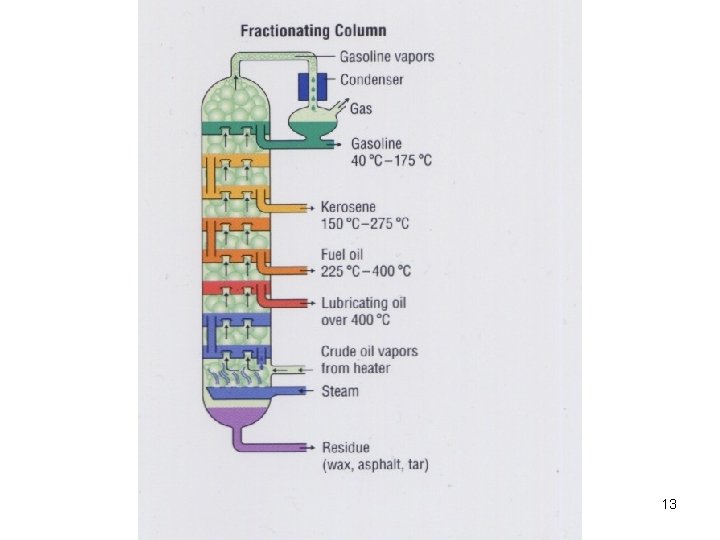
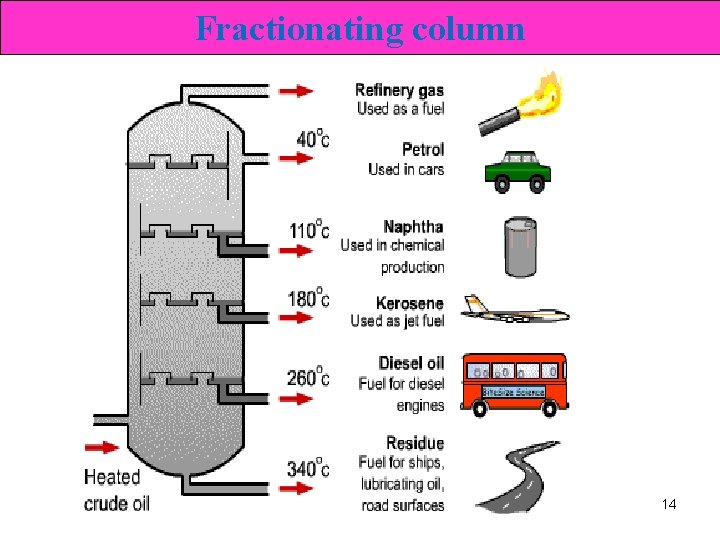
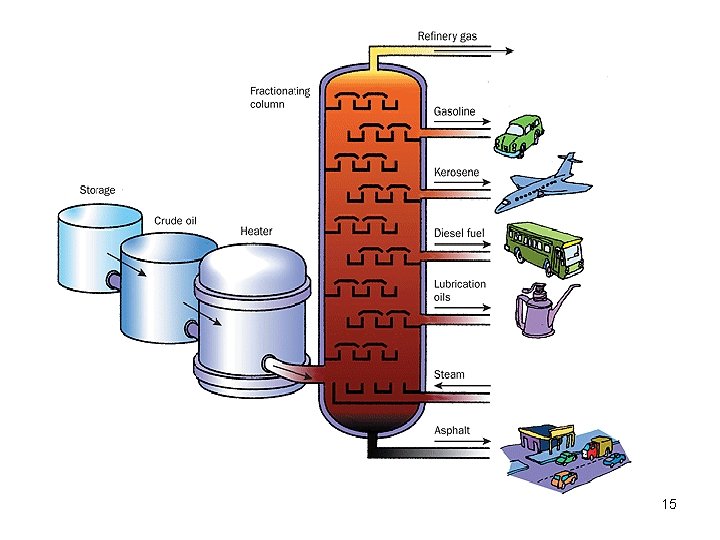
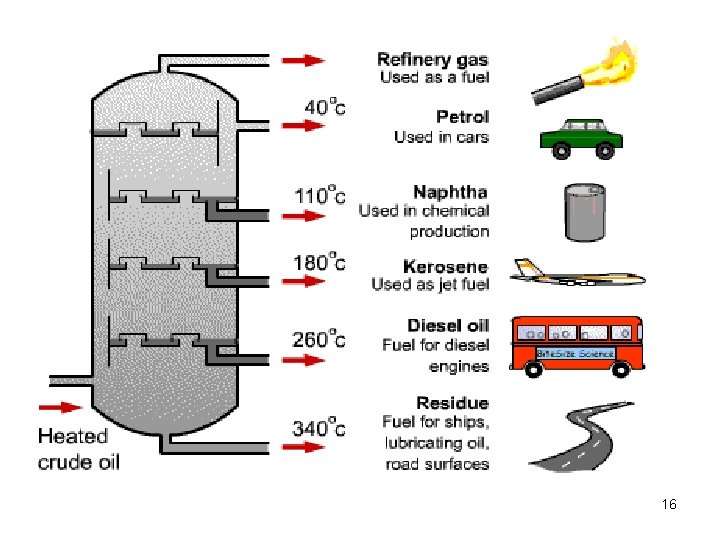
Fractionating column 14

15

16

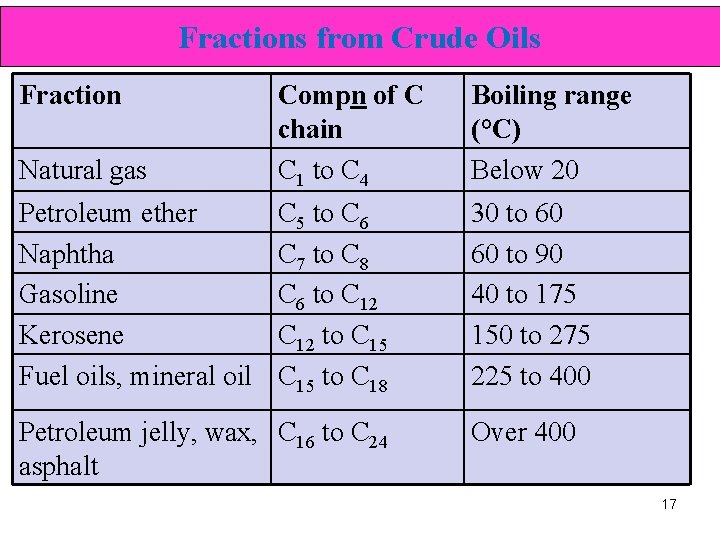
Fractions from Crude Oils Fraction Natural gas Compn of C chain C 1 to C 4 Boiling range (°C) Below 20 Petroleum ether Naphtha Gasoline Kerosene Fuel oils, mineral oil C 5 to C 6 C 7 to C 8 C 6 to C 12 to C 15 to C 18 30 to 60 60 to 90 40 to 175 150 to 275 225 to 400 Petroleum jelly, wax, C 16 to C 24 asphalt Over 400 17

Unsaturated Hydrocarbons Unsaturated hydrocarbon • A HC that has 1 or more −C=C− or −C≡C− bonds • The other bonds are single bonds (−C−) • Alkenes (−C=C−), alkynes (−C≡C−) 18

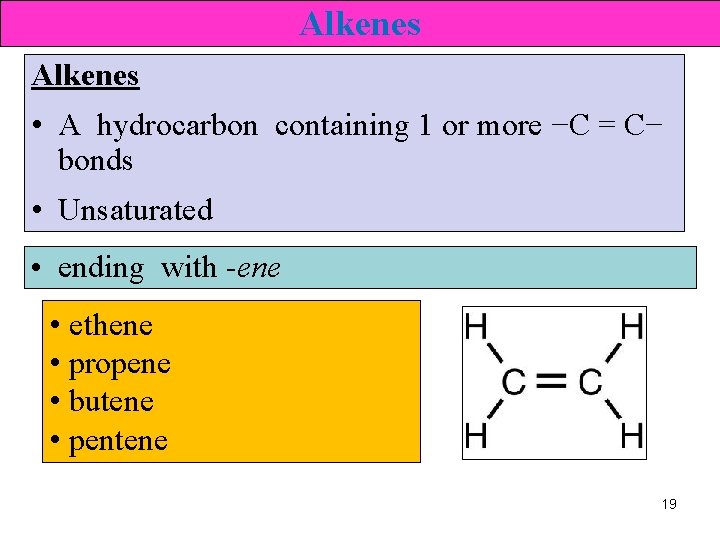
Alkenes • A hydrocarbon containing 1 or more −C = C− bonds • Unsaturated • ending with -ene • ethene • propene • butene • pentene 19

Alkenes • An unsaturated alkene can be converted into a saturated alkane by adding H to the C=C bond. • hydrogenation. 20

Alkynes • Another type of unsaturated HC—alkyne, contains a triple bond (−C≡C−) between 2 C atoms. • ending with -yne. • ethyne, propyne, butyne 21

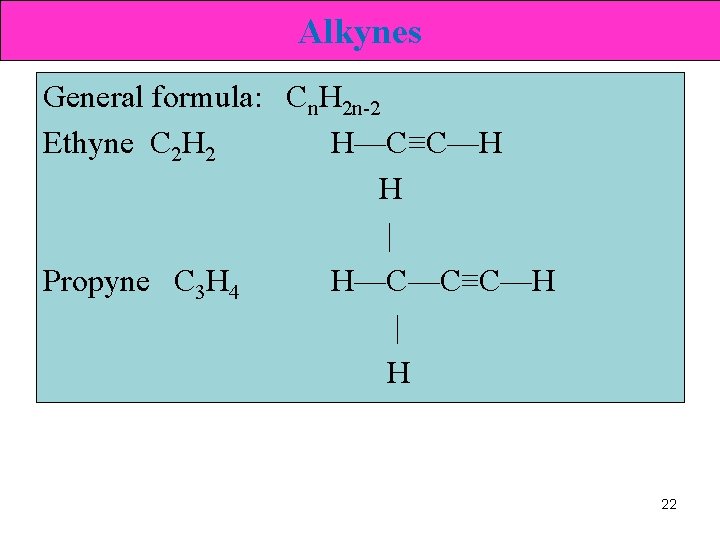
Alkynes General formula: Cn. H 2 n-2 Ethyne C 2 H 2 H—C≡C—H H | Propyne C 3 H 4 H—C—C≡C—H | H 22

Aromatic Hydrocarbons Aromatic hydrocarbon • has a benzene ring • most of them have distinctive aromas. 23

Monomers and Polymers A Polymer • a giant molecule formed by covalent bonds • hundreds or thousands of small individual repeating units (monomers), bonded together in chains. • Monomers may all be alike, or they may be different. • Properties of a polymer are different from those of the monomers that formed it. 24

Synthetic Polymers • Polymers are everywhere, making fabrics such as nylon and polyester, plastic wrap and bottles, rubber bands, and many more products you see every day. 25

Structure of Polymers 26

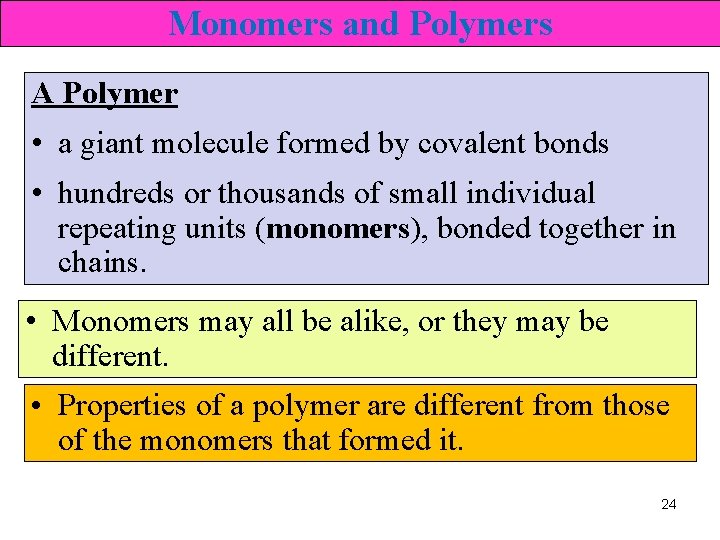
Structure of Polymers Cellulose Ø polymer found in the cell walls of plant cells • monomer : glucose 27

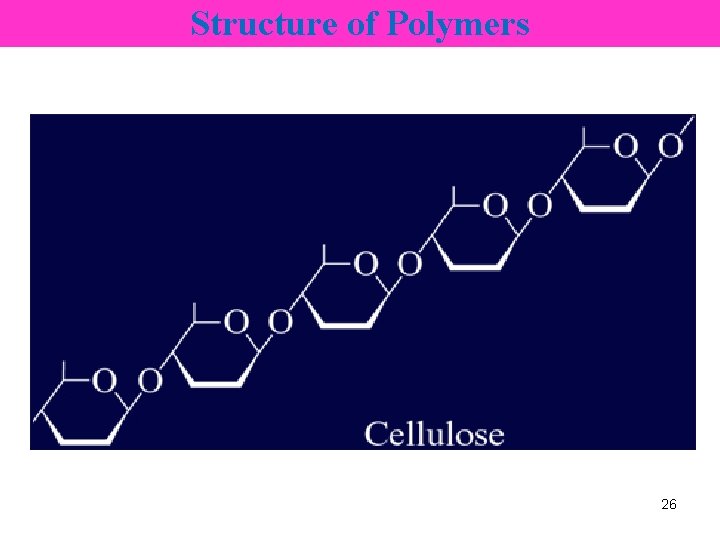
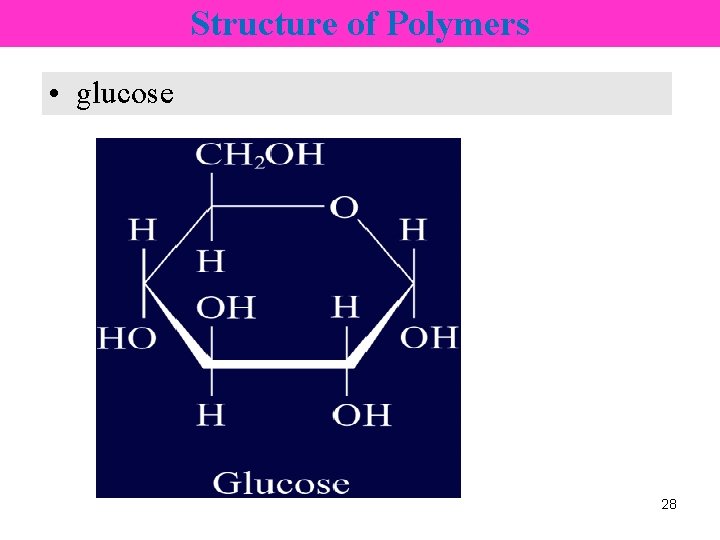
Structure of Polymers • glucose 28

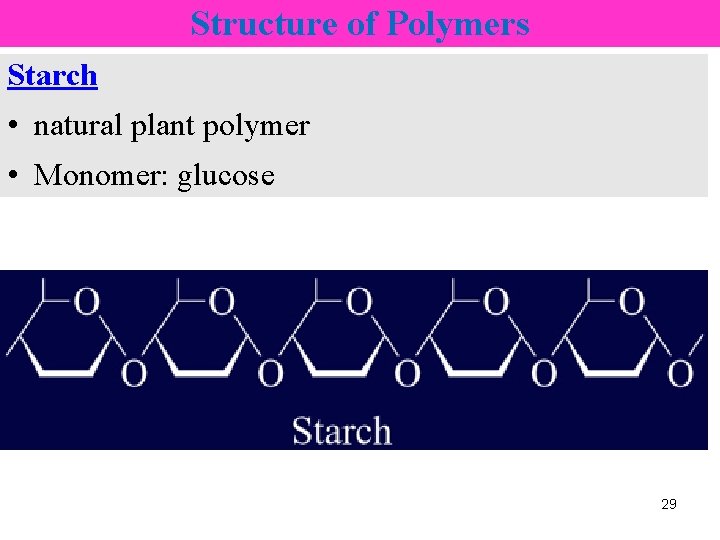
Structure of Polymers Starch • natural plant polymer • Monomer: glucose 29

Rubber • used in tires and rubber balls. 30

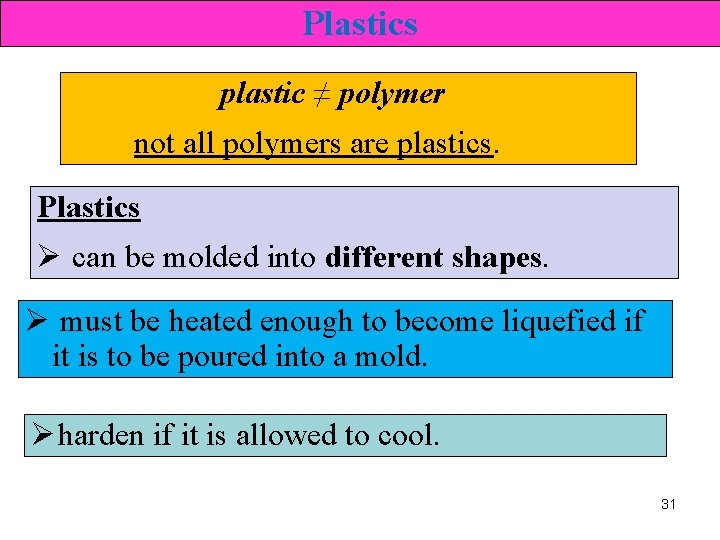
Plastics plastic ≠ polymer not all polymers are plastics. Plastics Ø can be molded into different shapes. Ø must be heated enough to become liquefied if it is to be poured into a mold. Ø harden if it is allowed to cool. 31

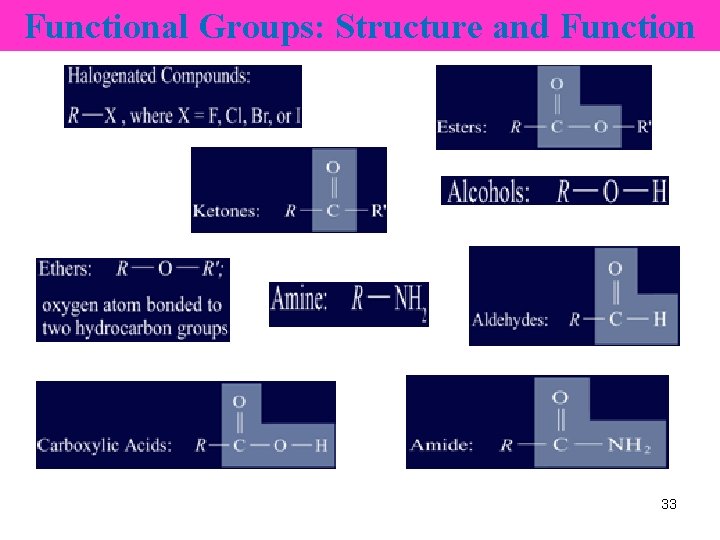
Functional Groups A functional group in an organic molecule • an atom or group of atoms that • always reacts in a certain way (similiar chem properties) 32

Functional Groups: Structure and Function 33

Functional Groups • The symbols R and R′ represent any C chains or rings bonded to the functional group. • * represents a H atom, C chain, or C ring. 34

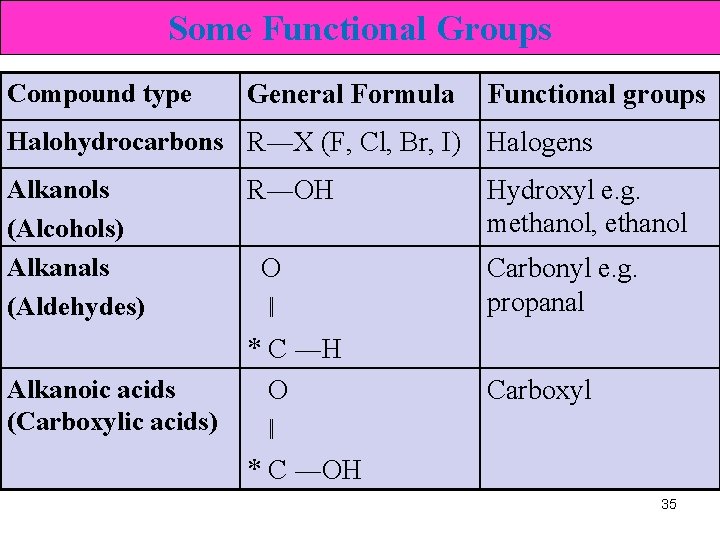
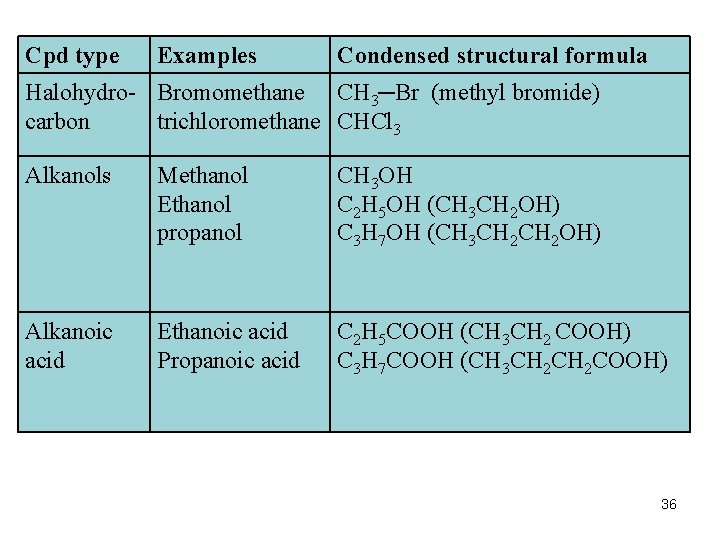
Some Functional Groups Compound type General Formula Functional groups Halohydrocarbons R―X (F, Cl, Br, I) Halogens Alkanols (Alcohols) Alkanals (Aldehydes) Alkanoic acids (Carboxylic acids) R―OH Hydroxyl e. g. methanol, ethanol O ‖ * C ―H O ‖ * C ―OH Carbonyl e. g. propanal Carboxyl 35

Cpd type Examples Condensed structural formula Halohydro- Bromomethane CH 3─Br (methyl bromide) carbon trichloromethane CHCl 3 Alkanols Methanol Ethanol propanol CH 3 OH C 2 H 5 OH (CH 3 CH 2 OH) C 3 H 7 OH (CH 3 CH 2 OH) Alkanoic acid Ethanoic acid Propanoic acid C 2 H 5 COOH (CH 3 CH 2 COOH) C 3 H 7 COOH (CH 3 CH 2 COOH) 36

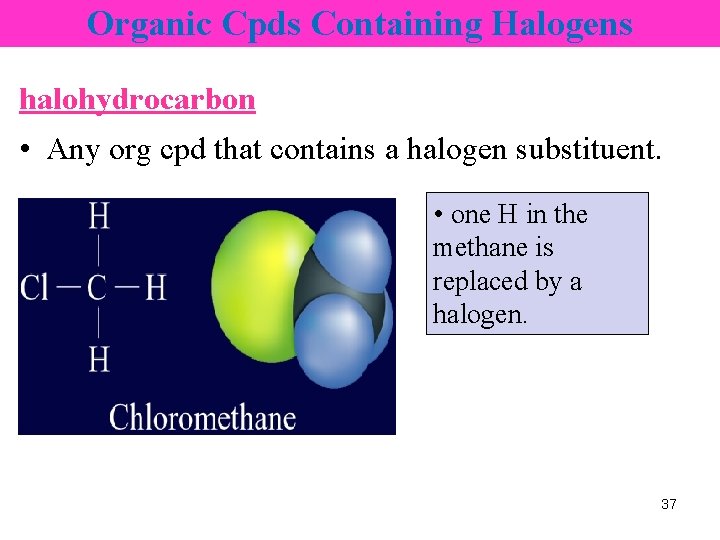
Organic Cpds Containing Halogens halohydrocarbon • Any org cpd that contains a halogen substituent. • one H in the methane is replaced by a halogen. 37

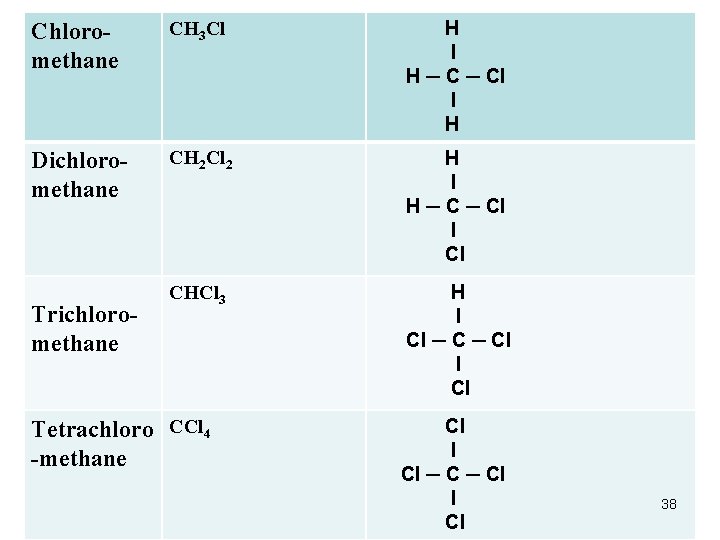
Chloromethane CH 3 Cl H ─ C ─ Cl l H Dichloromethane CH 2 Cl 2 H l H ─ Cl l Cl CHCl 3 H l Cl ─ Cl l Cl Trichloromethane Tetrachloro CCl 4 -methane Cl l Cl ─ Cl l Cl 38

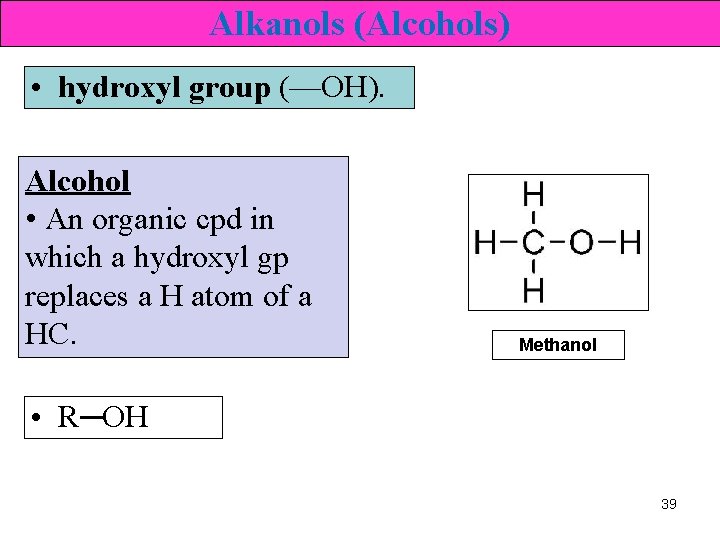
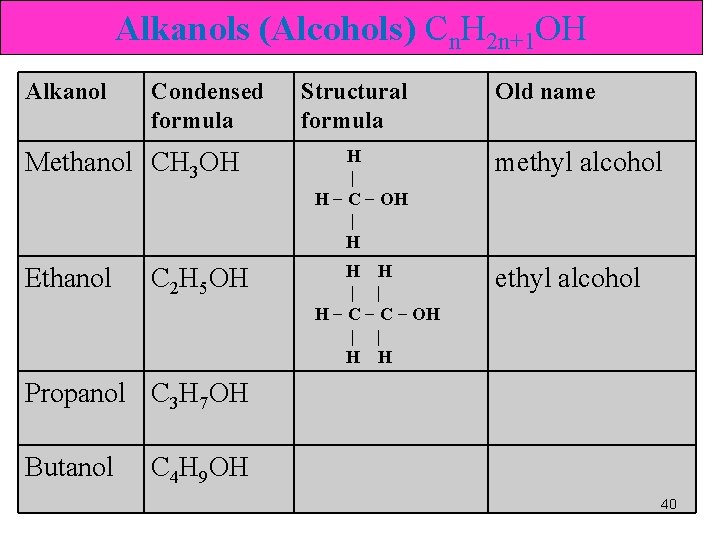
Alkanols (Alcohols) • hydroxyl group (—OH). Alcohol • An organic cpd in which a hydroxyl gp replaces a H atom of a HC. Methanol • R─OH 39

Alkanols (Alcohols) Cn. H 2 n+1 OH Alkanol Condensed formula Structural formula Old name Methanol CH 3 OH H | H − C − OH | H methyl alcohol Ethanol H H | | H − C − OH | | H H ethyl alcohol C 2 H 5 OH Propanol C 3 H 7 OH Butanol C 4 H 9 OH 40

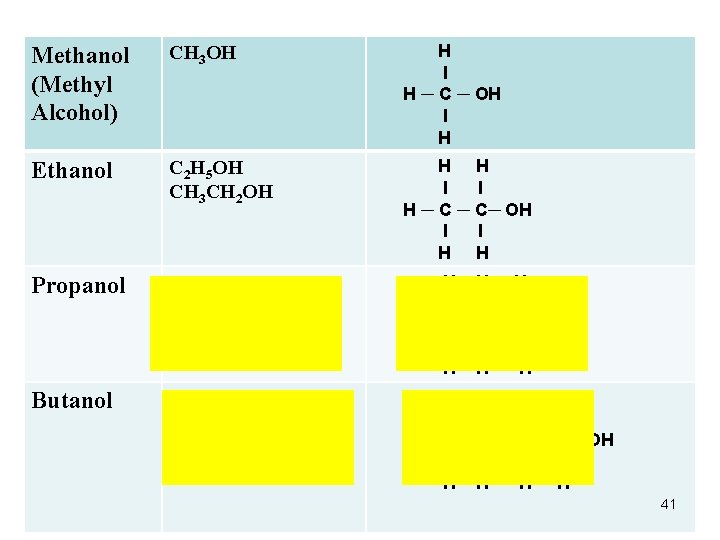
Methanol (Methyl Alcohol) CH 3 OH H l H ─ C ─ OH l H Ethanol C 2 H 5 OH CH 3 CH 2 OH H H l l H ─ C─ OH l l H H Propanol C 3 H 7 OH CH 3 CH 2 OH H l l l H ─ C ─ C ─ OH l l l H H H Butanol C 4 H 9 OH CH 3 CH 2 CH 2 OH H H l l H ─ C ─ C ─ OH l l H H 41

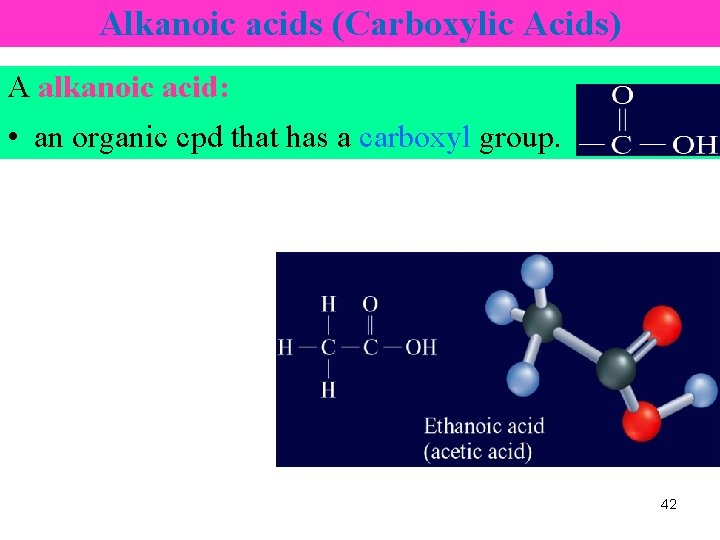
Alkanoic acids (Carboxylic Acids) A alkanoic acid: • an organic cpd that has a carboxyl group. 42

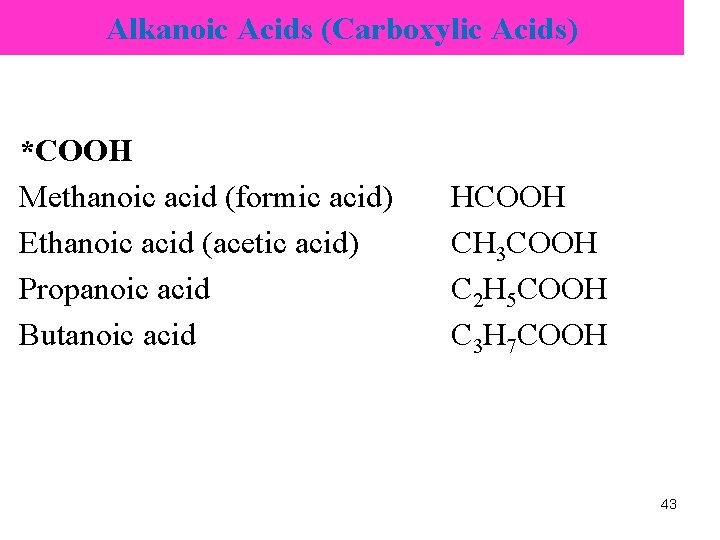
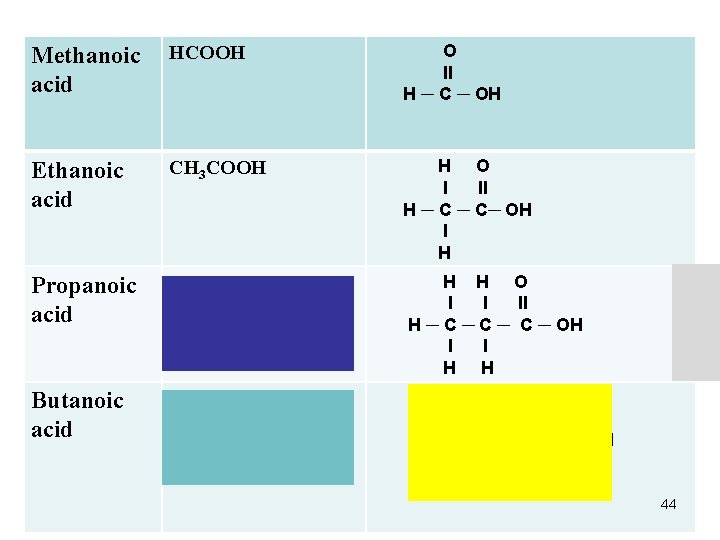
Alkanoic Acids (Carboxylic Acids) *COOH Methanoic acid (formic acid) Ethanoic acid (acetic acid) Propanoic acid Butanoic acid HCOOH CH 3 COOH C 2 H 5 COOH C 3 H 7 COOH 43

Methanoic acid HCOOH O ll H ─ C ─ OH Ethanoic acid CH 3 COOH H O l ll H ─ C─ OH l H Propanoic acid C 2 H 5 COOH CH 3 CH 2 COOH H H O l l ll H ─ C ─ C ─ OH l l H H Butanoic acid C 3 H 7 COOH CH 3 CH 2 COOH H O l ll H ─ C ─ C ─ OH l l l H H H 44

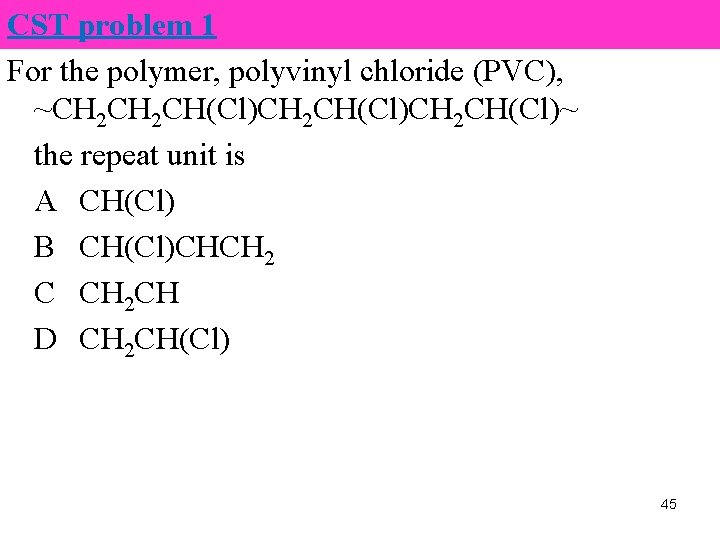
CST problem 1 For the polymer, polyvinyl chloride (PVC), ~CH 2 CH(Cl)CH 2 CH(Cl)~ the repeat unit is A CH(Cl) B CH(Cl)CHCH 2 C CH 2 CH D CH 2 CH(Cl) 45

The End 46