Chapter 20 Volumetric Analysis volumevolume stoichiometry solutions only








- Slides: 26

Chapter 20 Volumetric Analysis • volume-volume stoichiometry (solutions only) and application of volumetric analysis including the use of indicators, calculations related to preparation of standard solutions, dilution of solutions and use of acid-base titrations to determine the concentration of an acid or a base in a water sample.

20. 2 Standard Solutions Volumetric analysis is the technique used to accurately determine the concentrations of acids and bases. This involves reacting the solution of unknown concentration with a solution of accurately known concentration (a standard solution). A standard solution is prepared by dissolving an accurately measured mass of a primary standard in an accurately measured volume of water.

Primary Standards Pure substances are widely used in the laboratory to prepare solutions of accurately known concentrations. These substances are so pure that the amount of substance, in moles, can be calculated accurately from their mass. A primary standard should: • Be readily obtainable in pure form • Have a known chemical formula • Be easy to store without deteriorating or reacting with the atmosphere • Have a high molar mass to minimize the effect of errors in weighing • Be inexpensive

Preparing a standard solution • A volumetric flask is used to prepare a solution that has an accurately known concentration. Volumetric flasks of 50. 00 m. L, 100. 00 m. L or 250. 00 m. L are frequently used. • A standard flask is filled so that the bottom of the meniscus is level with the graduation line on the neck of the flask. Your eye should be level with the line to avoid parallax errors, • To prepare a standard solution from a primary standard you need to dissolve an accurately known amount of the substance in deionised water to produce a solution of known volume.

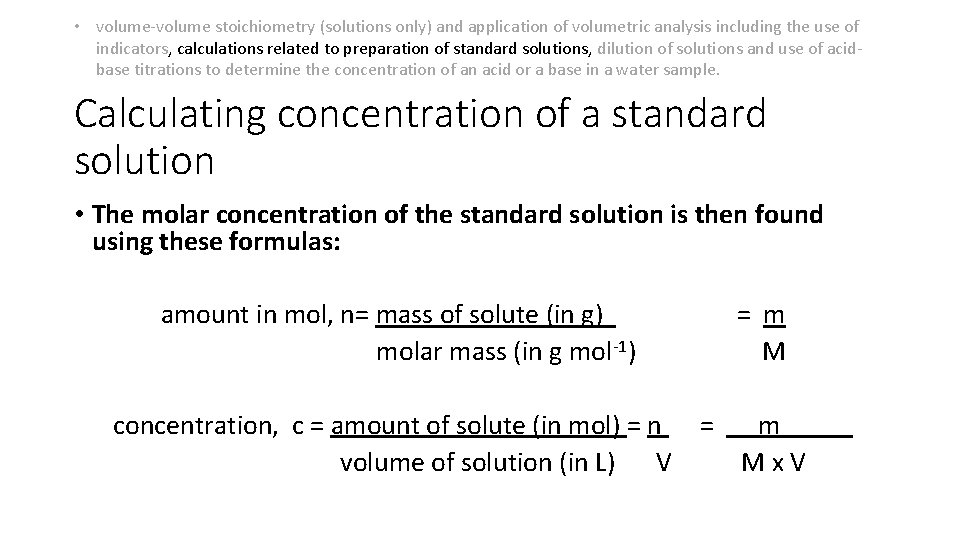
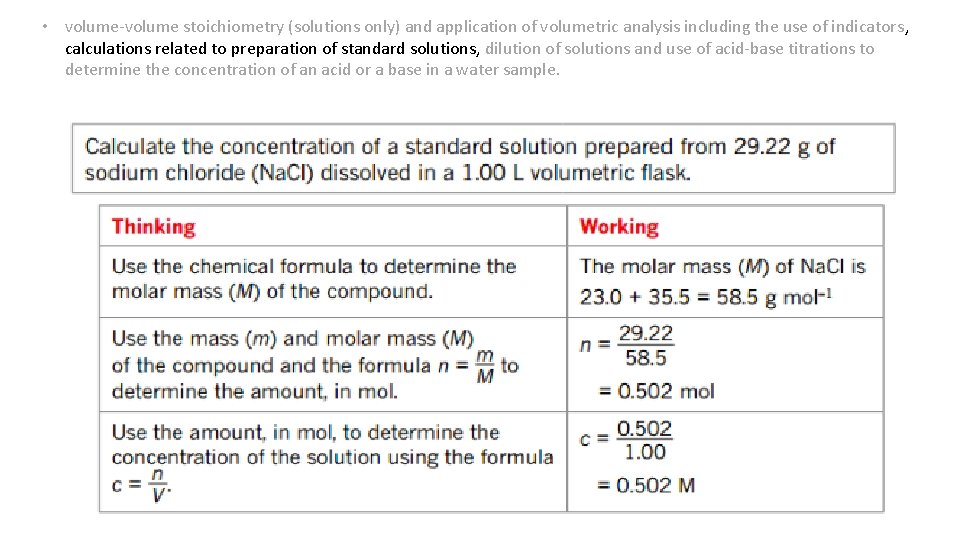
• volume-volume stoichiometry (solutions only) and application of volumetric analysis including the use of indicators, calculations related to preparation of standard solutions, dilution of solutions and use of acidbase titrations to determine the concentration of an acid or a base in a water sample. Calculating concentration of a standard solution • The molar concentration of the standard solution is then found using these formulas: amount in mol, n= mass of solute (in g) molar mass (in g mol-1) concentration, c = amount of solute (in mol) = n volume of solution (in L) V = m Mx. V

Steps for preparing a standard solution

• volume-volume stoichiometry (solutions only) and application of volumetric analysis including the use of indicators, calculations related to preparation of standard solutions, dilution of solutions and use of acid-base titrations to determine the concentration of an acid or a base in a water sample.

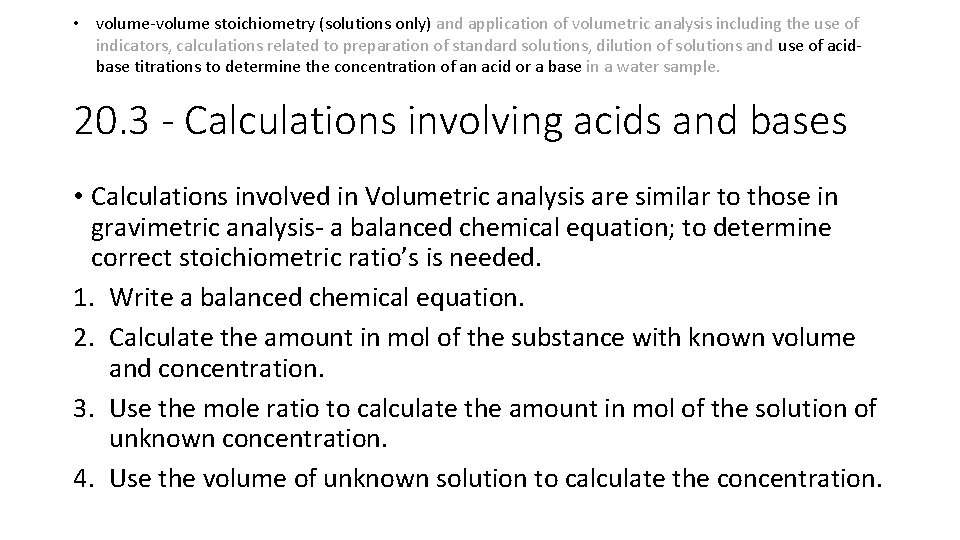
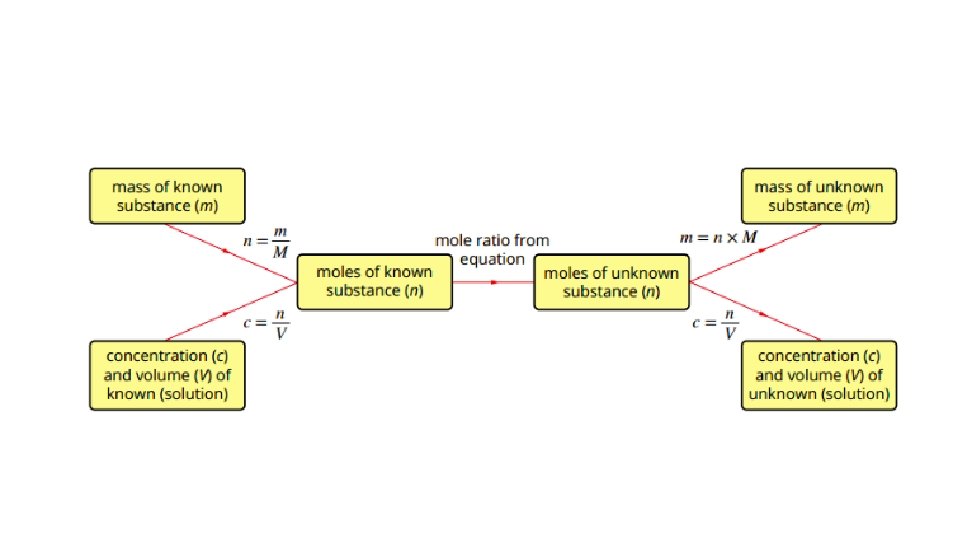
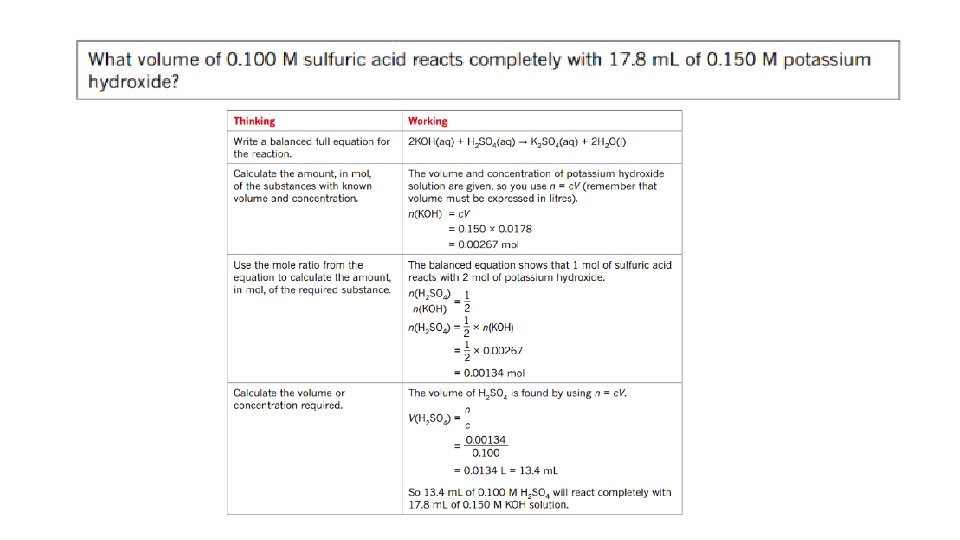
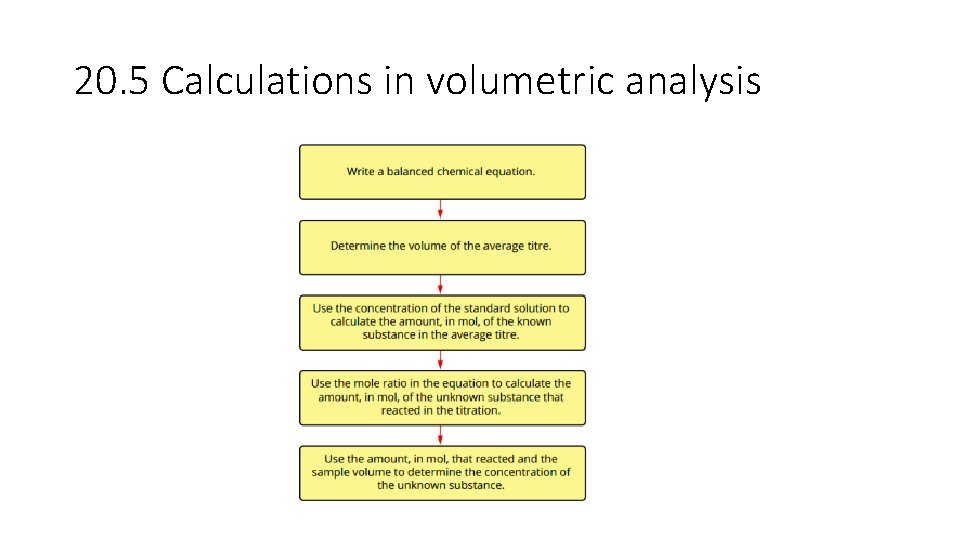
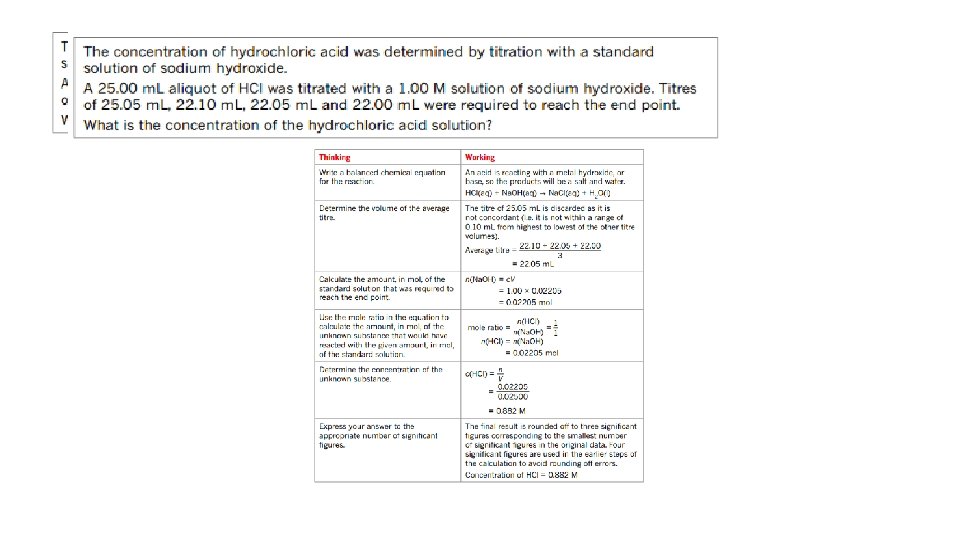
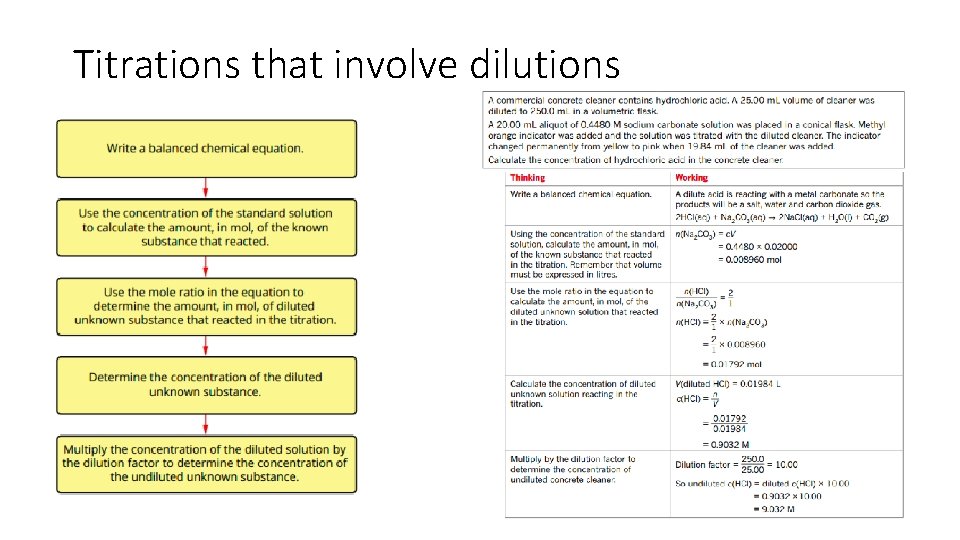
• volume-volume stoichiometry (solutions only) and application of volumetric analysis including the use of indicators, calculations related to preparation of standard solutions, dilution of solutions and use of acidbase titrations to determine the concentration of an acid or a base in a water sample. 20. 3 - Calculations involving acids and bases • Calculations involved in Volumetric analysis are similar to those in gravimetric analysis- a balanced chemical equation; to determine correct stoichiometric ratio’s is needed. 1. Write a balanced chemical equation. 2. Calculate the amount in mol of the substance with known volume and concentration. 3. Use the mole ratio to calculate the amount in mol of the solution of unknown concentration. 4. Use the volume of unknown solution to calculate the concentration.



volume-volume stoichiometry (solutions only) and application of volumetric analysis including the use of indicators, calculations related to preparation of standard solutions, dilution of solutions and use of acidbase titrations to determine the concentration of an acid or a base in a water sample. Indicator • Phenolphthalein is often used in titrations, it turns colourless in acidic solutions and pink in basic solutions.

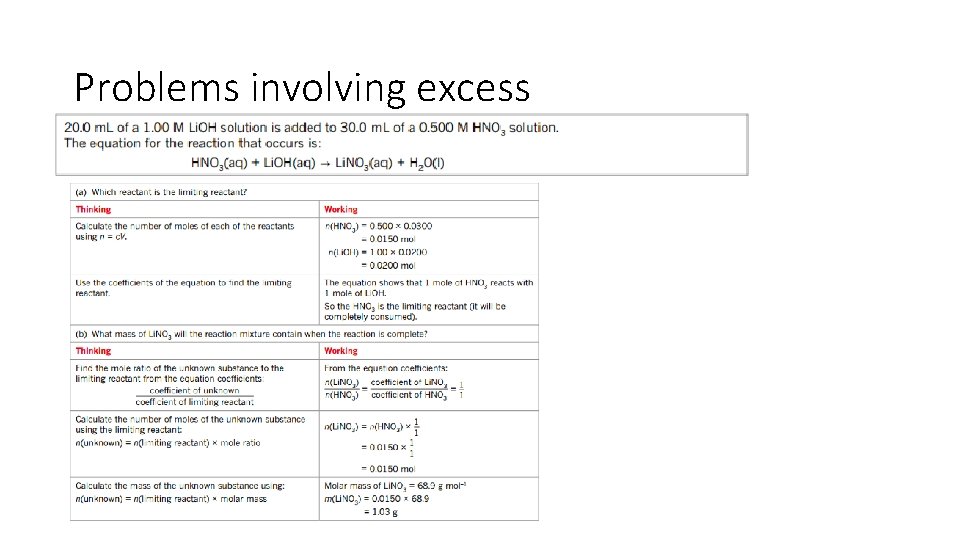
Problems involving excess

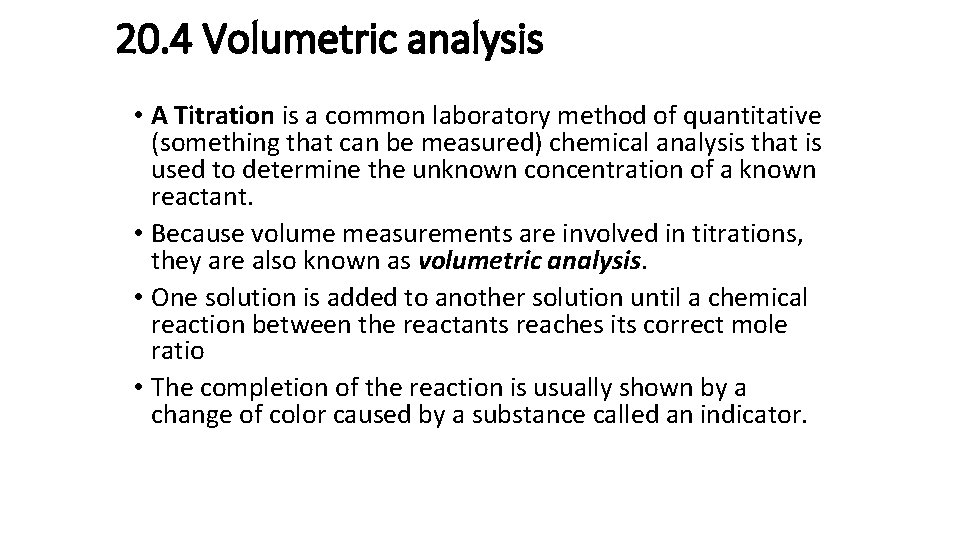
20. 4 Volumetric analysis • A Titration is a common laboratory method of quantitative (something that can be measured) chemical analysis that is used to determine the unknown concentration of a known reactant. • Because volume measurements are involved in titrations, they are also known as volumetric analysis. • One solution is added to another solution until a chemical reaction between the reactants reaches its correct mole ratio • The completion of the reaction is usually shown by a change of color caused by a substance called an indicator.

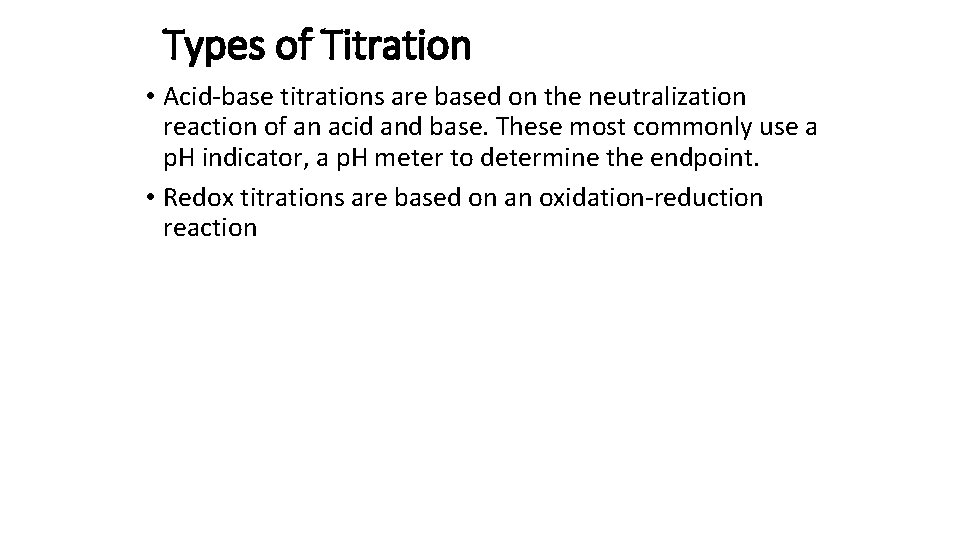
Types of Titration • Acid-base titrations are based on the neutralization reaction of an acid and base. These most commonly use a p. H indicator, a p. H meter to determine the endpoint. • Redox titrations are based on an oxidation-reduction reaction

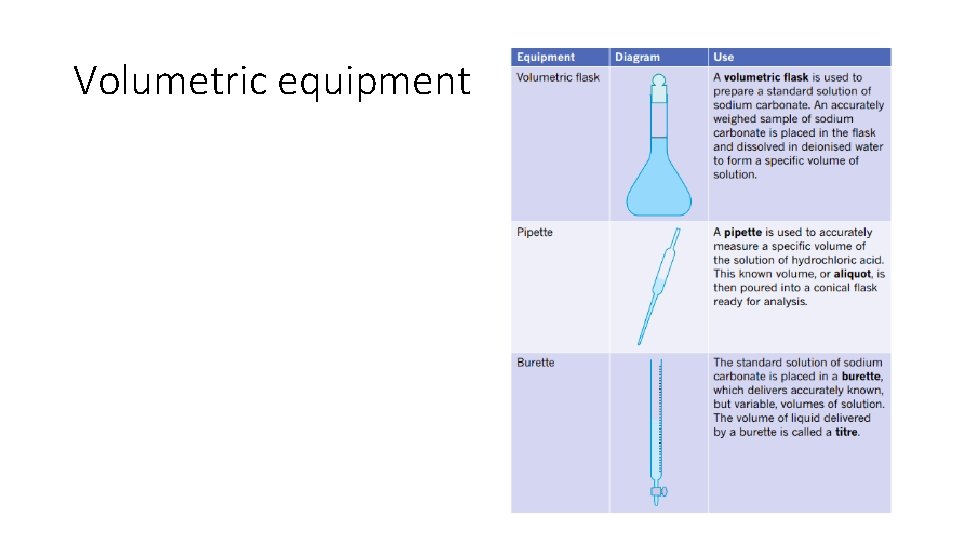
Volumetric equipment

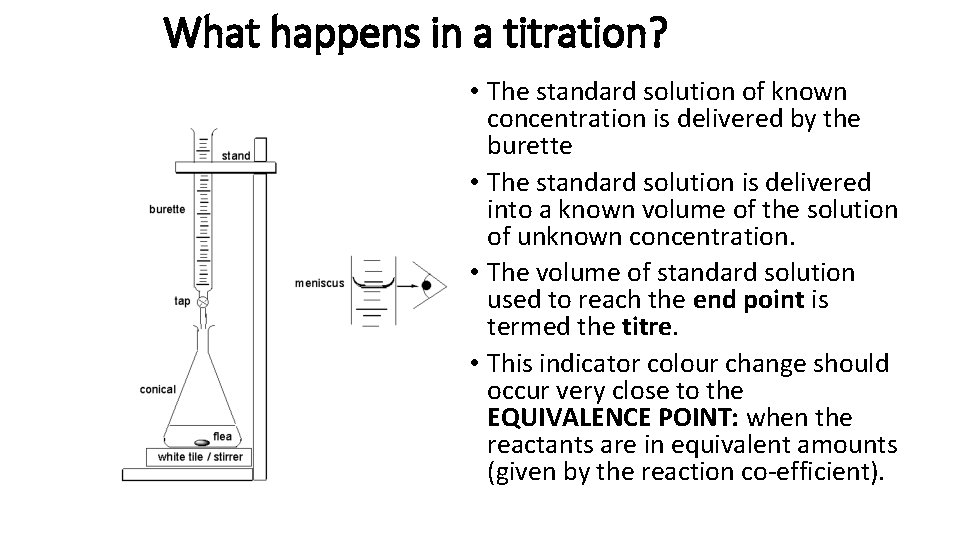
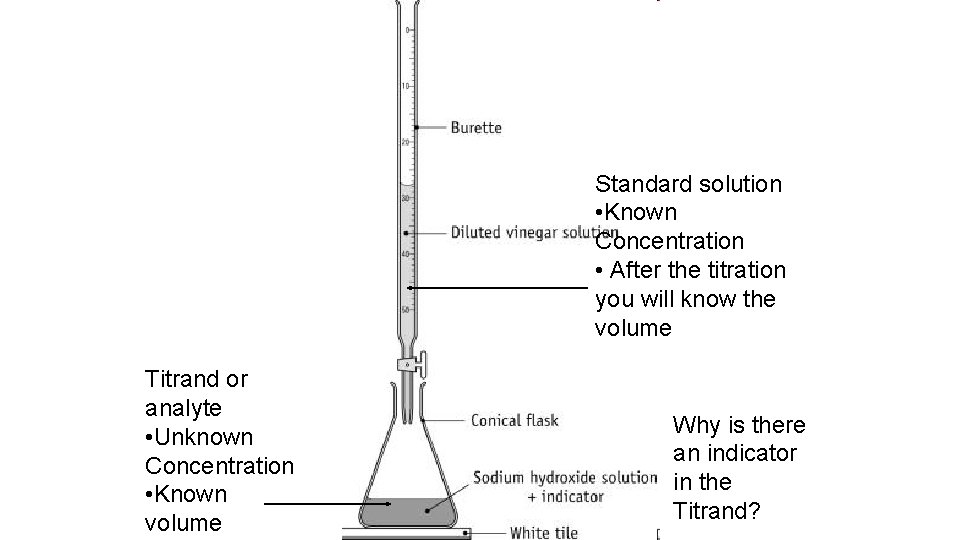
What happens in a titration? • The standard solution of known concentration is delivered by the burette • The standard solution is delivered into a known volume of the solution of unknown concentration. • The volume of standard solution used to reach the end point is termed the titre. • This indicator colour change should occur very close to the EQUIVALENCE POINT: when the reactants are in equivalent amounts (given by the reaction co-efficient).

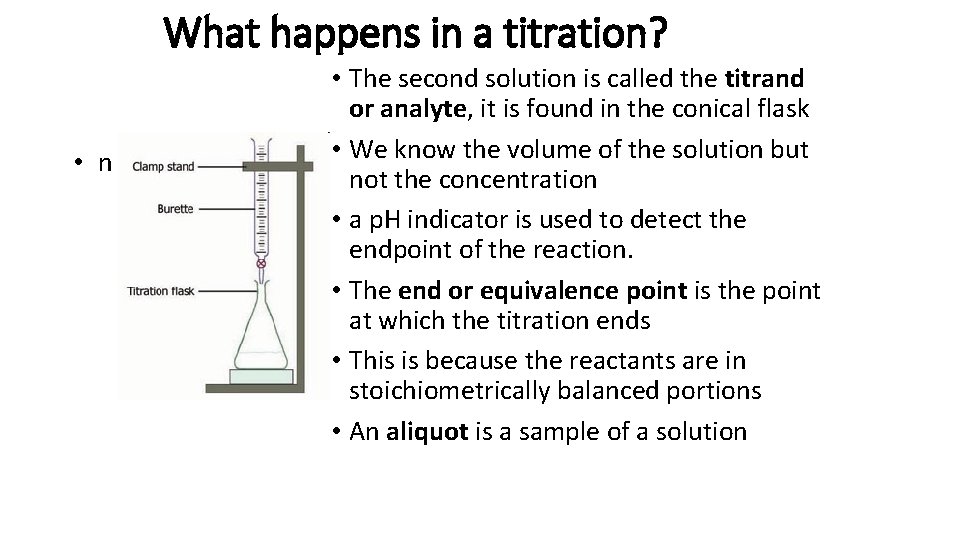
What happens in a titration? • n • The second solution is called the titrand or analyte, it is found in the conical flask • We know the volume of the solution but not the concentration • a p. H indicator is used to detect the endpoint of the reaction. • The end or equivalence point is the point at which the titration ends • This is because the reactants are in stoichiometrically balanced portions • An aliquot is a sample of a solution

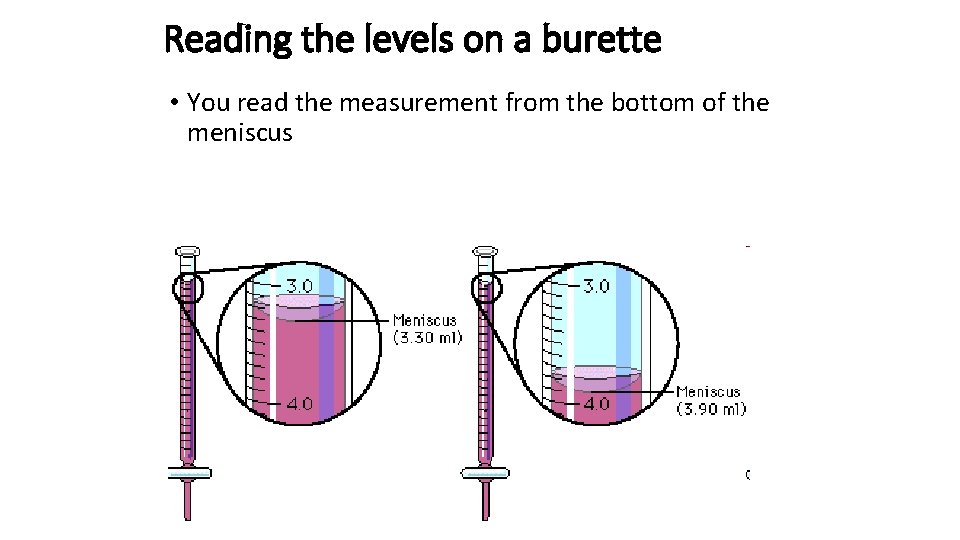
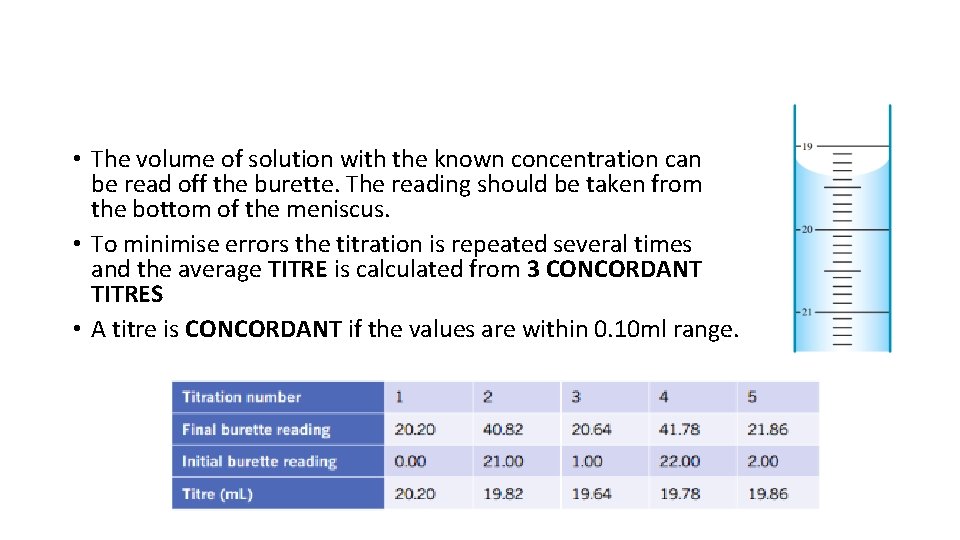
Reading the levels on a burette • You read the measurement from the bottom of the meniscus

• The volume of solution with the known concentration can be read off the burette. The reading should be taken from the bottom of the meniscus. • To minimise errors the titration is repeated several times and the average TITRE is calculated from 3 CONCORDANT TITRES • A titre is CONCORDANT if the values are within 0. 10 ml range.

Standard solution • Known Concentration • After the titration you will know the volume Titrand or analyte • Unknown Concentration • Known volume Why is there an indicator in the Titrand?

20. 5 Calculations in volumetric analysis


Titrations that involve dilutions

Uncertainties, Precision vs Accuracy and Errors • There always errors associated with measurements, even with precise measurement tools. Typical volumetric uncertainties are: • 20 ml pipette +/- 0. 03 ml • 50 ml burette +/- 0. 02 ml per reading • 250 ml volumetric flask +/- 0. 3 ml • PRECISION- all measurements are close together • ACCURACY – measurements are close to the “true” value • MISTAKES – are avoidable errors, careful experimentation will overcome i. e. misreading measurements, spills, DON’T REFER TO THESE AS ERRORS • SYSTEMATIC ERRORS – constant bias in measurement (same direction); will result in higher or lower than true value, minimised by calibration of equipment i. e. faulty balance, constant parallax error • RANDOM ERRORS – follow no regular pattern, reduced by taking multiple measurements and averaging

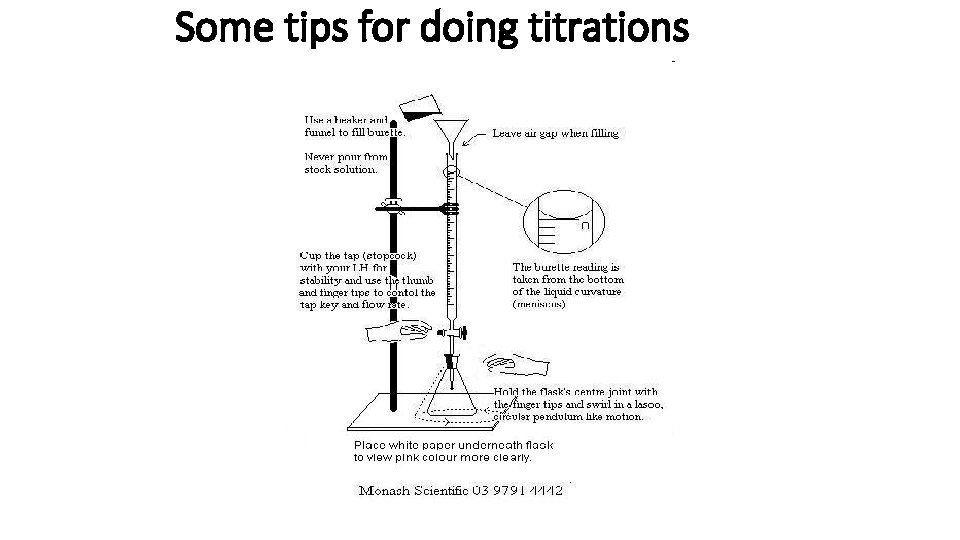
Some tips for doing titrations

Determining titre • You need to do several titrations to determine the amount needed • You then need to find the average of the titres • The titres you choose should be within 0. 1 m. L of each other (concordant with each other) • E. g. If i have 5 titres of 34 m. L, 45 m. L, 31 m. L, 32. 5 m. L, 33. 1 m. L Which three would you choose? • 31, 32. 5, 33. 1