Chapter 20 Heat Engine Entropy Heat Engine Refrigerator


















- Slides: 53

Chapter 20 Heat Engine Entropy, Heat Engine Refrigerator

Lecture - 1

Nature is Irreversible direction Time has only one direction: it always in the forward. Similarly heat flow also has only one direction: It always flow form hot body to cold body 3000 C 1000 C 2000 C direction The direction of heat flow is determined by a quantity known as “Entropy”

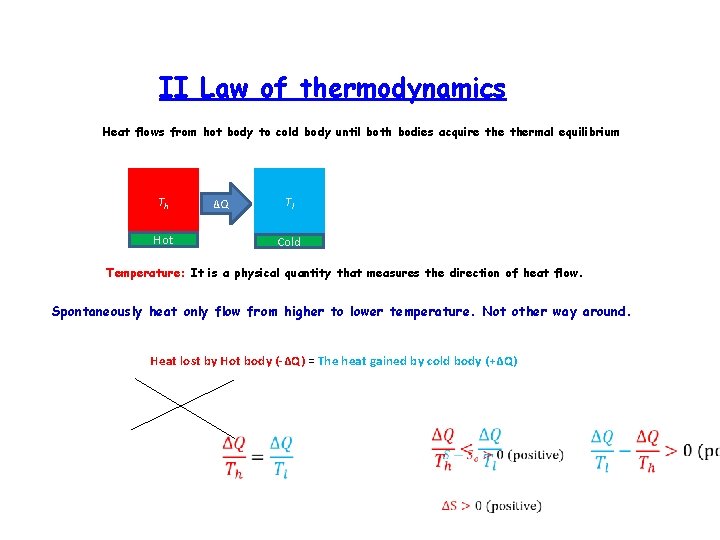
II Law of thermodynamics Heat flows from hot body to cold body until both bodies acquire thermal equilibrium Th Hot ΔQ Tl Cold Temperature: It is a physical quantity that measures the direction of heat flow. Spontaneously heat only flow from higher to lower temperature. Not other way around. Heat lost by Hot body (-ΔQ) = The heat gained by cold body (+ΔQ)

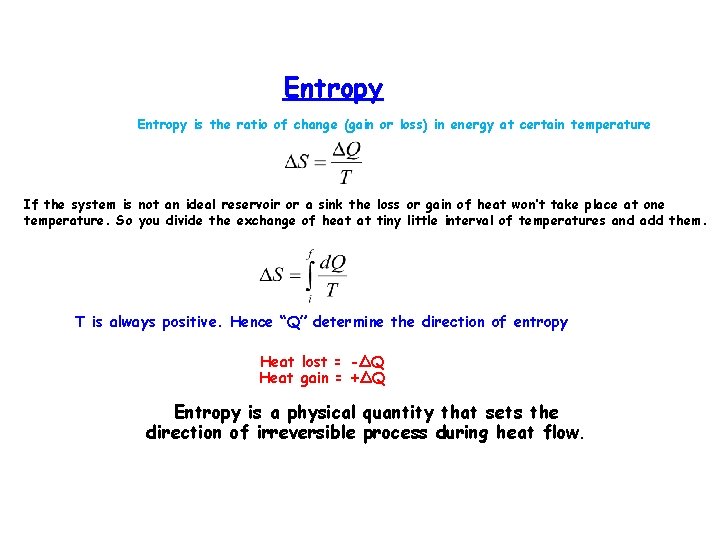
Entropy is the ratio of change (gain or loss) in energy at certain temperature If the system is not an ideal reservoir or a sink the loss or gain of heat won’t take place at one temperature. So you divide the exchange of heat at tiny little interval of temperatures and add them. T is always positive. Hence “Q” determine the direction of entropy Heat lost = -ΔQ Heat gain = +ΔQ Entropy is a physical quantity that sets the direction of irreversible process during heat flow.

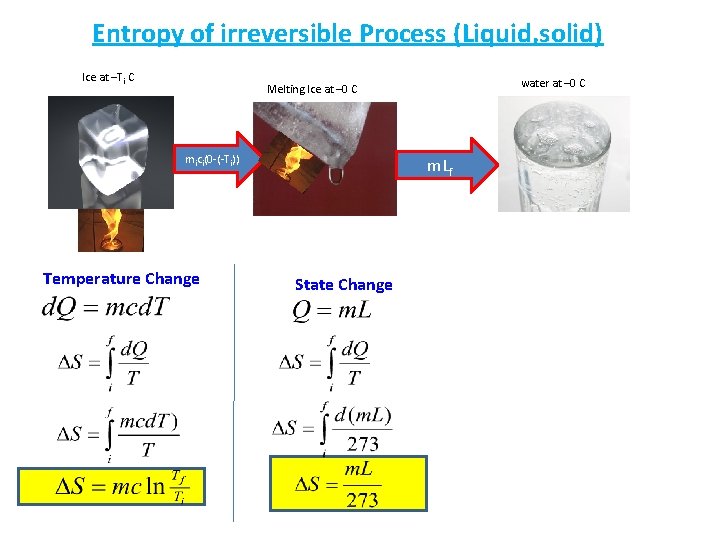
Entropy of irreversible Process (Liquid, solid) Ice at –Ti C water at – 0 C Melting Ice at – 0 C mici(0 -(-Ti)) Temperature Change m. Lf State Change

T 81 -Q 19. Calculate the change in entropy of 1. 0 kg of ice at 0. 0 °C when its temperature is increased to 20. 0 °C [ Lfusion-ice= 333 k. J/kg; cwater= 4190 J/kg. K. (Ans: 1. 5 × 103 J/K)

Checkpoint: Water is heated on a stove Rank the entropy changes of the water as the temperature rises a) From 20 o. C to 30 o. C b) From 30 o. C to 35 o. C c) From 80 o. C to 85 o. C T 072: Q 18: In an isolated container, a 0. 10 kg block of aluminum initially at 600 K is brought into thermal contact with a very large block of iron at 200 K until thermal equilibrium is reached. The iron block is so large that we can assume that its temperature does not change. What is the change in entropy of the iron block? (specific heat of iron is 0. 11 kcal/kg. K and specific heat of aluminum is 0. 22 kcal/kg. K) (Ans: 44 cal/K)

T 052: Q#5. A 100 g of ice at -10 o. C is placed in a lake whose temperature is 20 o. C. a) Calculate the change in entropy of the lake. b) Calculate the change in entropy of the ice c) Calculate the net change in entropy (Ans: – 150 J/K. )

T 103 -Q 18. A system consists of two large thermal reservoirs in contact with each other, one at a temperature of 300 °C and the other at a temperature 200 °C. If 600 J of heat is transferred from the 300 °C reservoir to the 200 °C reservoir, what is the change in entropy of this system? A) 0. 221 J/K T 101 -Q 20. One kilogram of water at 0 o. C (system A) is added to one kilogram of water at 100 o. C (system B) in an insulated container. Calculate the change in entropy of system B. A) – 603 J/K

Entropy of Gas

Reversible Process 1 0 o. C ice P Water at 00 C 2 T ±d. Q conductor We have the information only about Initial and final states not about the states between them If you remove tiny little masses one by one you can track the pressure, temperature and volume. If the bottom is conductor you can exchange heat at constant temperature.

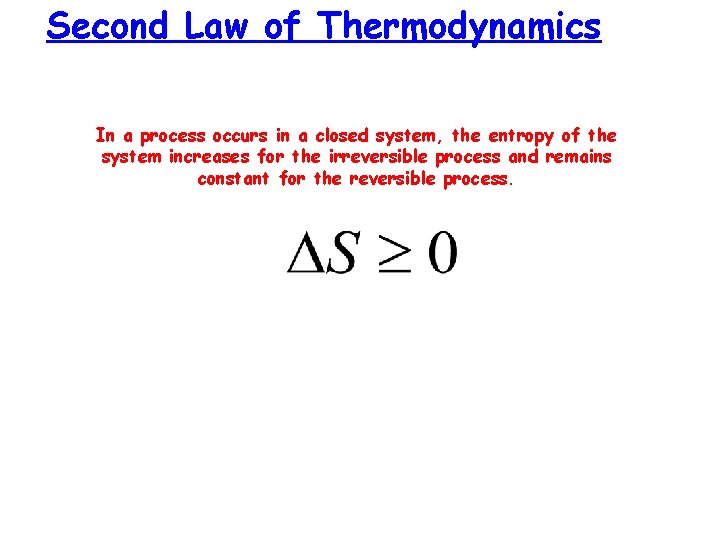
Second Law of Thermodynamics In a process occurs in a closed system, the entropy of the system increases for the irreversible process and remains constant for the reversible process.

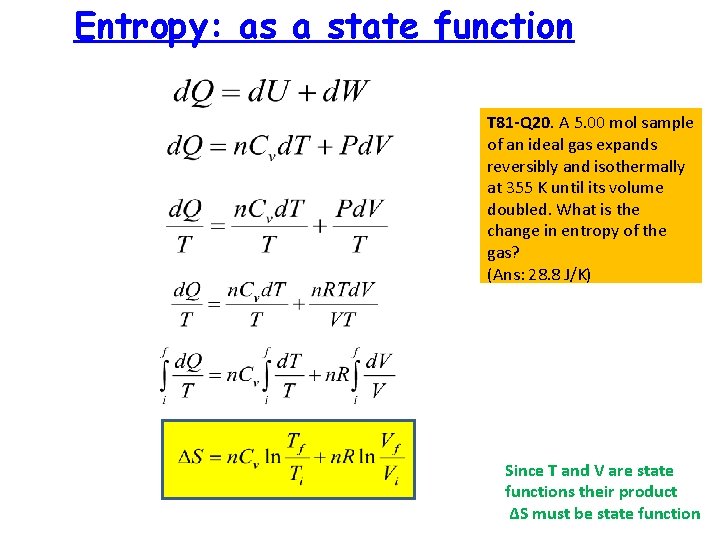
Entropy: as a state function T 81 -Q 20. A 5. 00 mol sample of an ideal gas expands reversibly and isothermally at 355 K until its volume doubled. What is the change in entropy of the gas? (Ans: 28. 8 J/K) Since T and V are state functions their product ΔS must be state function

T 81 -Q 20. A 5. 00 mol sample of an ideal gas expands reversibly and isothermally at 355 K until its volume doubled. What is the change in entropy of the gas? (Ans: 28. 8 J/K)

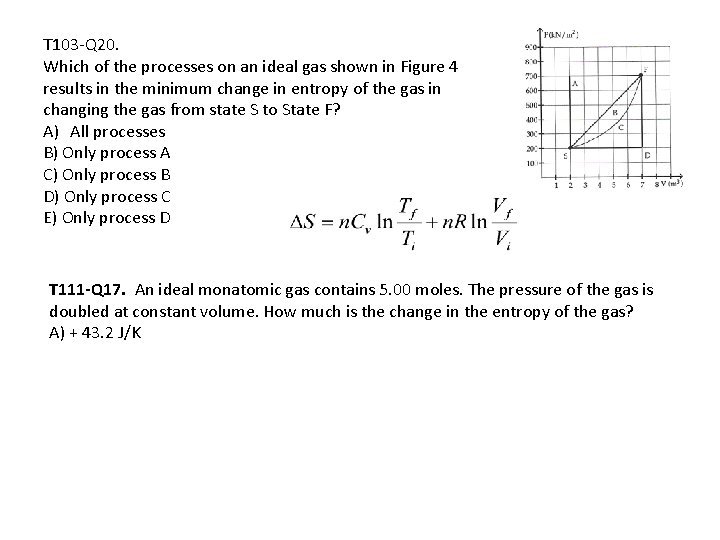
T 103 -Q 20. Which of the processes on an ideal gas shown in Figure 4 results in the minimum change in entropy of the gas in changing the gas from state S to State F? A) All processes B) Only process A C) Only process B D) Only process C E) Only process D T 111 -Q 17. An ideal monatomic gas contains 5. 00 moles. The pressure of the gas is doubled at constant volume. How much is the change in the entropy of the gas? A) + 43. 2 J/K

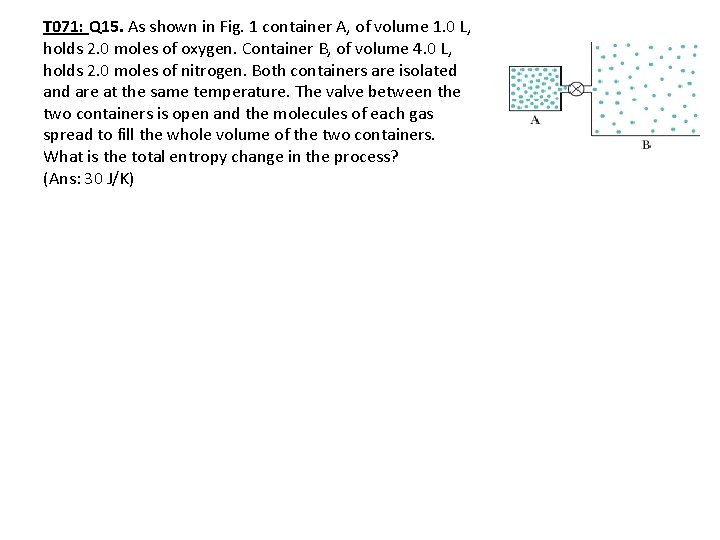
T 071: Q 15. As shown in Fig. 1 container A, of volume 1. 0 L, holds 2. 0 moles of oxygen. Container B, of volume 4. 0 L, holds 2. 0 moles of nitrogen. Both containers are isolated and are at the same temperature. The valve between the two containers is open and the molecules of each gas spread to fill the whole volume of the two containers. What is the total entropy change in the process? (Ans: 30 J/K)

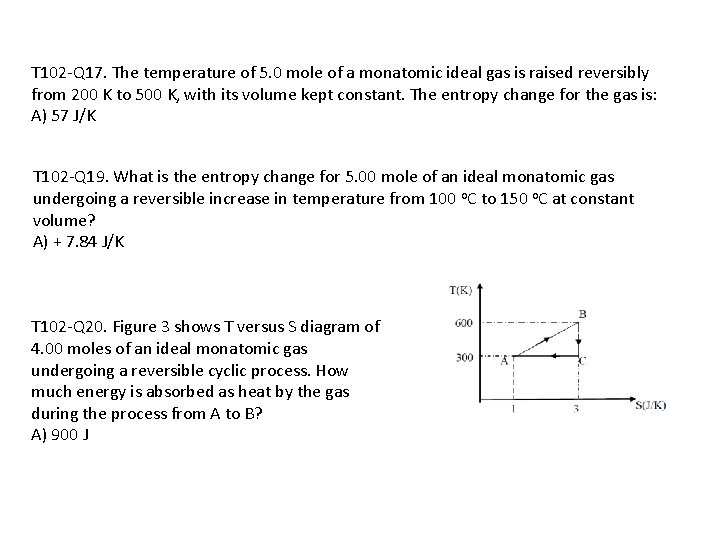
T 102 -Q 17. The temperature of 5. 0 mole of a monatomic ideal gas is raised reversibly from 200 K to 500 K, with its volume kept constant. The entropy change for the gas is: A) 57 J/K T 102 -Q 19. What is the entropy change for 5. 00 mole of an ideal monatomic gas undergoing a reversible increase in temperature from 100 o. C to 150 o. C at constant volume? A) + 7. 84 J/K T 102 -Q 20. Figure 3 shows T versus S diagram of 4. 00 moles of an ideal monatomic gas undergoing a reversible cyclic process. How much energy is absorbed as heat by the gas during the process from A to B? A) 900 J

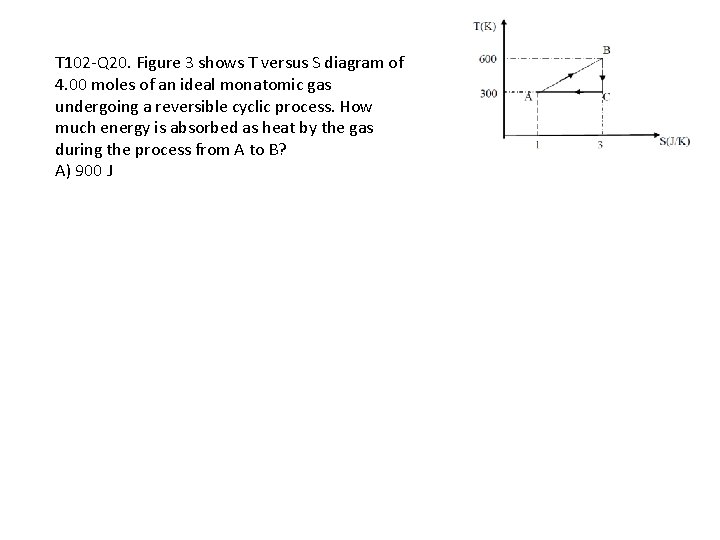
T 102 -Q 20. Figure 3 shows T versus S diagram of 4. 00 moles of an ideal monatomic gas undergoing a reversible cyclic process. How much energy is absorbed as heat by the gas during the process from A to B? A) 900 J

Lecture - 2

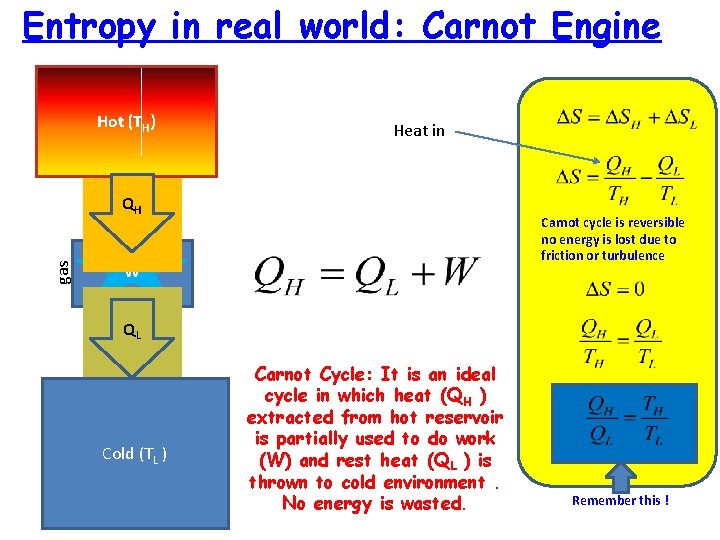
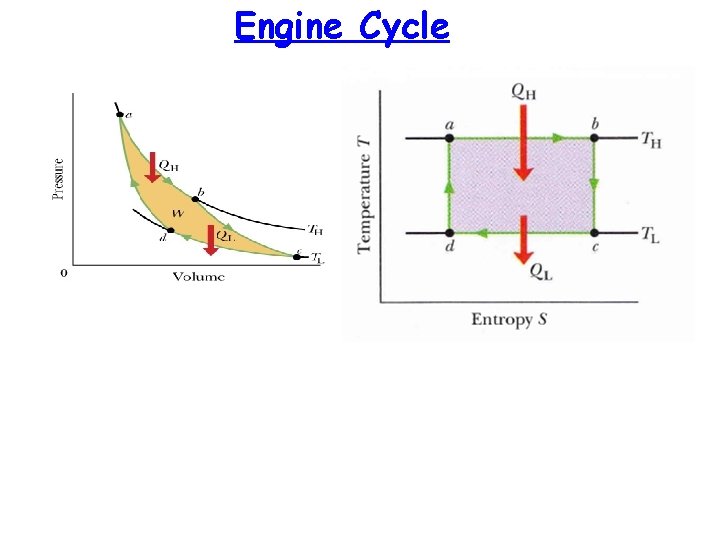
Entropy in real world: Carnot Engine Hot (TH) Heat in gas QH Carnot cycle is reversible no energy is lost due to friction or turbulence W gas QL Cold (TL ) Carnot Cycle: It is an ideal cycle in which heat (QH ) extracted from hot reservoir is partially used to do work (W) and rest heat (QL ) is thrown to cold environment. No energy is wasted. Remember this !

Engine Cycle

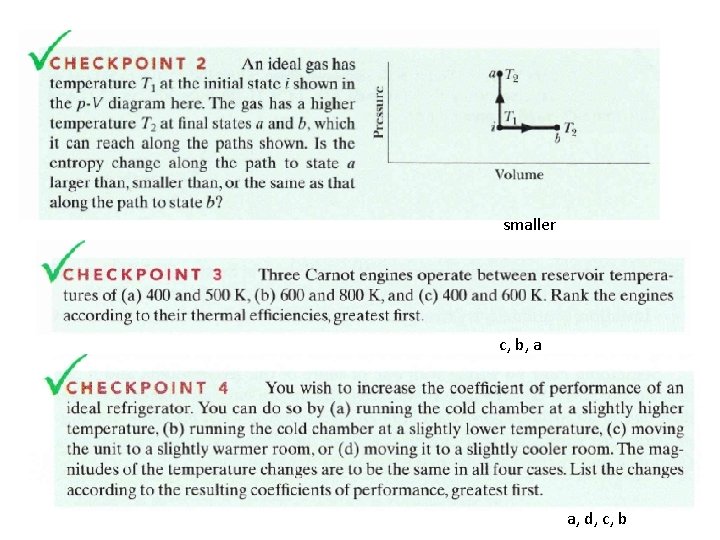
smaller c, b, a a, d, c, b


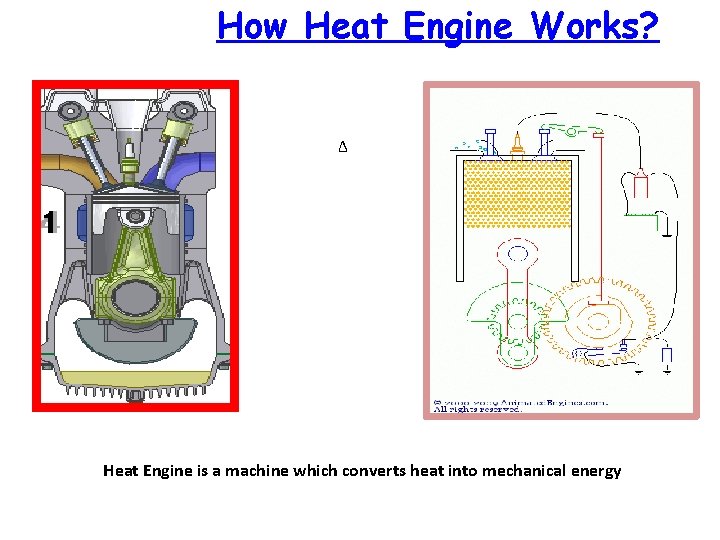
How Heat Engine Works? Δ Heat Engine is a machine which converts heat into mechanical energy

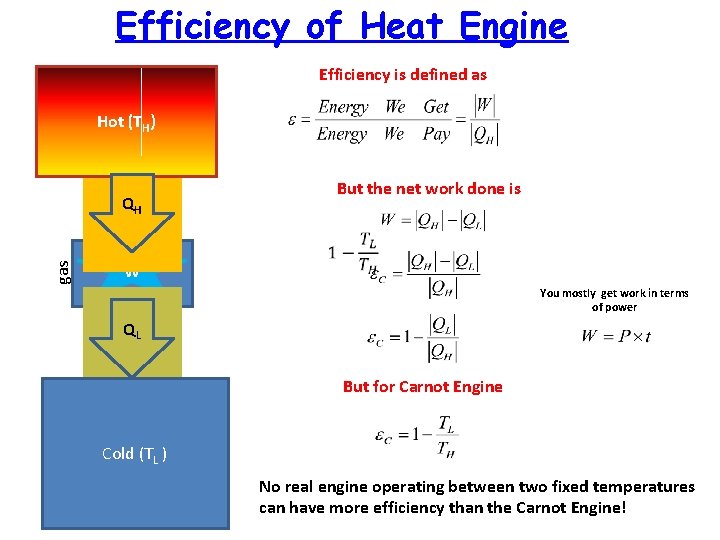
Efficiency of Heat Engine Efficiency is defined as Hot (TH) gas QH But the net work done is W gas You mostly get work in terms of power QL But for Carnot Engine Cold (TL ) No real engine operating between two fixed temperatures can have more efficiency than the Carnot Engine!

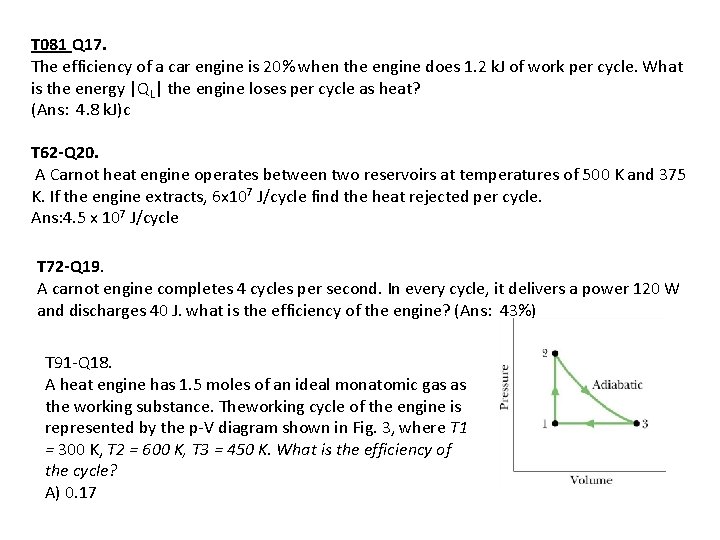
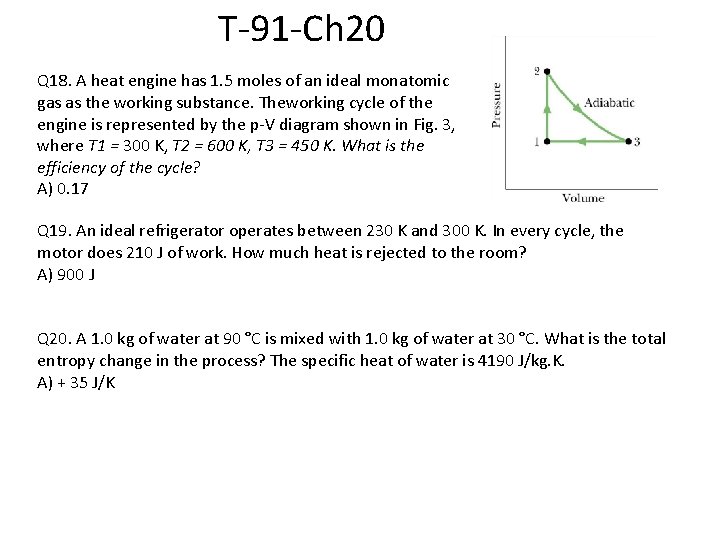
T 081 Q 17. The efficiency of a car engine is 20% when the engine does 1. 2 k. J of work per cycle. What is the energy |QL| the engine loses per cycle as heat? (Ans: 4. 8 k. J)c T 62 -Q 20. A Carnot heat engine operates between two reservoirs at temperatures of 500 K and 375 K. If the engine extracts, 6 x 107 J/cycle find the heat rejected per cycle. Ans: 4. 5 x 107 J/cycle T 72 -Q 19. A carnot engine completes 4 cycles per second. In every cycle, it delivers a power 120 W and discharges 40 J. what is the efficiency of the engine? (Ans: 43%) T 91 -Q 18. A heat engine has 1. 5 moles of an ideal monatomic gas as the working substance. Theworking cycle of the engine is represented by the p-V diagram shown in Fig. 3, where T 1 = 300 K, T 2 = 600 K, T 3 = 450 K. What is the efficiency of the cycle? A) 0. 17

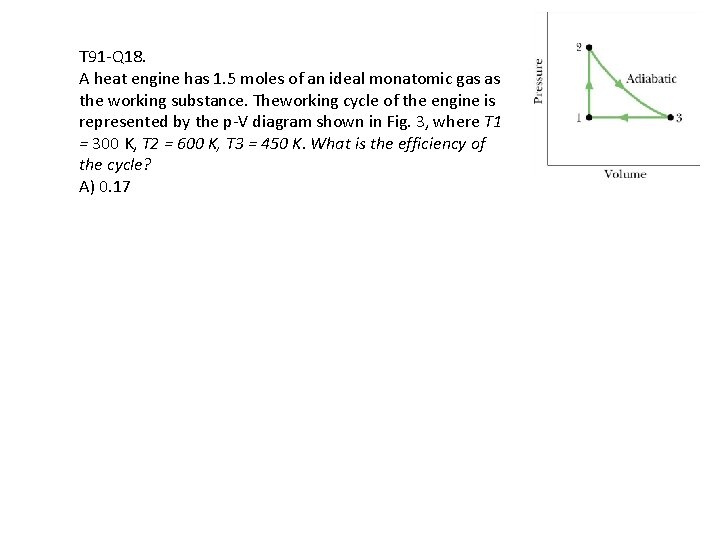
T 91 -Q 18. A heat engine has 1. 5 moles of an ideal monatomic gas as the working substance. Theworking cycle of the engine is represented by the p-V diagram shown in Fig. 3, where T 1 = 300 K, T 2 = 600 K, T 3 = 450 K. What is the efficiency of the cycle? A) 0. 17




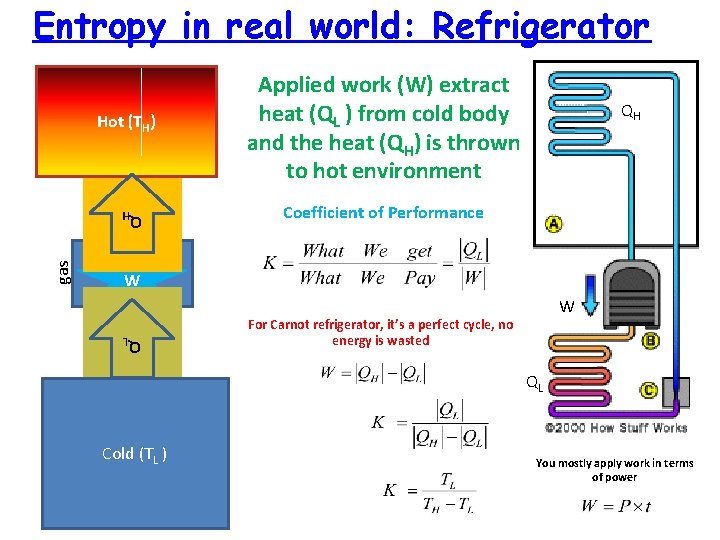
Entropy in real world: Refrigerator Hot (TH) QH QH Coefficient of Performance gas W QL gas Applied work (W) extract heat (QL ) from cold body and the heat (QH) is thrown to hot environment W For Carnot refrigerator, it’s a perfect cycle, no energy is wasted QL Cold (TL ) You mostly apply work in terms of power

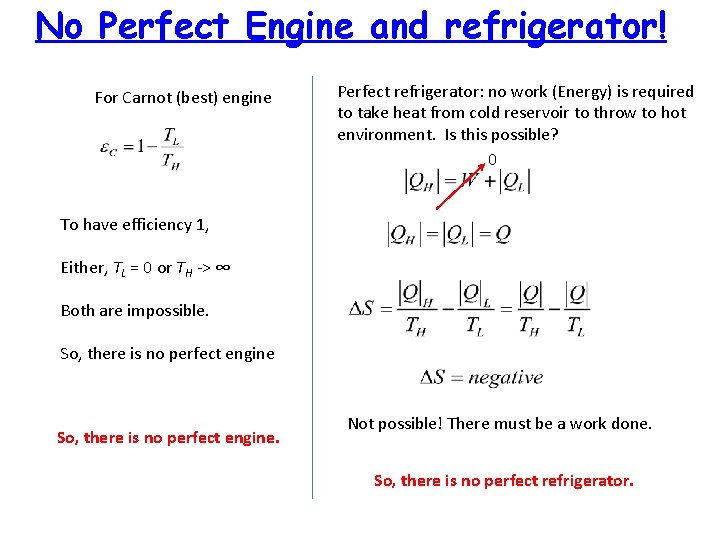
No Perfect Engine and refrigerator! For Carnot (best) engine Perfect refrigerator: no work (Energy) is required to take heat from cold reservoir to throw to hot environment. Is this possible? 0 To have efficiency 1, Either, TL = 0 or TH -> ∞ Both are impossible. So, there is no perfect engine. Not possible! There must be a work done. So, there is no perfect refrigerator.

T 72 -Q 20. A refrigerator converts 7. 0 kg of water at 0 o. C inti ice at 0 o. C in one hour. What is the coefficient of performance of the refrigerator if its power input is 300 W? Heat of fusion for water is 333 k. J/kg. (Ans: 2. 2) T 71 -Q 18. A carnot refrigerator operating between -20 o. C and +20 o. C extracts heat from the cold reservoir at the rate 200 J/s. What is the rate at which work is done on the refrigerator? (Ans: 32 J/s) T 81 -Q 18. The freezing compartment of a Carnot refrigerator is at 269 K while outside air in the room is at 298 K. If the power of refrigerator motor is 150 W, what is maximum amount of energy that can be extracted as heat from the freezing compartment in 10. 0 min? (Ans: 8. 35 × 105 J)

Old Exams






T-111 -Ch 20 Q 17. An ideal monatomic gas contains 5. 00 moles. The pressure of the gas is doubled at constant volume. How much is the change in the entropy of the gas? A) + 43. 2 J/K Q 18. A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K. During each cycle, 1. 0 × 105 J of heat is removed from the hot reservoir and 5. 0 × 104 J of work is performed. The actual efficiency of this engine is: A) 75 % of the maximum efficiency Q 19. A Carnot refrigerator is placed in a kitchen. The temperature inside the refrigerator is 2. 0 °C, and the temperature of the kitchen is 22 °C. The rate of heat flow from the refrigerator to the kitchen is 24. 7 k. W. What power is needed to operate this refrigerator? A) 1. 8 k. W Q 20. A system consists of two thermal reservoirs in contact with each other, one at a temperature of 300 o. C and the other at a temperature of 200 o. C. If 6000 J of heat is transferred from the 300 o. C reservoir to the 200 o. C reservoir, what is the change in entropy of this system? A) +2. 2 J/K

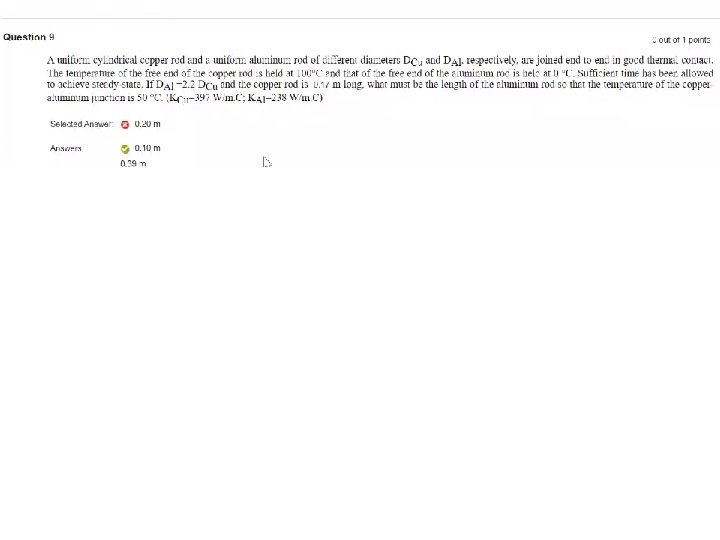
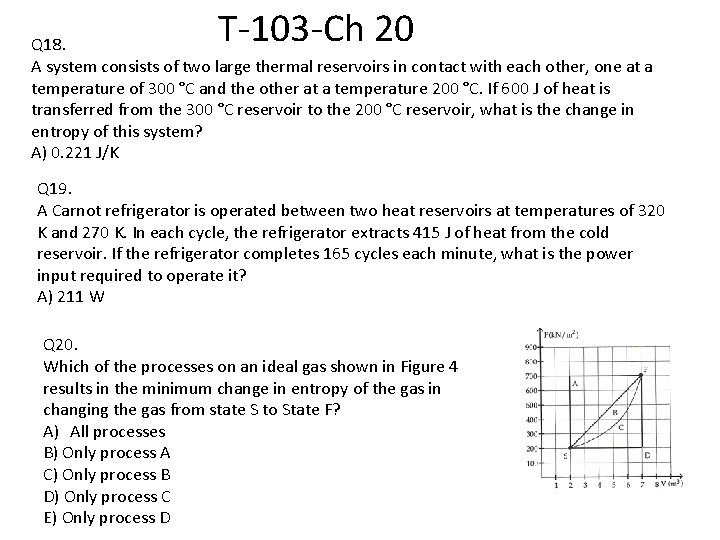
T-103 -Ch 20 Q 18. A system consists of two large thermal reservoirs in contact with each other, one at a temperature of 300 °C and the other at a temperature 200 °C. If 600 J of heat is transferred from the 300 °C reservoir to the 200 °C reservoir, what is the change in entropy of this system? A) 0. 221 J/K Q 19. A Carnot refrigerator is operated between two heat reservoirs at temperatures of 320 K and 270 K. In each cycle, the refrigerator extracts 415 J of heat from the cold reservoir. If the refrigerator completes 165 cycles each minute, what is the power input required to operate it? A) 211 W Q 20. Which of the processes on an ideal gas shown in Figure 4 results in the minimum change in entropy of the gas in changing the gas from state S to State F? A) All processes B) Only process A C) Only process B D) Only process C E) Only process D

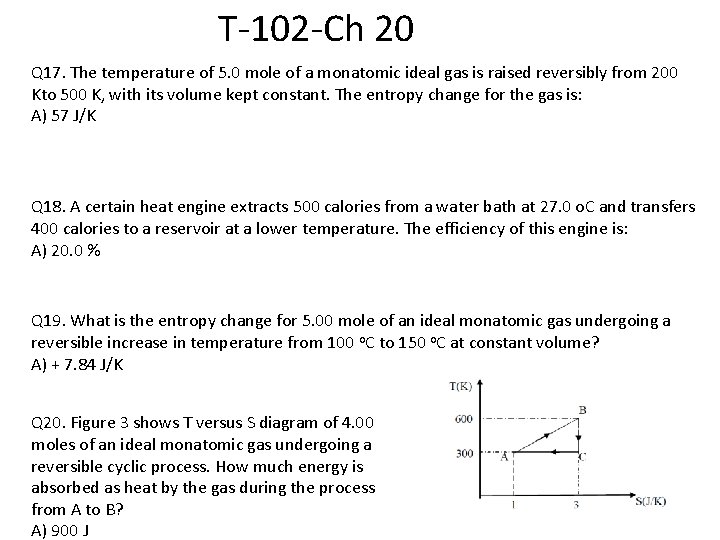
T-102 -Ch 20 Q 17. The temperature of 5. 0 mole of a monatomic ideal gas is raised reversibly from 200 Kto 500 K, with its volume kept constant. The entropy change for the gas is: A) 57 J/K Q 18. A certain heat engine extracts 500 calories from a water bath at 27. 0 o. C and transfers 400 calories to a reservoir at a lower temperature. The efficiency of this engine is: A) 20. 0 % Q 19. What is the entropy change for 5. 00 mole of an ideal monatomic gas undergoing a reversible increase in temperature from 100 o. C to 150 o. C at constant volume? A) + 7. 84 J/K Q 20. Figure 3 shows T versus S diagram of 4. 00 moles of an ideal monatomic gas undergoing a reversible cyclic process. How much energy is absorbed as heat by the gas during the process from A to B? A) 900 J

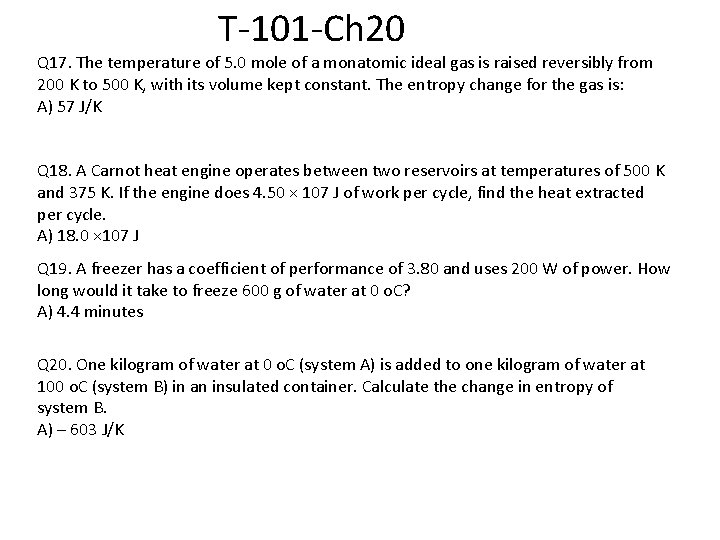
T-101 -Ch 20 Q 17. The temperature of 5. 0 mole of a monatomic ideal gas is raised reversibly from 200 K to 500 K, with its volume kept constant. The entropy change for the gas is: A) 57 J/K Q 18. A Carnot heat engine operates between two reservoirs at temperatures of 500 K and 375 K. If the engine does 4. 50 × 107 J of work per cycle, find the heat extracted per cycle. A) 18. 0 × 107 J Q 19. A freezer has a coefficient of performance of 3. 80 and uses 200 W of power. How long would it take to freeze 600 g of water at 0 o. C? A) 4. 4 minutes Q 20. One kilogram of water at 0 o. C (system A) is added to one kilogram of water at 100 o. C (system B) in an insulated container. Calculate the change in entropy of system B. A) – 603 J/K

T-91 -Ch 20 Q 18. A heat engine has 1. 5 moles of an ideal monatomic gas as the working substance. Theworking cycle of the engine is represented by the p-V diagram shown in Fig. 3, where T 1 = 300 K, T 2 = 600 K, T 3 = 450 K. What is the efficiency of the cycle? A) 0. 17 Q 19. An ideal refrigerator operates between 230 K and 300 K. In every cycle, the motor does 210 J of work. How much heat is rejected to the room? A) 900 J Q 20. A 1. 0 kg of water at 90 °C is mixed with 1. 0 kg of water at 30 °C. What is the total entropy change in the process? The specific heat of water is 4190 J/kg. K. A) + 35 J/K

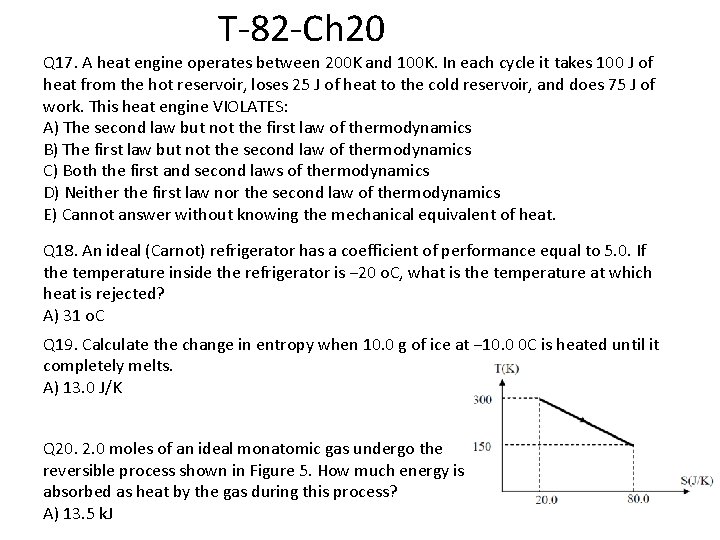
T-82 -Ch 20 Q 17. A heat engine operates between 200 K and 100 K. In each cycle it takes 100 J of heat from the hot reservoir, loses 25 J of heat to the cold reservoir, and does 75 J of work. This heat engine VIOLATES: A) The second law but not the first law of thermodynamics B) The first law but not the second law of thermodynamics C) Both the first and second laws of thermodynamics D) Neither the first law nor the second law of thermodynamics E) Cannot answer without knowing the mechanical equivalent of heat. Q 18. An ideal (Carnot) refrigerator has a coefficient of performance equal to 5. 0. If the temperature inside the refrigerator is − 20 o. C, what is the temperature at which heat is rejected? A) 31 o. C Q 19. Calculate the change in entropy when 10. 0 g of ice at − 10. 0 0 C is heated until it completely melts. A) 13. 0 J/K Q 20. 2. 0 moles of an ideal monatomic gas undergo the reversible process shown in Figure 5. How much energy is absorbed as heat by the gas during this process? A) 13. 5 k. J

T-81 -Ch 20 Q 17. The efficiency of a car engine is 20% when the engine does 1. 2 k. J of work per cycle. What is the energy |QL| the engine loses per cycle as heat? (Ans: 4. 8 k. J) Q 18. The freezing compartment of a Carnot refrigerator is at 269 K while outside air in the room is at 298 K. If the power of refrigerator motor is 150 W, what is maximum amount of energy that can be extracted as heat from the freezing compartment in 10. 0 min? (Ans: 8. 35 × 105 J) Q 19. Calculate the change in entropy of 1. 0 kg of ice at 0. 0 °C when its temperature is increased to 20. 0 °C [ Lfusion-ice= 333 k. J/kg; cwater= 4190 J/kg. K. (Ans: 1. 5 × 103 J/K) Q 20. A 5. 00 mol sample of an ideal gas expands reversibly and isothermally at 355 K until its volume doubled. What is the change in entropy of the gas? (Ans: 28. 8 J/K)

T-72 -Ch 20 Q 18: In an isolated container, a 0. 10 kg block of aluminum initially at 600 K is brought into thermal contact with a very large block of iron at 200 K until thermal equilibrium is reached. The iron block is so large that we can assume that its temperature does not change. What is the change in entropy of the iron block? (specific heat of iron is 0. 11 kcal/kg. K and specific heat of aluminum is 0. 22 kcal/kg. K) (Ans: 44 cal/K) Q 19. A carnot engine completes 4 cycles per second. In every cycle, it delivers a power 120 W and discharges 40 J. what is the efficiency of the engine? (Ans: 43%) Q 20. A refrigerator converts 7. 0 kg of water at 0 o. C inti ice at 0 o. C in one hour. What is the coefficient of performance of the refrigerator if its power input is 300 W? Heat of fusion for water is 333 k. J/kg. (Ans: 2. 2)

T-71 -Ch 16 Q 15. As shown in Fig. 1 container A, of volume 1. 0 L, holds 2. 0 moles of oxygen. Container B, of volume 4. 0 L, holds 2. 0 moles of nitrogen. Both containers are isolated and are at the same temperature. The valve between the two containers is open and the molecules of each gas spread to fill the whole volume of the two containers. What is the total entropy change in the process? (Ans: 30 J/K) Q 16. An ice cube of mass 400 g at temperature of 0 o. C melts to water at 0 o. C. The process takes place very slowly, so it is reversible. What is the change in entropy of the ice when it has all melted. (Ans: 488 J/K) Q 17. A carnot heat engine operates between reservoirs at temperatures of 700 K and 300 K. In one cycle it absorbs 1500 J heat. How much work is done by the engine? (857 J) Q 18. A carnot refrigerator operating between -20 o. C and +20 o. C extracts heat from the cold reservoir at the rate 200 J/s. What is the rate at which work is done on the refrigerator? (Ans: 32 J/s)

T-62 -Ch 20 Q 19. The change in entropy for melting 6. 0 kg of a solid which melts at 27 o. C is: [The latent heat of fusion of the solid is ] (Ans: 5. 0 x 102 J/K) Q 20. A Carnot heat engine operates between two reservoirs at temperatures of 500 K and 375 K. If the engine extracts , find the heat rejected per cycle. (Ans: 4. 5 x 107 cycle/s)

T-61 -Ch 20 Q 18. Liquid water having a mass of 50 grams was initially at 0 o. C. Heat was added to the water so that its entropy increases by 94. 0 J/K, what is the final temperature of the water? (Ans: 428 K ) Q 19. A 2. 50 -mole sample of an ideal monatomic gas was initially at a temperature of 300 K. The gas is compressed isobarically to half of its original volume, what is the change of entropy of the gas? (Ans: – 36. 0 J/K ) Q 20. A Carnot heat engine operates between two reservoirs at temperatures of 400 K and 500 K. What is the ratio of the work done by the engine to the heat expelled to the lowtemperature reservoir? (Ans: 0. 25 )

T-52 -Ch 20 Q#5. A 100 g of ice at -10 o. C is placed in a lake whose temperature is 20 o. C. Calculate the change in entropy of the lake. (Ans: – 150 J/K. ) Q#17. The temperature of 2. 0 mole of a monatomic ideal gas is raised reversibly from 100 K to 300 K, with its volume kept constant. The entropy change for the gas is: (Ans: 27 J/K )

T-51 -Ch 20 Q#17. One mole of an ideal gas expands reversibly and isothermally at temperature T =27 o. C until its volume is doubled. The change of entropy of this gas for this process is: (Ans: 5. 8 J/K ) Q#18. A 300 g of lead melts at 327 C. The latent heat of fusion of lead is 24. 5 k. J/kg. The change in entropy is (Ans: 12. 3 J/K Q#19. Two moles of an ideal monatomic gas are taken around the cycle shown in the figure 1. If Ta= 60 K, Tb=30 K and Tc=38 K, find the efficiency? (Cv=12. 5 and Cp=20. 75 J/mol. K). (Ans: 56% )