CHAPTER 2 VERTICAL STRUCTURE OF THE ATMOSPHERE Measurement

- Slides: 9

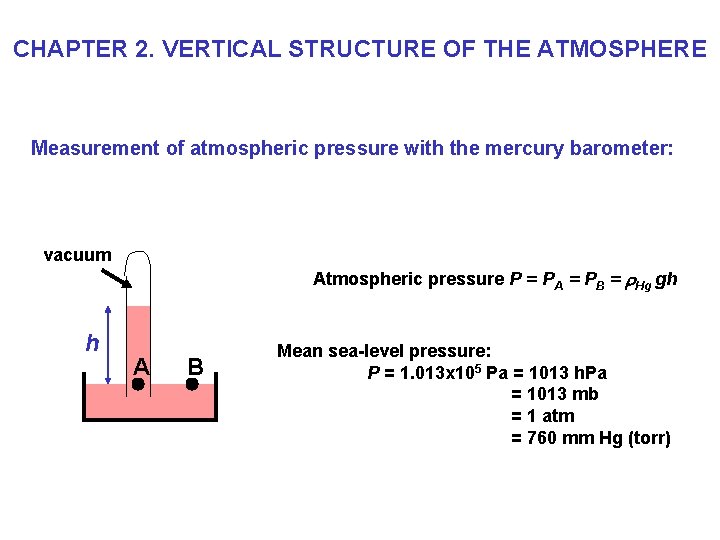
CHAPTER 2. VERTICAL STRUCTURE OF THE ATMOSPHERE Measurement of atmospheric pressure with the mercury barometer: vacuum Atmospheric pressure P = PA = PB = r. Hg gh h A B Mean sea-level pressure: P = 1. 013 x 105 Pa = 1013 h. Pa = 1013 mb = 1 atm = 760 mm Hg (torr)

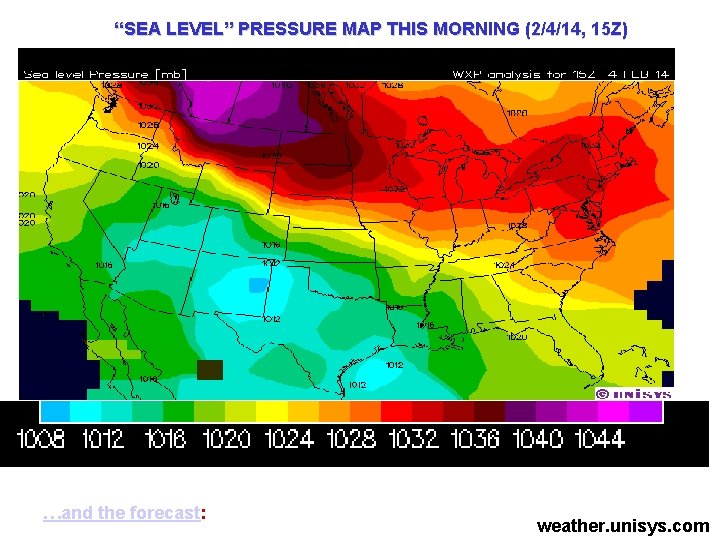
“SEA LEVEL” PRESSURE MAP THIS MORNING (2/4/14, 15 Z) …and the forecast: weather. unisys. com

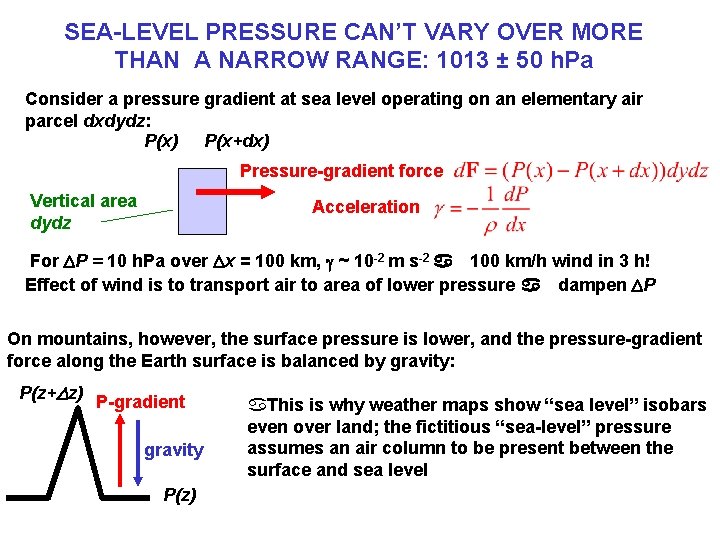
SEA-LEVEL PRESSURE CAN’T VARY OVER MORE THAN A NARROW RANGE: 1013 ± 50 h. Pa Consider a pressure gradient at sea level operating on an elementary air parcel dxdydz: P(x) P(x+dx) Pressure-gradient force Vertical area dydz Acceleration For DP = 10 h. Pa over Dx = 100 km, g ~ 10 -2 m s-2 a 100 km/h wind in 3 h! Effect of wind is to transport air to area of lower pressure a dampen DP On mountains, however, the surface pressure is lower, and the pressure-gradient force along the Earth surface is balanced by gravity: P(z+Dz) P-gradient gravity P(z) a. This is why weather maps show “sea level” isobars even over land; the fictitious “sea-level” pressure assumes an air column to be present between the surface and sea level

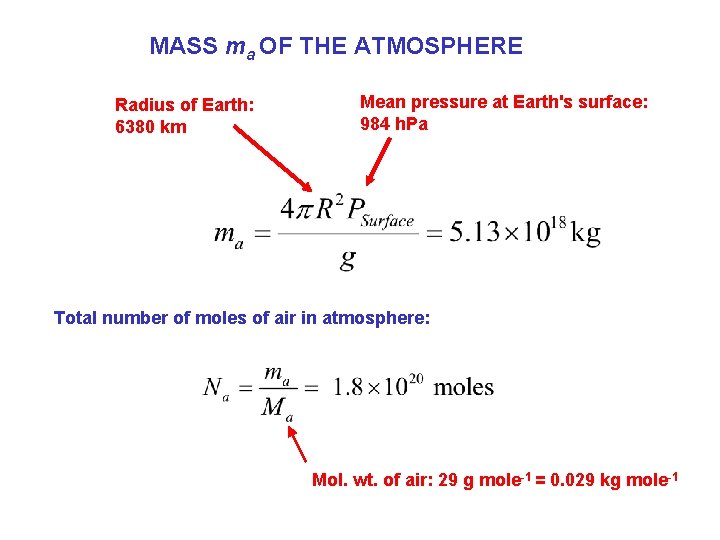
MASS ma OF THE ATMOSPHERE Radius of Earth: 6380 km Mean pressure at Earth's surface: 984 h. Pa Total number of moles of air in atmosphere: Mol. wt. of air: 29 g mole-1 = 0. 029 kg mole-1

QUESTIONS 1. What is the fractional increase in volume of water-soluble aerosol particles when relative humidity increases from 90% to 95%? (assume that the particles are mainly water, so neglect the contribution of the solute to particle volume). Assuming that visibility degradation is proportional to the cross-sectional area of the particles, what is the resulting percentage decrease in visibility? 2. Why does it take longer to boil an egg in Denver than in Boston?

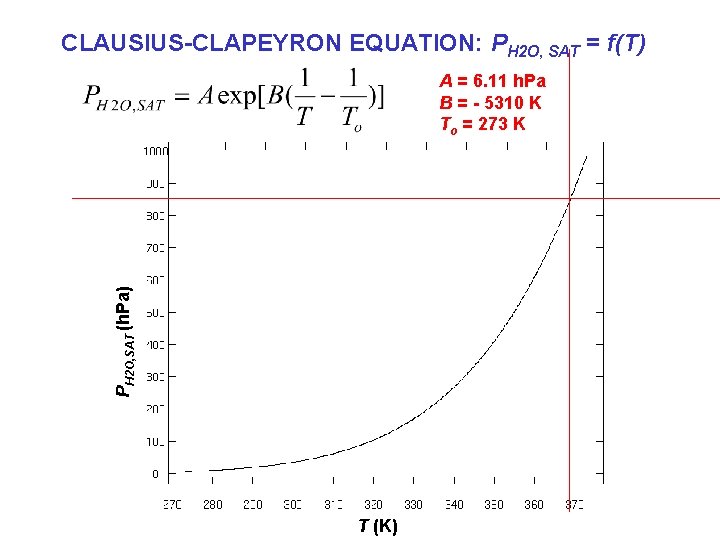
CLAUSIUS-CLAPEYRON EQUATION: PH 2 O, SAT = f(T) PH 2 O, SAT (h. Pa) A = 6. 11 h. Pa B = - 5310 K To = 273 K T (K)

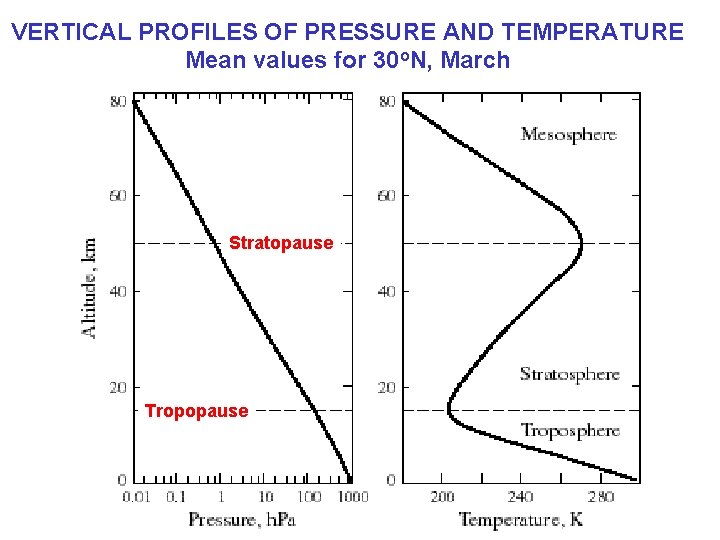
VERTICAL PROFILES OF PRESSURE AND TEMPERATURE Mean values for 30 o. N, March Stratopause Tropopause

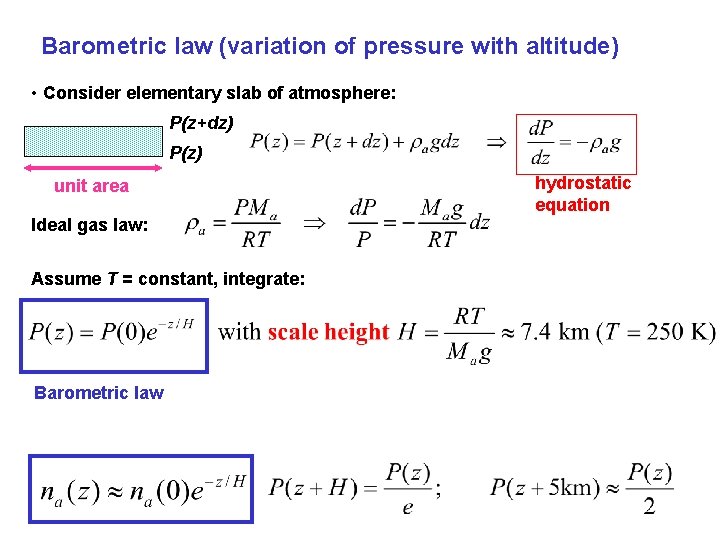
Barometric law (variation of pressure with altitude) • Consider elementary slab of atmosphere: P(z+dz) P(z) unit area Ideal gas law: Assume T = constant, integrate: Barometric law hydrostatic equation

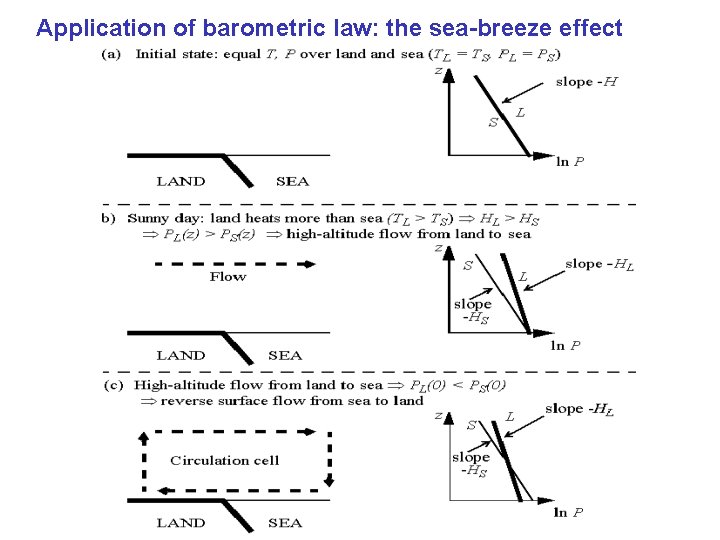
Application of barometric law: the sea-breeze effect