Chapter 2 The Structure and Function of Macromolecules







- Slides: 27

Chapter 2: The Structure and Function of Macromolecules

Polymer Principles • POLYMER: large molecule consisting of many identical or similar subunits connected together • MONOMER: subunit or building block molecule of a polymer • MACROMOLECULE: large organic polymer *Examples: carbohydrates, lipids, proteins, nucleic acids

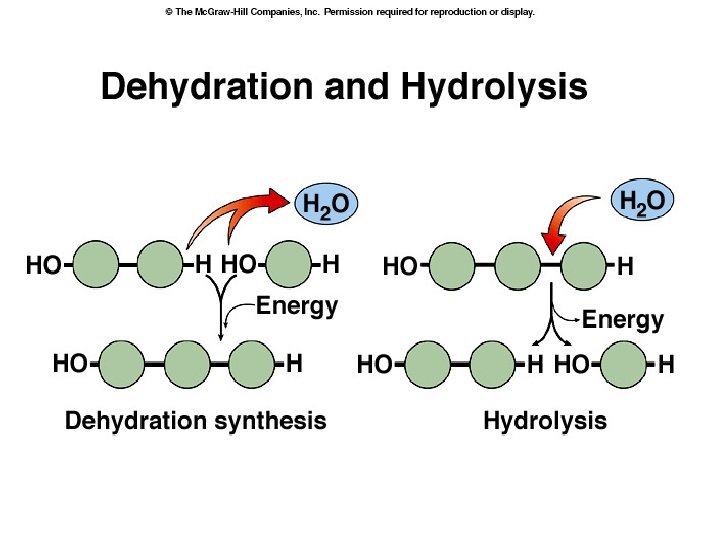
• POLYMERIZATION REACTIONS: chemical reactions that link 2 or more small molecules (monomers) to form larger molecules (polymers) • DEHYDRATION SYNTHESIS REACTIONS (or CONDENSATION): reactions during which monomers are linked together; an –H and –OH are removed, producing net removal of a water molecule for each covalent linkage

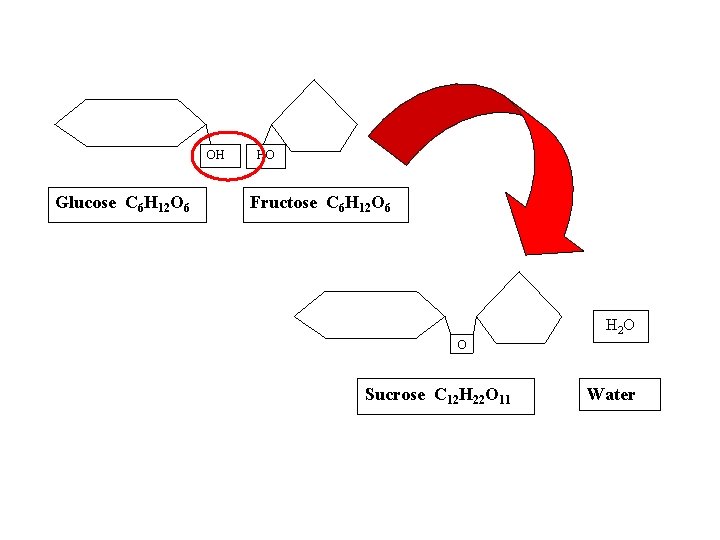
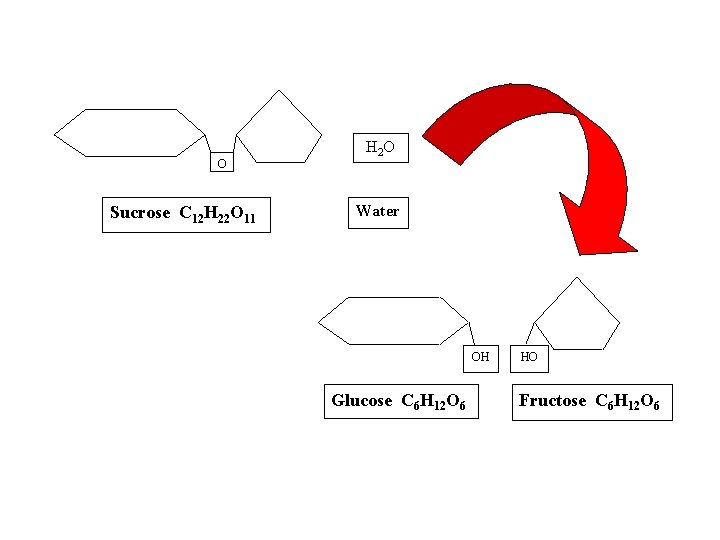
OH Glucose C 6 H 12 O 6 HO Fructose C 6 H 12 O 6 H 2 O O Sucrose C 12 H 22 O 11 Water

• HYDROLYSIS: process that breaks the covalent bonds between monomers by the addition of water molecules *Example: DIGESTION

O Sucrose C 12 H 22 O 11 H 2 O Water OH Glucose C 6 H 12 O 6 HO Fructose C 6 H 12 O 6



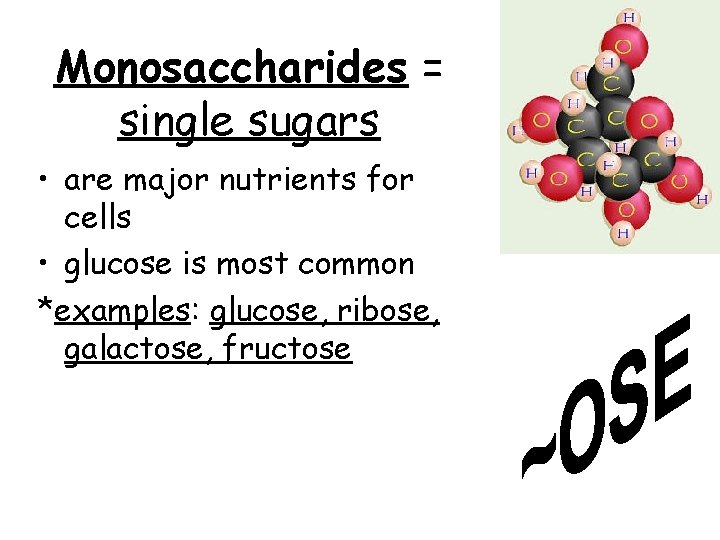
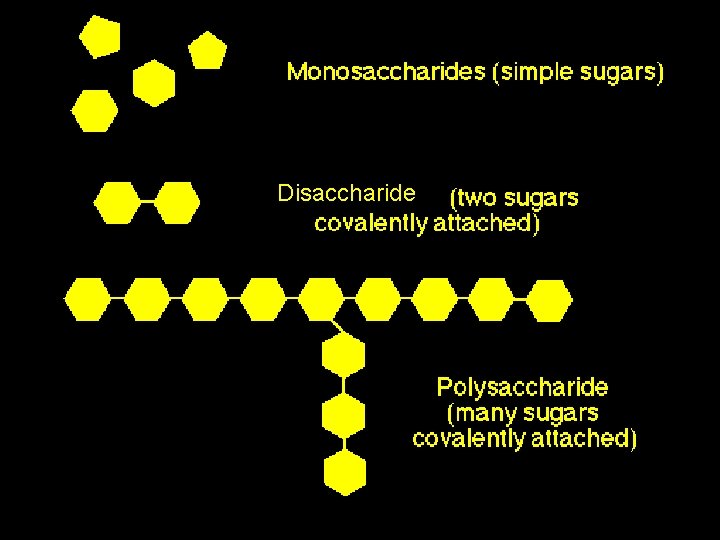
Monosaccharides = single sugars • are major nutrients for cells • glucose is most common *examples: glucose, ribose, galactose, fructose

Disaccharides = double sugars • Also a source of energy • Formed when 2 monosaccharides combine in a dehydration reaction; • Examples: lactose (milk sugar): glucose + galactose sucrose (table sugar): glucose + fructose

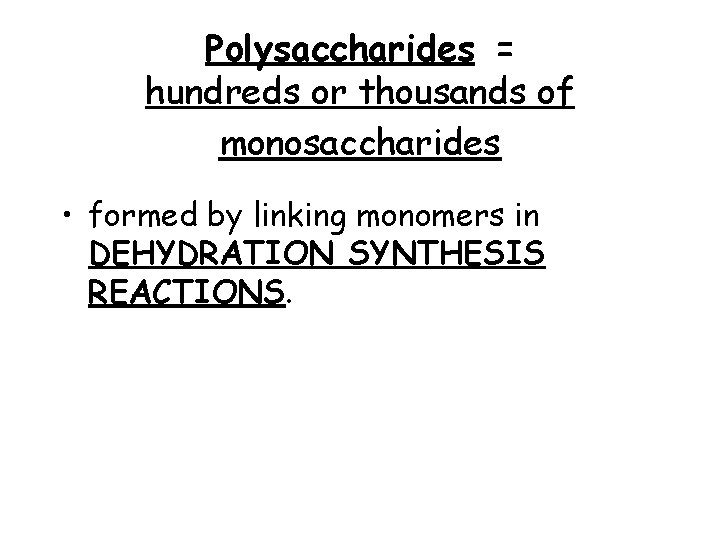
Polysaccharides = hundreds or thousands of monosaccharides • formed by linking monomers in DEHYDRATION SYNTHESIS REACTIONS.

Disaccharide

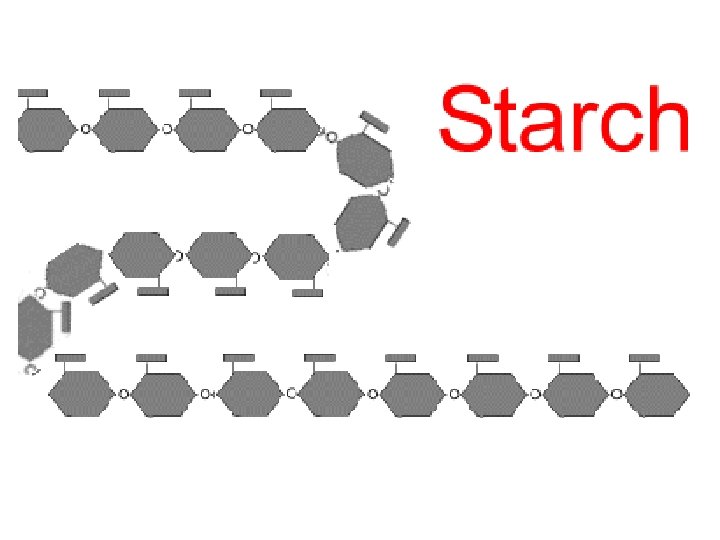
Examples of energy storage polysaccharides: • starch = glucose polymer in plants used for energy storage (in roots, tubers, etc. ) • glycogen = glucose polymer in animals stored in skeletal muscles and liver of humans & other vertebrates


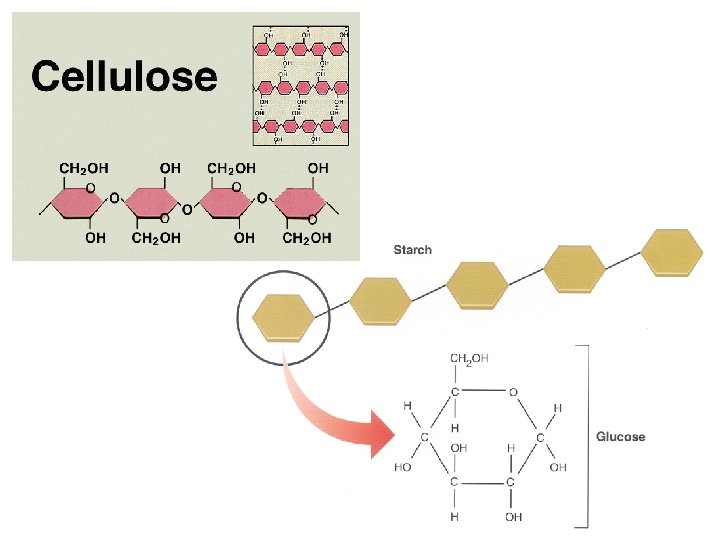
Examples of structural support polysaccharides: • cellulose = structural component of plant cell walls that cannot be digested by most organisms • chitin = forms exoskeletons of arthropods



LIPIDS insoluble in water (because they are NONPOLAR, or HYDROPHOBIC) include: 1. Fats 2. Phospholipids 3. Steroids

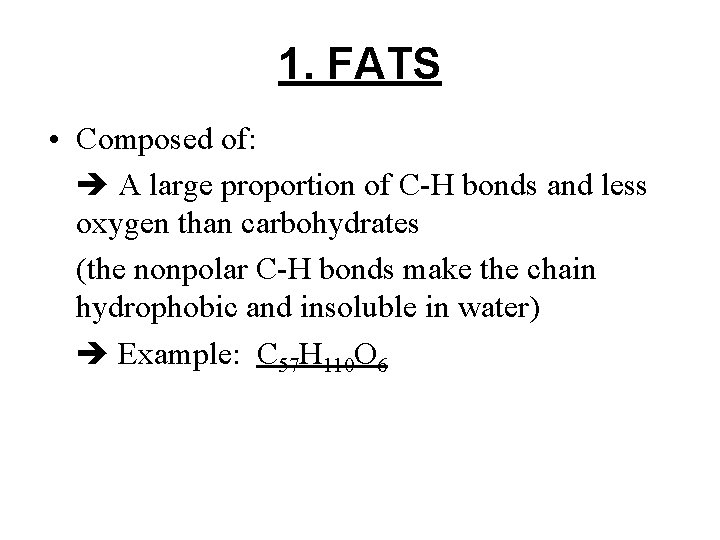
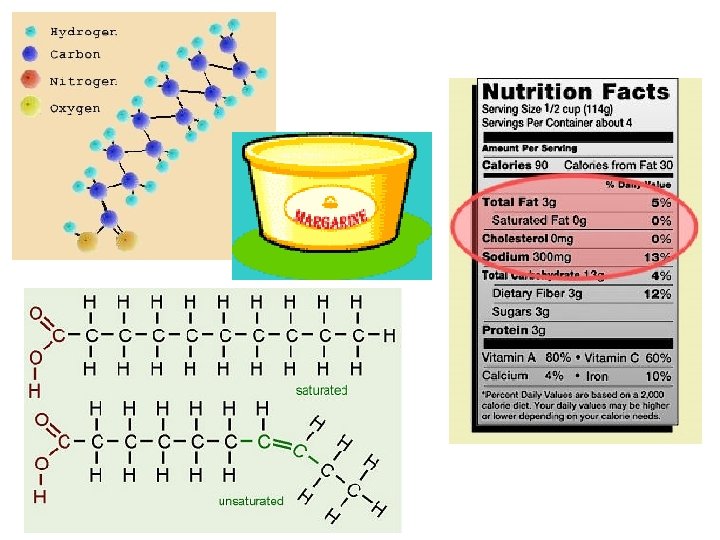
1. FATS • Composed of: A large proportion of C-H bonds and less oxygen than carbohydrates (the nonpolar C-H bonds make the chain hydrophobic and insoluble in water) Example: C 57 H 110 O 6

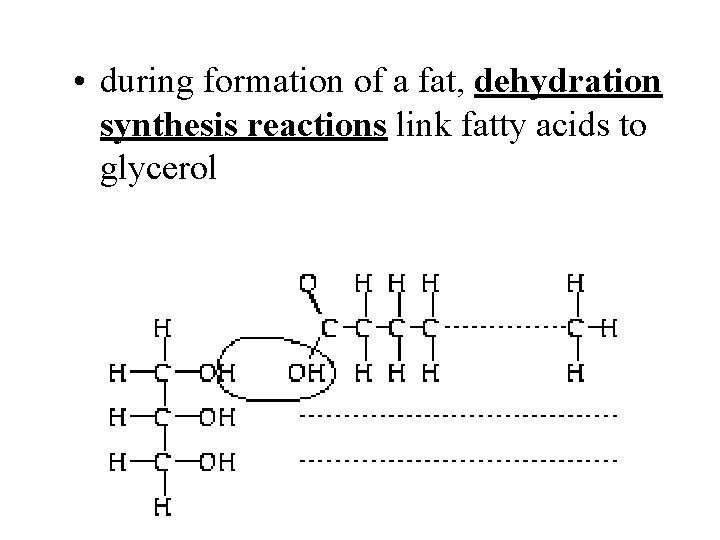
• during formation of a fat, dehydration synthesis reactions link fatty acids to glycerol

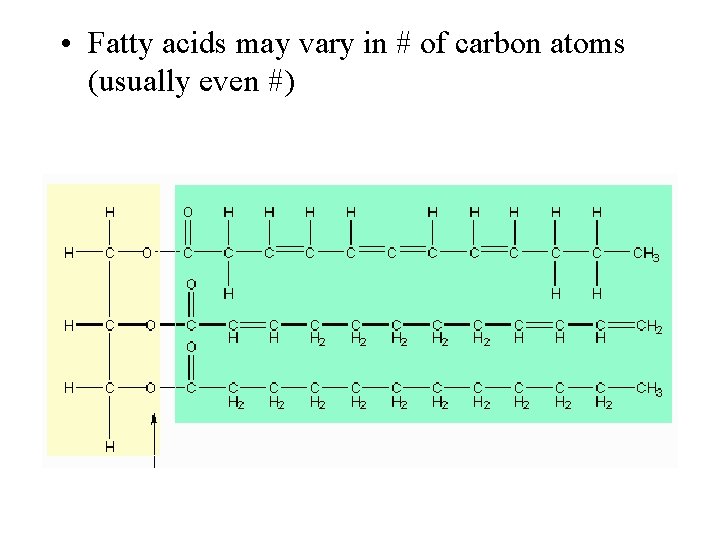
• Fatty acids may vary in # of carbon atoms (usually even #)

Saturated vs. Unsaturated Fats SATURATED FAT UNSATURATED FAT no C-C double bonds in fatty acid tail usually solid at room temp. most animal fats e. g. , bacon grease, lard, butter one or more C-C double bonds in fatty acid tail usually a liquid at room temp. most plant fats e. g. , corn, peanut, olive oils


Functions of Fats • energy storage (1 g of fat stores 2 x as much energy as 1 g of carbohydrate) • cushions vital organs in mammals (e. g. kidney) • insulates against heat loss (e. g. whales, seals)

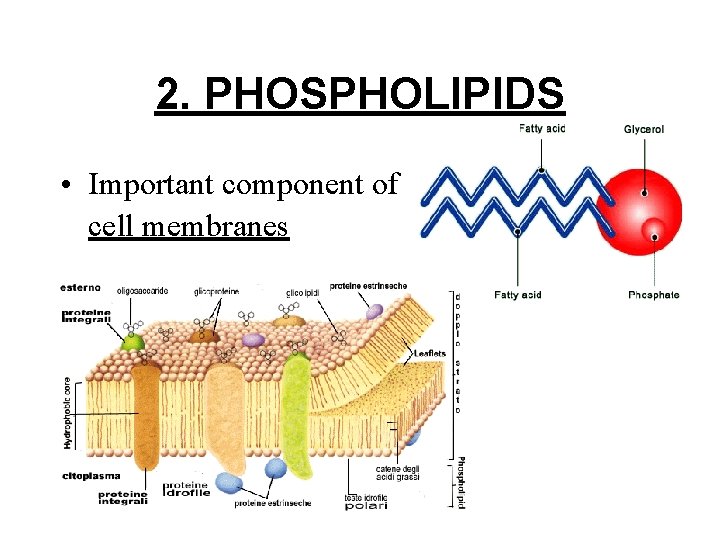
2. PHOSPHOLIPIDS • Important component of cell membranes

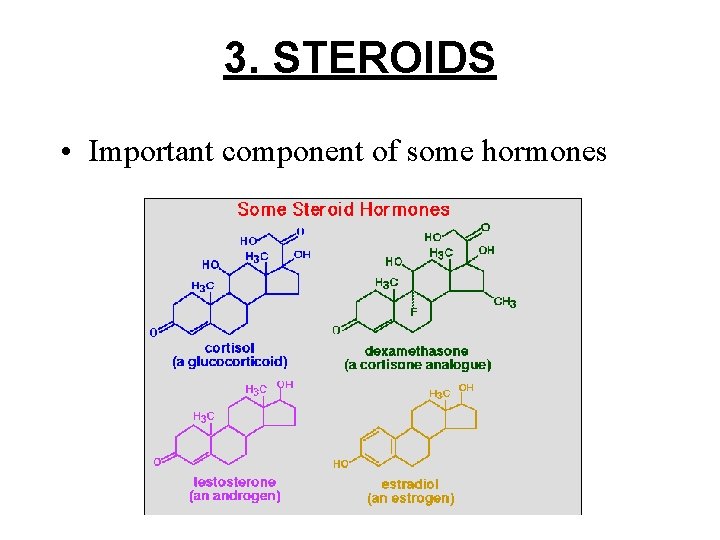
3. STEROIDS • Important component of some hormones

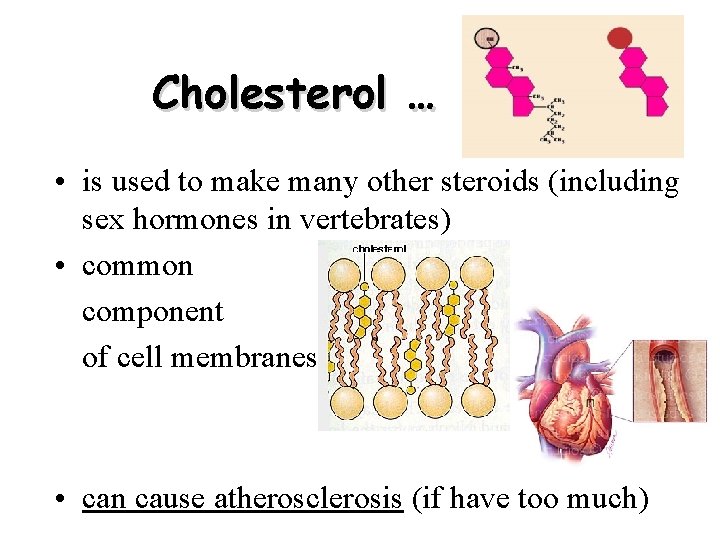
Cholesterol … • is used to make many other steroids (including sex hormones in vertebrates) • common component of cell membranes • can cause atherosclerosis (if have too much)