Chapter 2 The Chemistry of Life The Nature







- Slides: 24

Chapter 2 The Chemistry of Life

The Nature of Matter

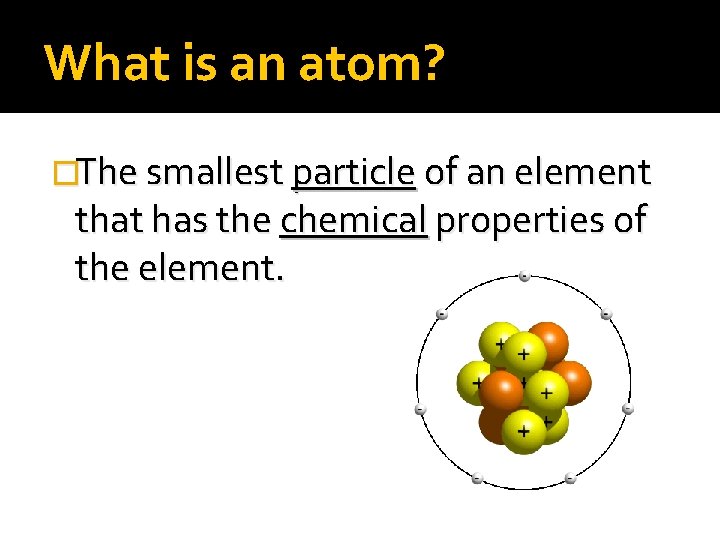
What is an atom? �The smallest particle of an element that has the chemical properties of the element.

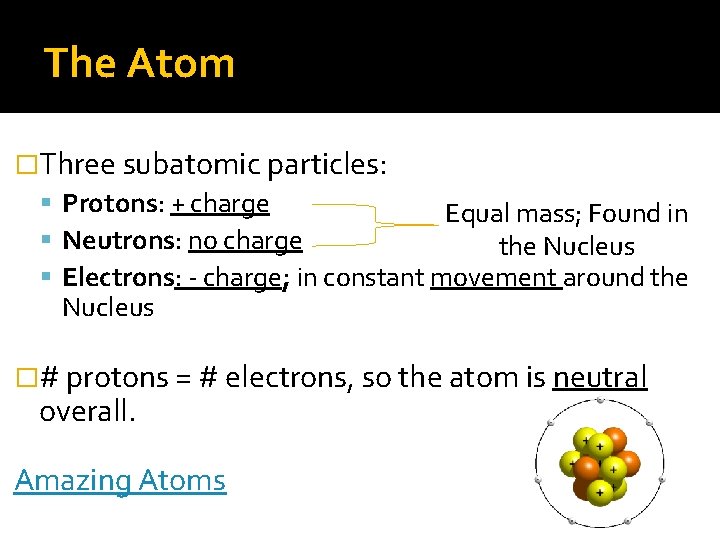
The Atom �Three subatomic particles: Protons: + charge Equal mass; Found in Neutrons: no charge the Nucleus Electrons: - charge; in constant movement around the Nucleus �# protons = # electrons, so the atom is neutral overall. Amazing Atoms

Elements �A pure substance that consists entirely of one type of atom. Listed on the periodic table. Ex: hydrogen (H)

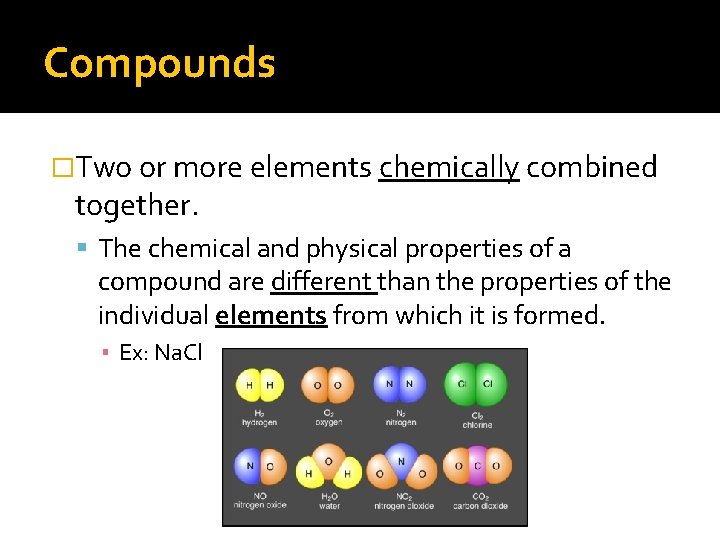
Compounds �Two or more elements chemically combined together. The chemical and physical properties of a compound are different than the properties of the individual elements from which it is formed. ▪ Ex: Na. Cl

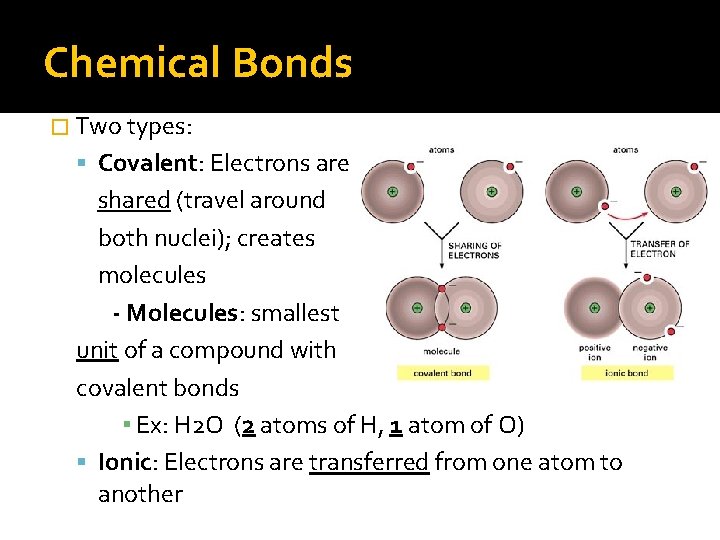
Chemical Bonds � Two types: Covalent: Electrons are shared (travel around both nuclei); creates molecules - Molecules: smallest unit of a compound with covalent bonds ▪ Ex: H 2 O (2 atoms of H, 1 atom of O) Ionic: Electrons are transferred from one atom to another

Properties of Water A Good Review

Mixture �Composed of two or more elements or compounds. �NOT chemically combined. �Solution: a mixture with components evenly distributed throughout �Two parts: Solute: the part that is dissolved Solvent: the part that is dissolving

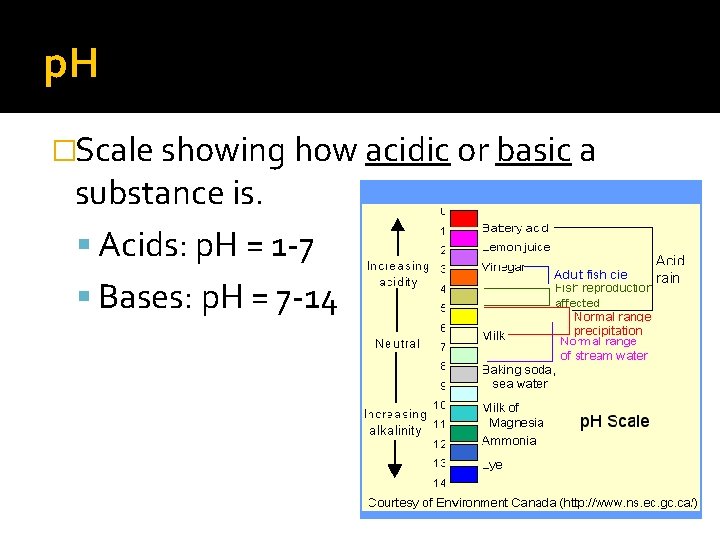
p. H �Scale showing how acidic or basic a substance is. Acids: p. H = 1 -7 Bases: p. H = 7 -14

Carbon Compounds

Why is carbon in all life? �Carbon has 4 electrons for bonding, so it can form strong covalent bonds with many other elements (like H, O, P, S, and N). �Carbon can form single, double and triple bonds with itself. �Organic = contains Carbon

Macromolecules = giant molecules �Monomers: smaller unit �Polymers: larger compound �Types of Macromolecules: carbohydrates, lipids, nucleic acids, proteins

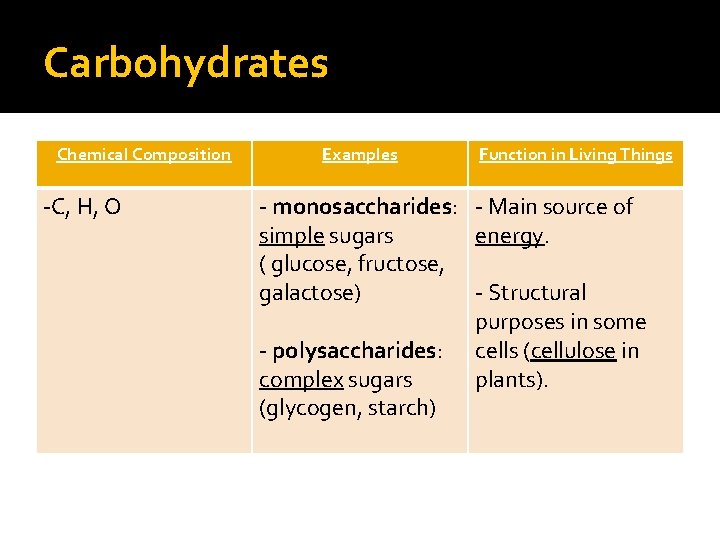
Carbohydrates Chemical Composition -C, H, O Examples Function in Living Things - monosaccharides: - Main source of simple sugars energy. ( glucose, fructose, galactose) - Structural purposes in some - polysaccharides: cells (cellulose in complex sugars plants). (glycogen, starch)

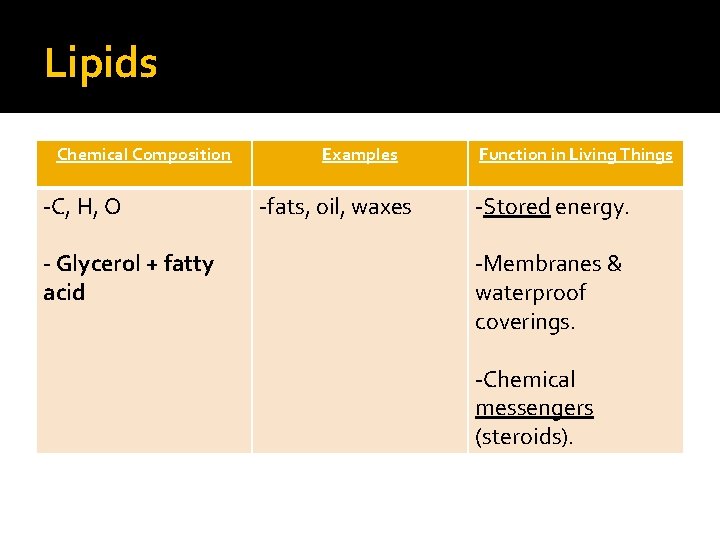
Lipids Chemical Composition -C, H, O - Glycerol + fatty acid Examples -fats, oil, waxes Function in Living Things -Stored energy. -Membranes & waterproof coverings. -Chemical messengers (steroids).

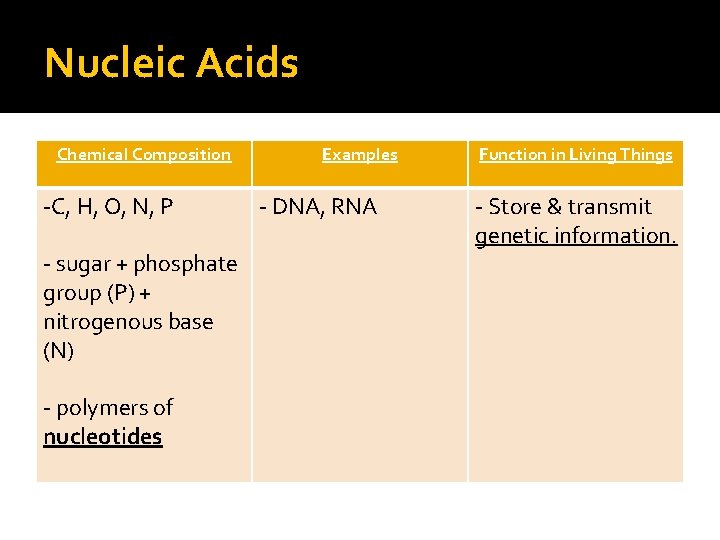
Nucleic Acids Chemical Composition -C, H, O, N, P - sugar + phosphate group (P) + nitrogenous base (N) - polymers of nucleotides Examples - DNA, RNA Function in Living Things - Store & transmit genetic information.

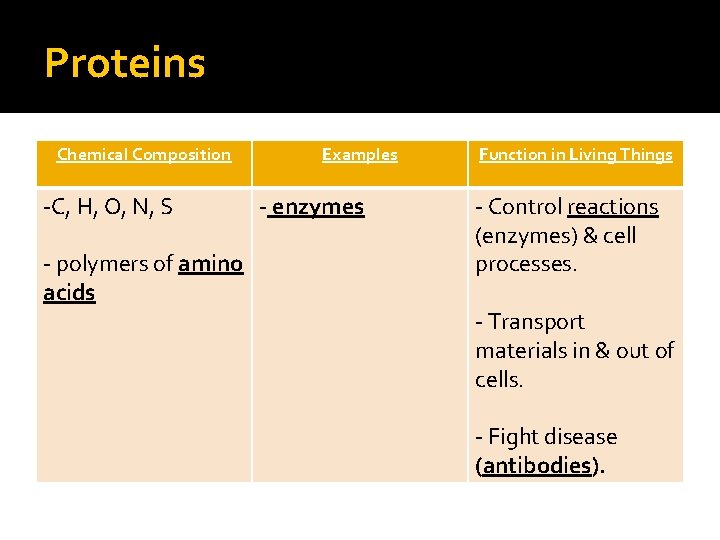
Proteins Chemical Composition -C, H, O, N, S - polymers of amino acids Examples - enzymes Function in Living Things - Control reactions (enzymes) & cell processes. - Transport materials in & out of cells. - Fight disease (antibodies).

Hunting the Elements �Video Clip: 58 min- 1: 18

Chemical Reactions & Enzymes

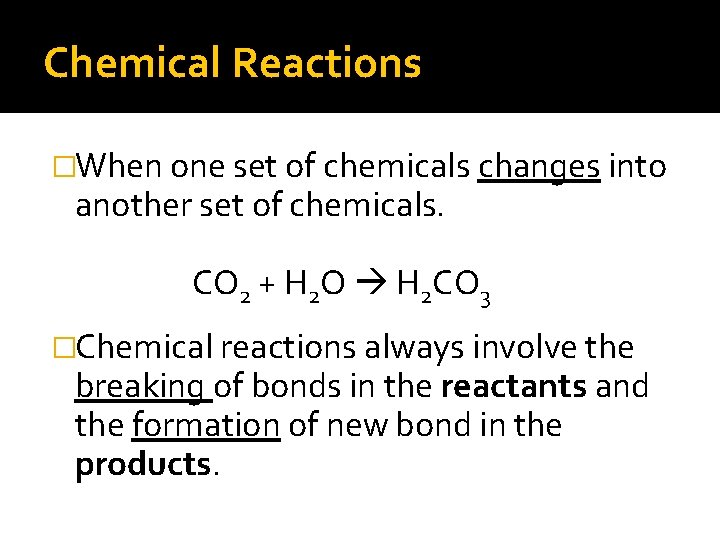
Chemical Reactions �When one set of chemicals changes into another set of chemicals. CO 2 + H 2 O H 2 CO 3 �Chemical reactions always involve the breaking of bonds in the reactants and the formation of new bond in the products.

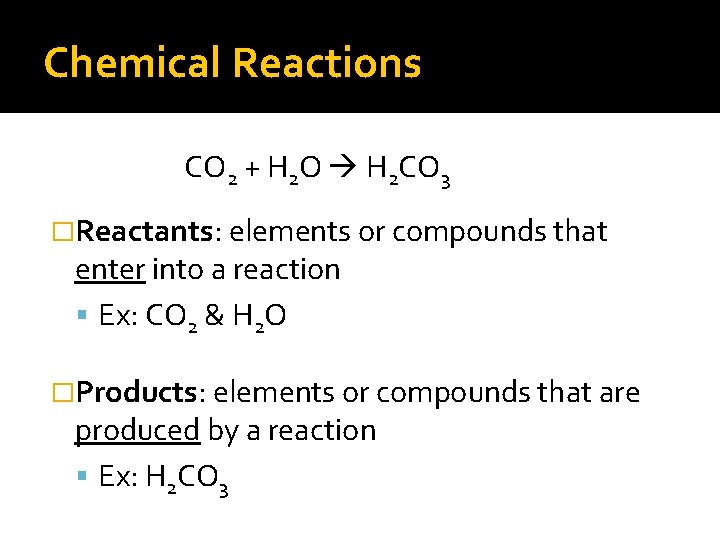
Chemical Reactions CO 2 + H 2 O H 2 CO 3 �Reactants: elements or compounds that enter into a reaction Ex: CO 2 & H 2 O �Products: elements or compounds that are produced by a reaction Ex: H 2 CO 3

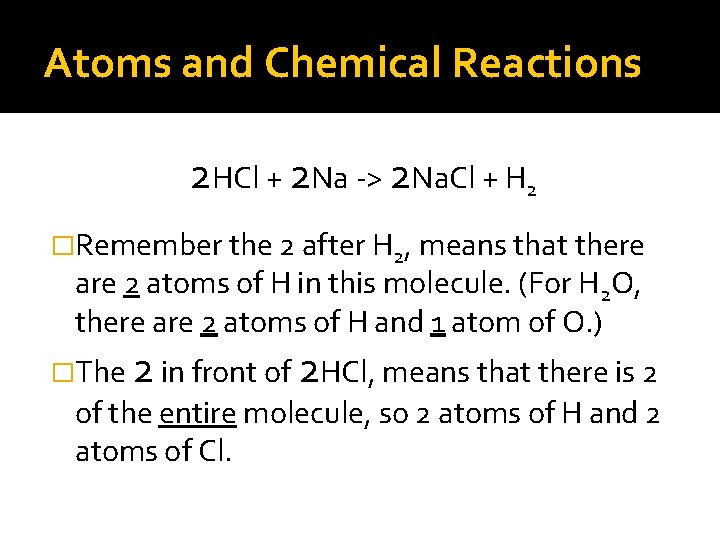
Atoms and Chemical Reactions 2 HCl + 2 Na -> 2 Na. Cl + H 2 �Remember the 2 after H 2, means that there are 2 atoms of H in this molecule. (For H 2 O, there are 2 atoms of H and 1 atom of O. ) �The 2 in front of 2 HCl, means that there is 2 of the entire molecule, so 2 atoms of H and 2 atoms of Cl.

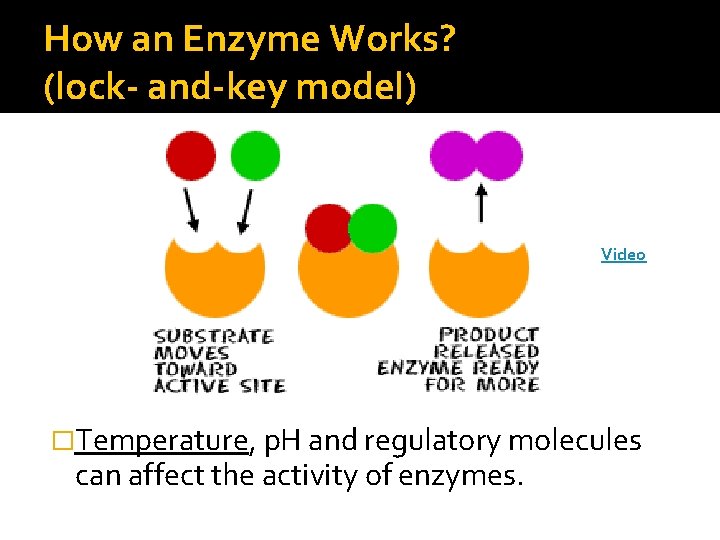
Enzymes �Enzymes: proteins that act as catalysts in cells. �Substrates: the reactants of an enzymecatalyzed reaction �Active site: spot on the enzyme where the substrate binds

How an Enzyme Works? (lock- and-key model) Video �Temperature, p. H and regulatory molecules can affect the activity of enzymes.