Chapter 2 The Chemistry of Life Chemistry Vocabulary

Chapter 2 The Chemistry of Life
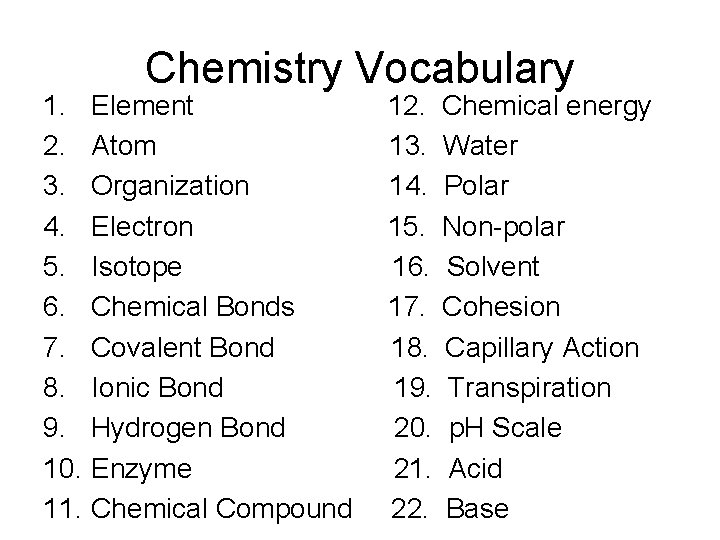
Chemistry Vocabulary 1. Element 12. Chemical energy 2. Atom 13. Water 3. Organization 14. Polar 4. Electron 15. Non-polar 5. Isotope 16. Solvent 6. Chemical Bonds 17. Cohesion 7. Covalent Bond 18. Capillary Action 8. Ionic Bond 19. Transpiration 9. Hydrogen Bond 20. p. H Scale 10. Enzyme 21. Acid 11. Chemical Compound 22. Base

Composition of an Atom • The subatomic particles that make up atoms are protons(+) neutrons, and electrons (-). Atoms molecules cells tissues organ systems
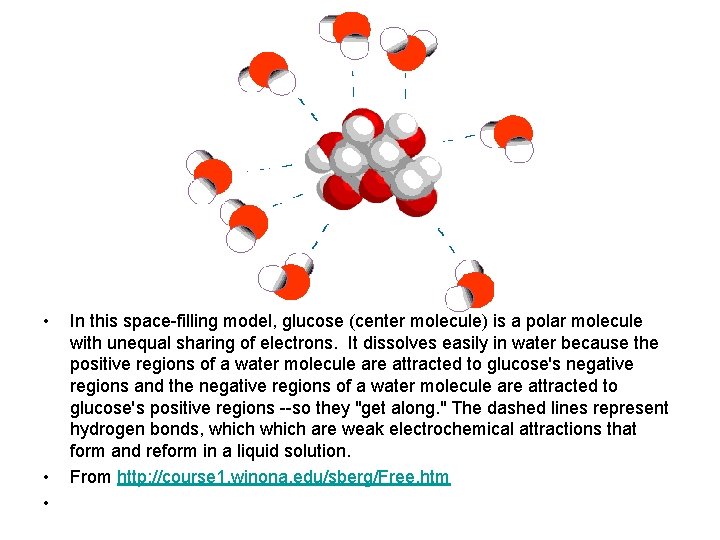
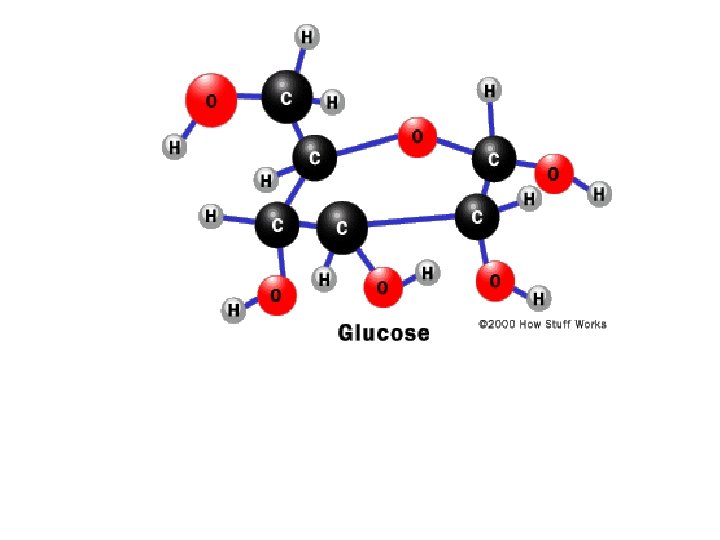
• • • In this space-filling model, glucose (center molecule) is a polar molecule with unequal sharing of electrons. It dissolves easily in water because the positive regions of a water molecule are attracted to glucose's negative regions and the negative regions of a water molecule are attracted to glucose's positive regions --so they "get along. " The dashed lines represent hydrogen bonds, which are weak electrochemical attractions that form and reform in a liquid solution. From http: //course 1. winona. edu/sberg/Free. htm
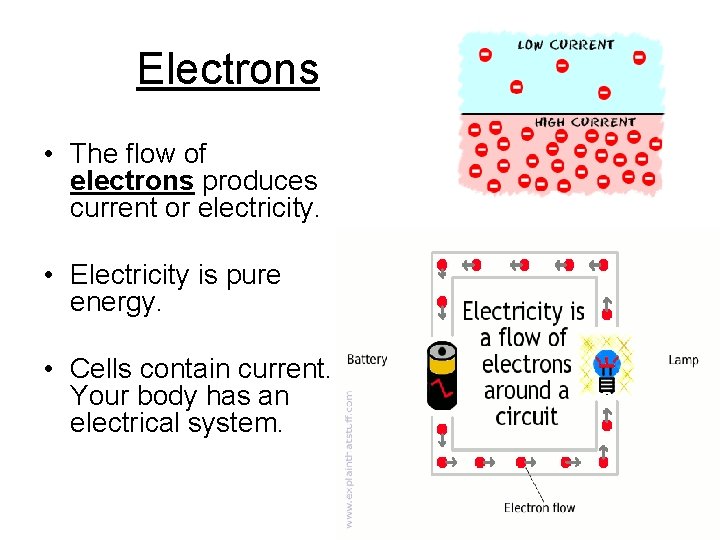
Electrons • The flow of electrons produces current or electricity. • Electricity is pure energy. • Cells contain current. Your body has an electrical system.
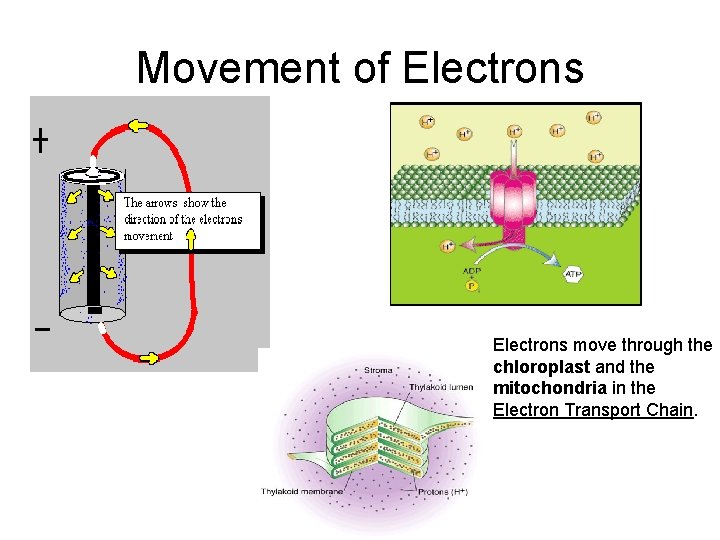
Movement of Electrons move through the chloroplast and the mitochondria in the Electron Transport Chain.
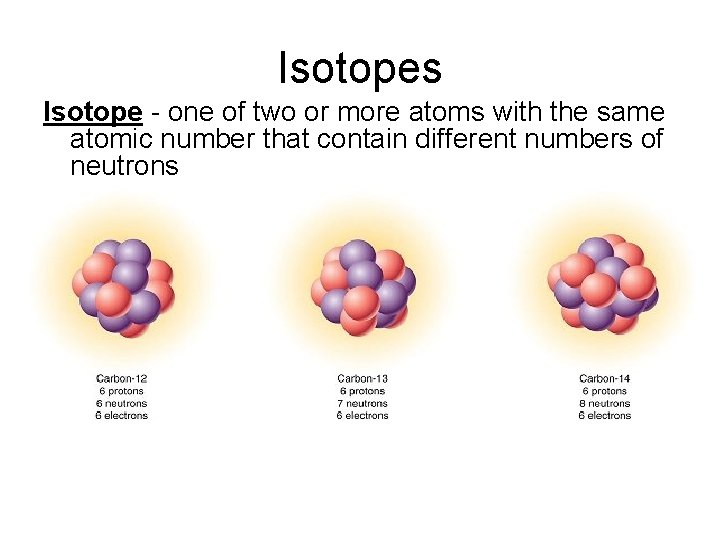
Isotopes Isotope - one of two or more atoms with the same atomic number that contain different numbers of neutrons
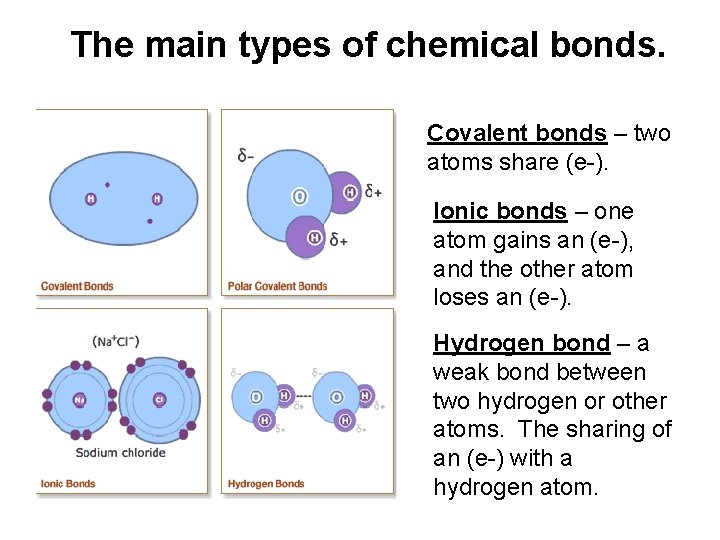
The main types of chemical bonds. Covalent bonds – two atoms share (e-). Ionic bonds – one atom gains an (e-), and the other atom loses an (e-). Hydrogen bond – a weak bond between two hydrogen or other atoms. The sharing of an (e-) with a hydrogen atom.
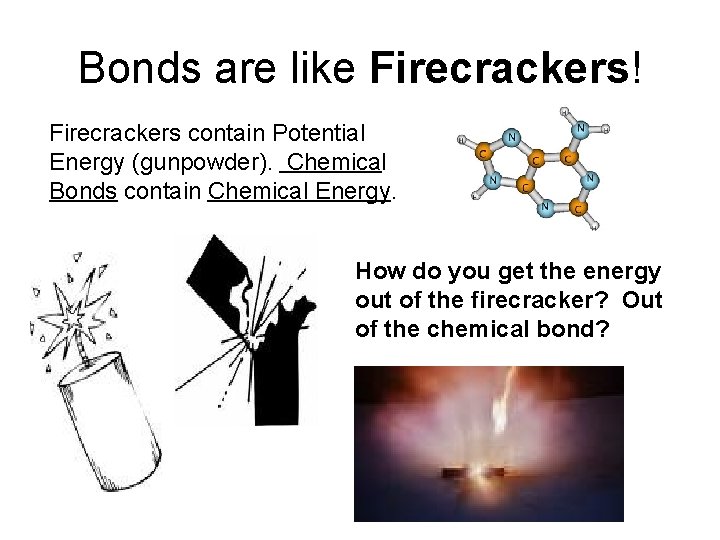
Bonds are like Firecrackers! Firecrackers contain Potential Energy (gunpowder). Chemical Bonds contain Chemical Energy. How do you get the energy out of the firecracker? Out of the chemical bond?
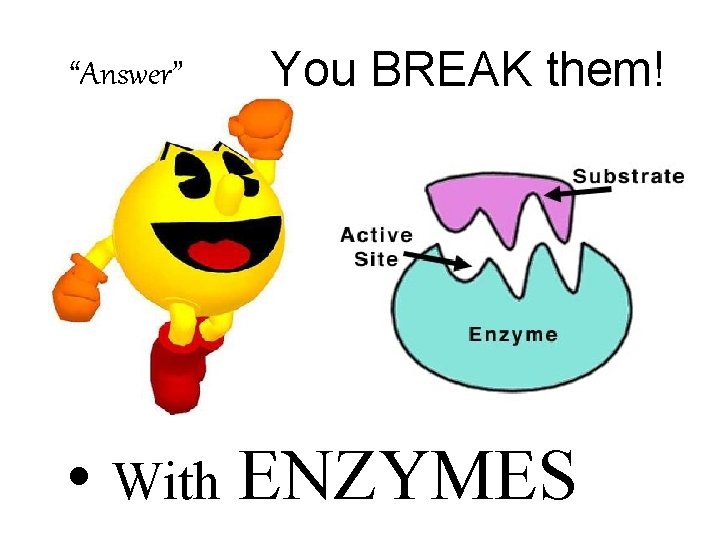
“Answer” You BREAK them! • With ENZYMES
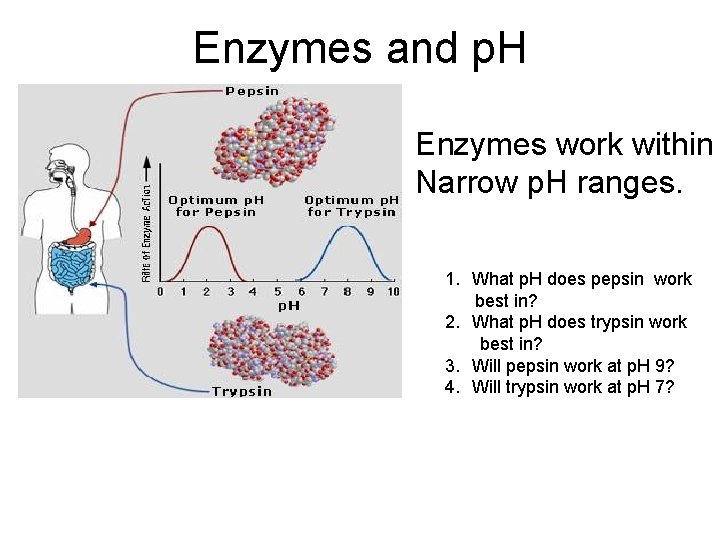
Enzymes and p. H Enzymes work within Narrow p. H ranges. 1. What p. H does pepsin work best in? 2. What p. H does trypsin work best in? 3. Will pepsin work at p. H 9? 4. Will trypsin work at p. H 7?
• A chemical compound is a substance formed by the chemical combination of two or more elements in definite proportions

• Chemical energy is the energy that’s stored in the bonds and atoms that make up molecules. • If different chemicals are allowed to react, these bonds can rearrange themselves. • Sometimes they need extra energy to do this, so they soak up some from their surroundings. These reactions are called ’endothermic’. Exothermic example – combustion • But sometimes they’ll release some extra energy into the environment, heating it up. These are called ’exothermic’ reactions. Endothermic example – Melting of ice

Where does all energy originally come from?
Why study about water? • All life occurs in water • Inside and outside the cell
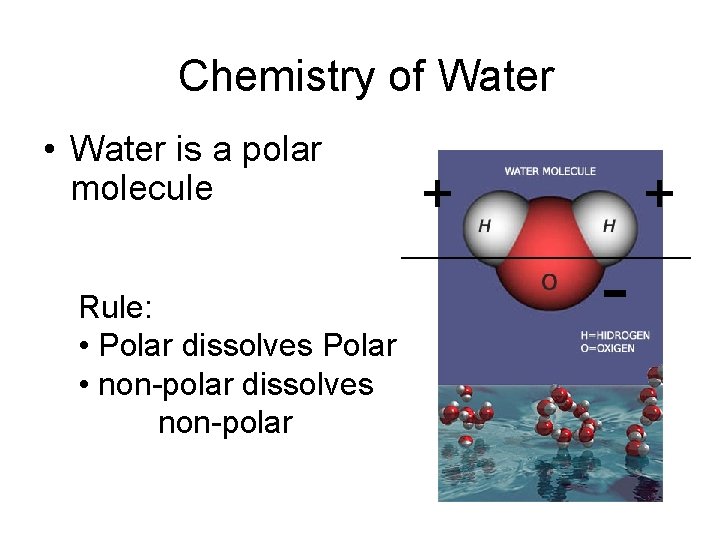
Chemistry of Water • Water is a polar molecule + + _______________ Rule: • Polar dissolves Polar • non-polar dissolves non-polar -
Water is the universal solvent of life • Polar – molecule that is asymmetrical charged (-) charge at one end and (+) charge at the other • Solute + Solvent Solution (substance being dissolved) (doing the dissolving) (Mixture of both) Sugar + Water sugar water Salt + water salt water Kool aid + water kool aid solution Grease (non-polar) + water
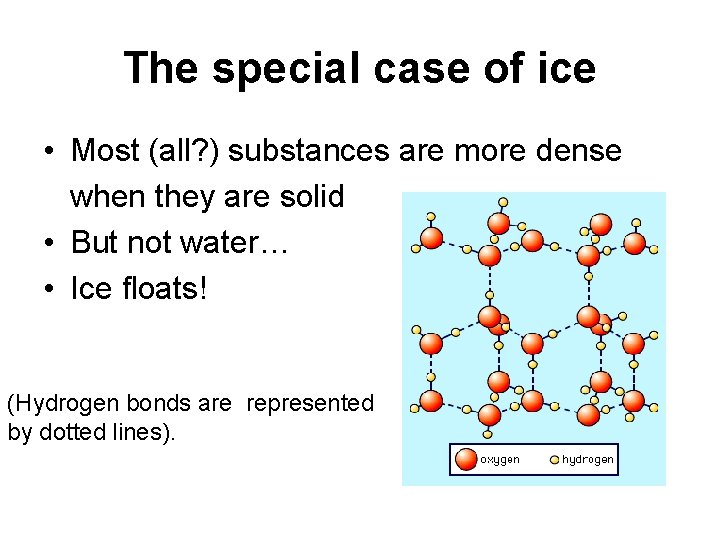
The special case of ice • Most (all? ) substances are more dense when they are solid • But not water… • Ice floats! (Hydrogen bonds are represented by dotted lines).

Why is “ice floats” important? • Oceans and lakes don’t freeze solid • Surface insulates water below allowing life to survive the winter • Seasonal turnover of lakes (cycling nutrients)
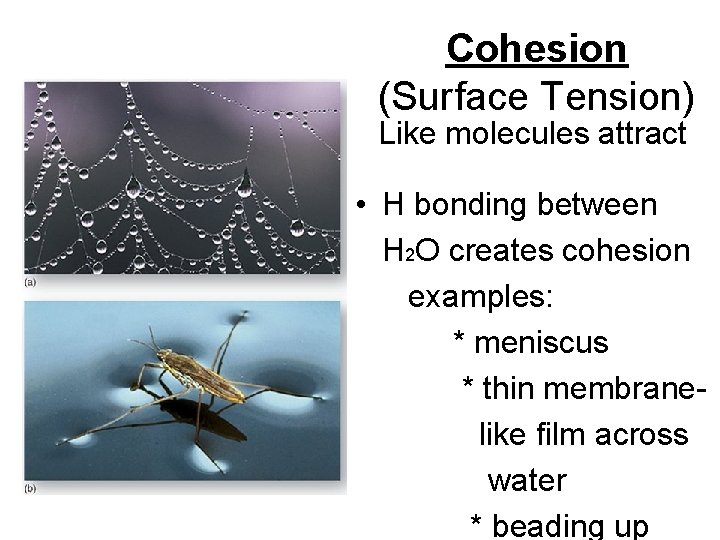
Cohesion (Surface Tension) Like molecules attract • H bonding between H 2 O creates cohesion examples: * meniscus * thin membrane like film across water * beading up

Adhesion- unlike molecules attract to each other. Example water and glass, water and pastic, water and paper towel.
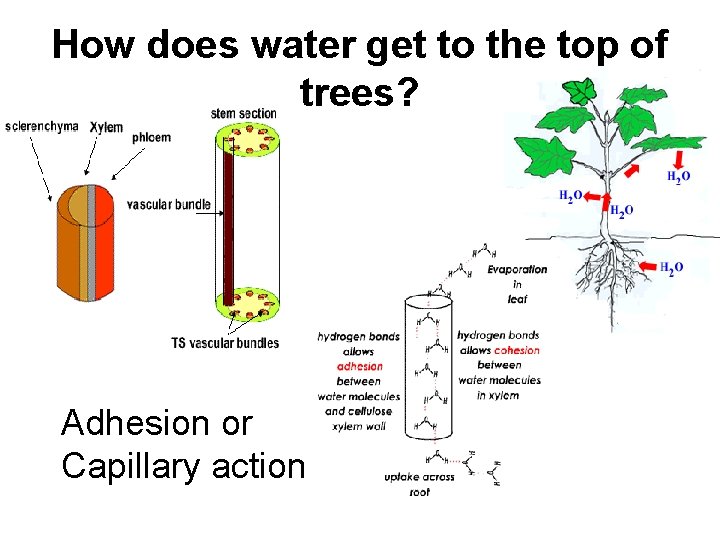
How does water get to the top of trees? Adhesion or Capillary action
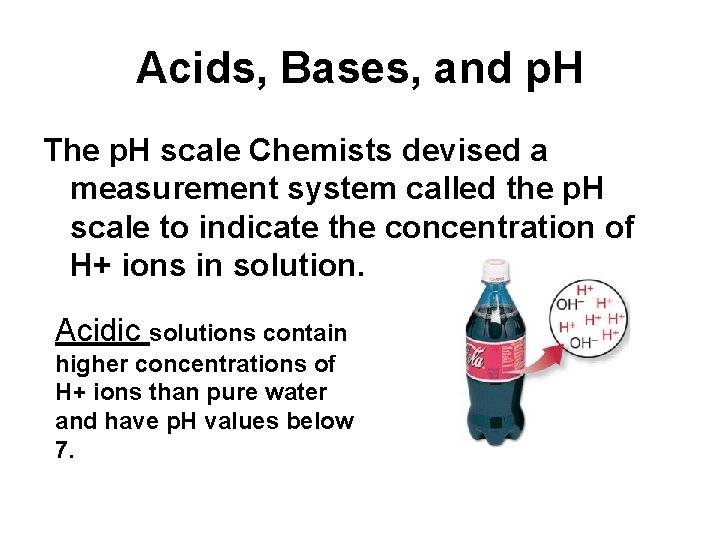
Acids, Bases, and p. H The p. H scale Chemists devised a measurement system called the p. H scale to indicate the concentration of H+ ions in solution. Acidic solutions contain higher concentrations of H+ ions than pure water and have p. H values below 7.
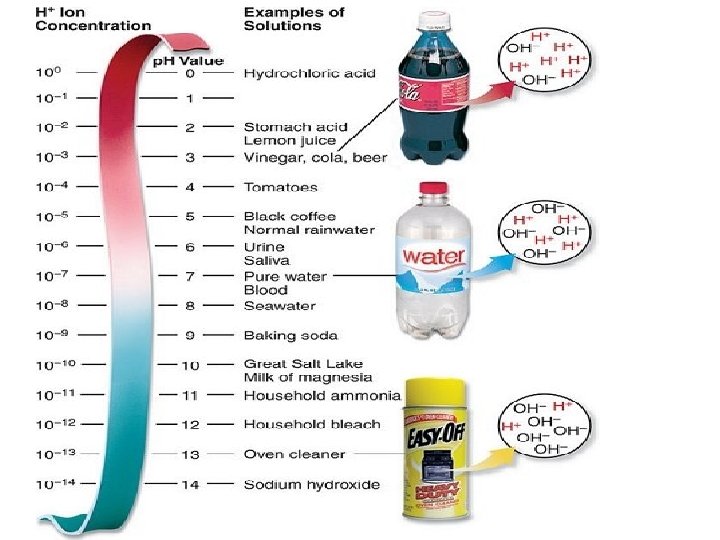

Acids -releases (H+) hydrogen ions when placed in water • • Have a p. H of 0 to 6. 9 Taste sour Feel oily Corrosive and poisonous

The effects of Acid Rain. • Acid rain damages the tissues of plants. • Acid rain leaches nutrients from soil. • Changes the p. H in pond, lakes, and rivers killing wildlife.

BASE • Bases is a compound that produces hydroxide ions (OH− ions) in solution. • Basic or alkaline, solutions contain lower concentrations of H+ ions than pure water and have p. H values above 7. • p. H values ranging from 7. 1 to 14.
Neutral • p. H 7 • Neither an acid or base • Salt • Contains equal Numbers of OH And H+ Acid + Base Salt + water
Buffers • Buffer: system of molecules that absorb or release H+ —maintaining a relatively stable p. H
Macromolecules (basic structure) • Macromolecule - giant molecules (many atoms, not just a few) • Monomers (one unit) – monomers make up polymers • Polymers are assembled by dehydration synthesis • Polymers are disassembled by hydrolysis
Carbohydrate • Saccharides (C 6 H 12 O 6)—sugar groups that make up many carbohydrates – Monosaccharides—just one saccharide group • Examples: glucose, fructose, galactose • Also called "simple sugars" or "single sugars” – Disaccharides—have two saccharide groups • Examples: sucrose, maltose, lactose • Also called "double sugars" – Polysaccharides—have many saccharide groups • Example: glycogen • Examples of function: fuel, fuel storage

- Slides: 34