Chapter 2 STRUCTURE AND PROPERTIES OF ORGANIC MOLECULES


- Slides: 15

Chapter 2 STRUCTURE AND PROPERTIES OF ORGANIC MOLECULES Helpful web site: http: //cw. prenhall. com/bookbind/pubbooks/wade/ Chapter 2: Structure and Properties of Organic Molecules

LINEAR COMBINATION OF ATOMIC ORBITALS (LCAO) Atomic orbitals can combine via addition or subtraction (hence, linear combination) and form: • MOLECULAR ORBITALS, if the starting atomic orbitals belong to different atoms • HYBRID ORBITALS, if the starting atomic orbitals belong to the same atom GENERAL RULE: The number of new orbitals (molecular or hybrid) is ALWAYS EQUAL to the number of starting orbitals Chapter 2: Structure and Properties of Organic Molecules

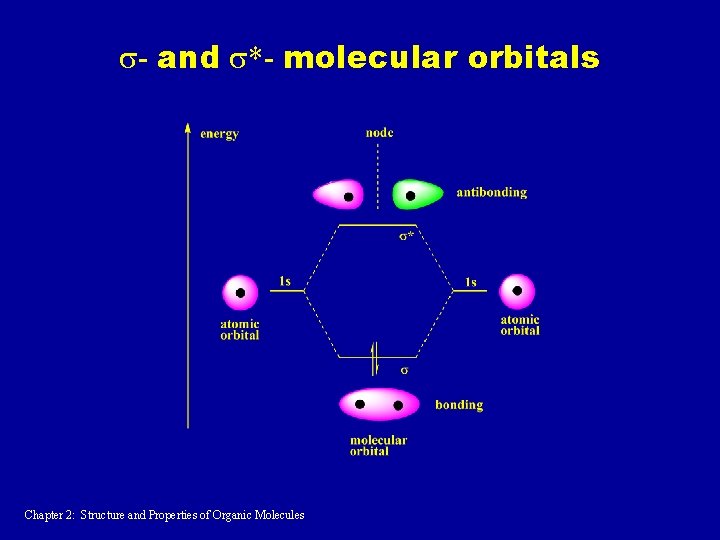
s- and s*- molecular orbitals Chapter 2: Structure and Properties of Organic Molecules

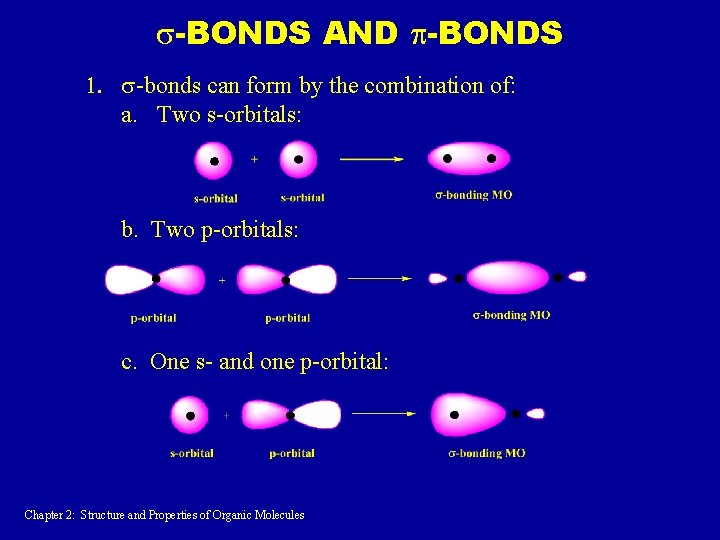
s-BONDS AND p-BONDS 1. s-bonds can form by the combination of: a. Two s-orbitals: b. Two p-orbitals: c. One s- and one p-orbital: Chapter 2: Structure and Properties of Organic Molecules

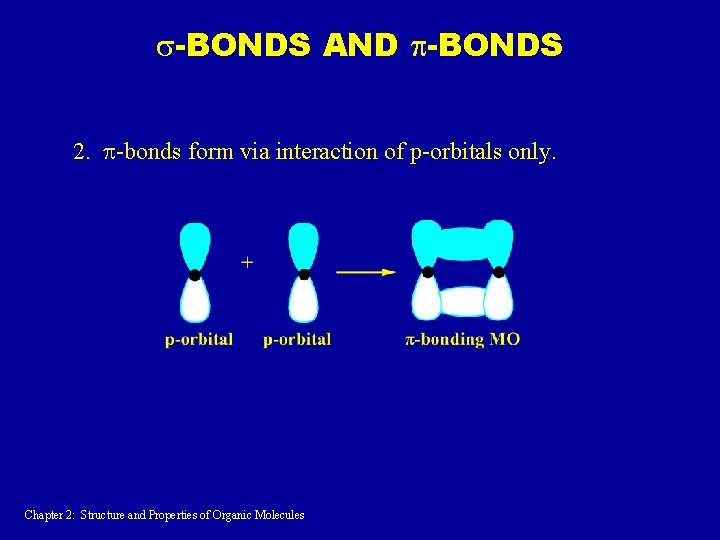
s-BONDS AND p-BONDS 2. p-bonds form via interaction of p-orbitals only. Chapter 2: Structure and Properties of Organic Molecules

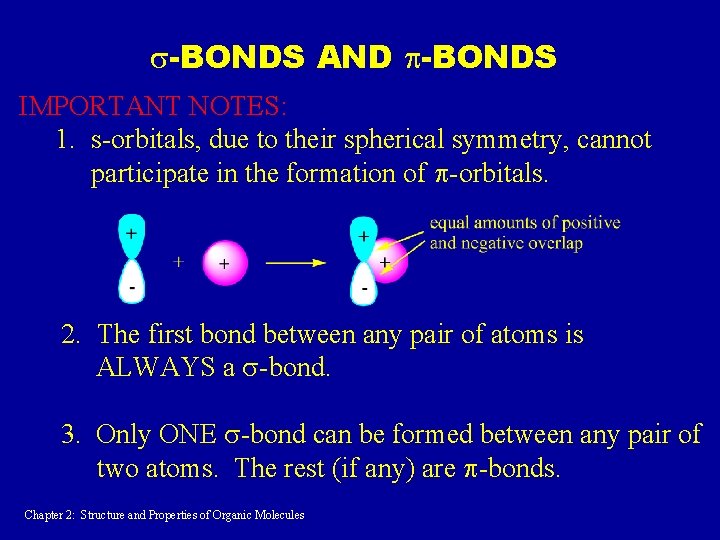
s-BONDS AND p-BONDS IMPORTANT NOTES: 1. s-orbitals, due to their spherical symmetry, cannot participate in the formation of p-orbitals. 2. The first bond between any pair of atoms is ALWAYS a s-bond. 3. Only ONE s-bond can be formed between any pair of two atoms. The rest (if any) are p-bonds. Chapter 2: Structure and Properties of Organic Molecules

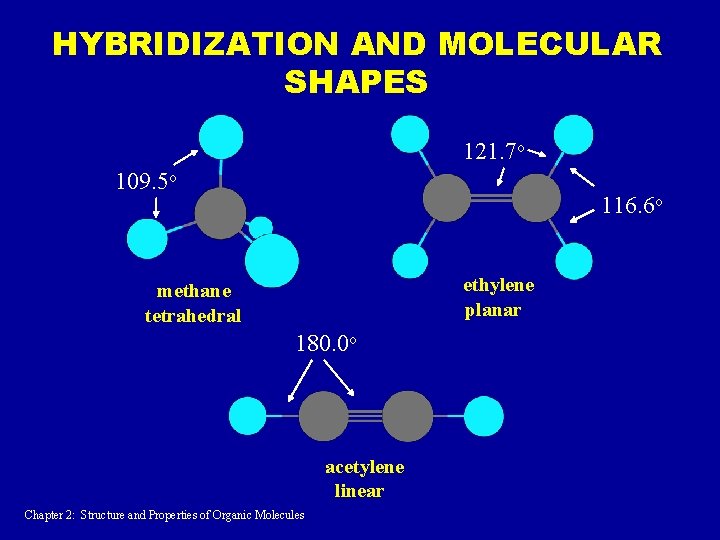
PREDICTIONS BASED ON ELECTRONIC STRUCTURE OF CARBON 1. There is one 2 s and three 2 p orbitals, to form four bonds total. It would be therefore expected that one of the bonds would be different from the other three. 2. The 2 s orbital is spherical (non-directional). The 2 p orbitals are perpendicular to each other. One would therefore expect that the newly formed bonds would be at a 90 o angle. BOTH PREDICTIONS ARE FALSE!!!!! Chapter 2: Structure and Properties of Organic Molecules

HYBRIDIZATION AND MOLECULAR SHAPES 121. 7 o 109. 5 o 116. 6 o ethylene planar methane tetrahedral 180. 0 o acetylene linear Chapter 2: Structure and Properties of Organic Molecules

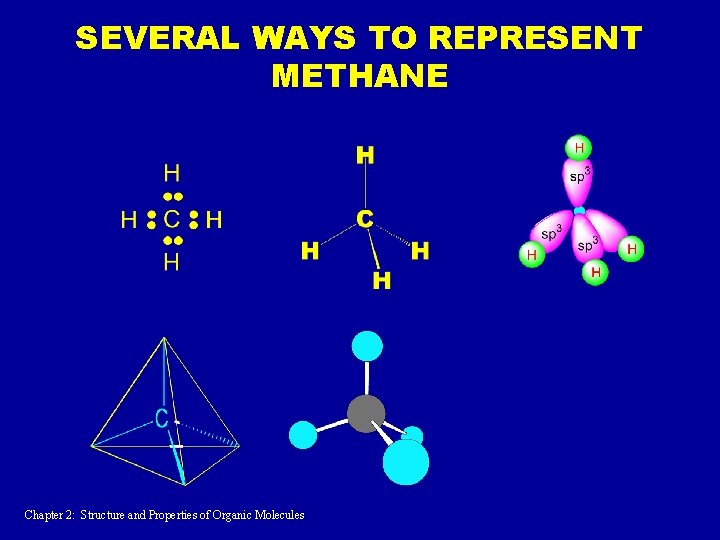
SEVERAL WAYS TO REPRESENT METHANE Chapter 2: Structure and Properties of Organic Molecules

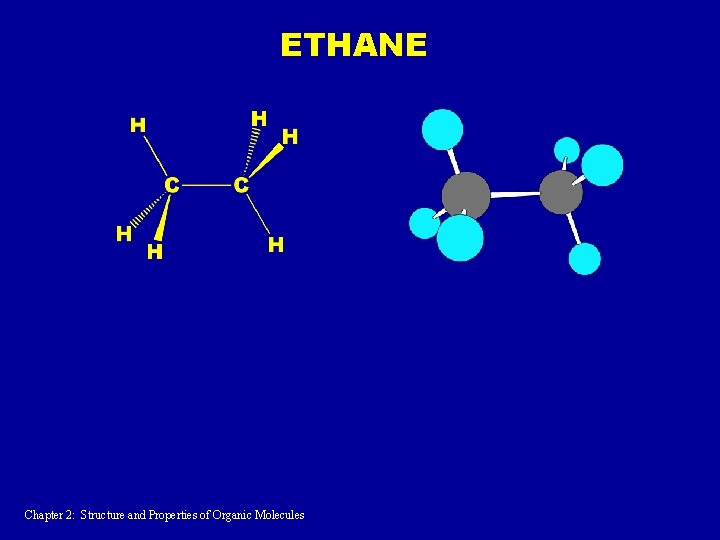
ETHANE Chapter 2: Structure and Properties of Organic Molecules

ISOMERS 1. Constitutional (structural) isomers. Same molecular formula, but different structures. 2. Stereoisomers. Same molecular formula, same structure, different orientation of atoms or groups in space. Chapter 2: Structure and Properties of Organic Molecules

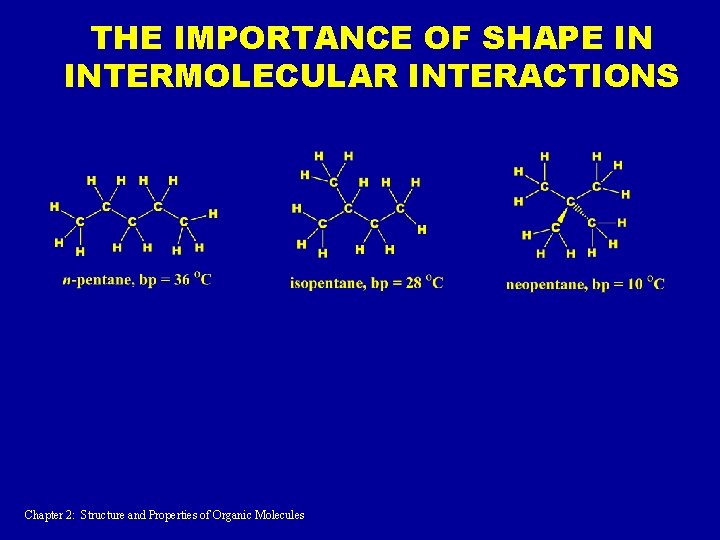
THE IMPORTANCE OF SHAPE IN INTERMOLECULAR INTERACTIONS Chapter 2: Structure and Properties of Organic Molecules

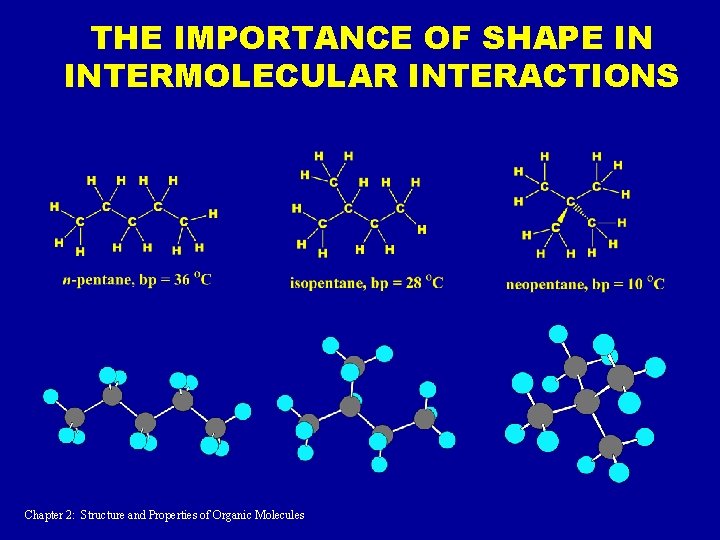
THE IMPORTANCE OF SHAPE IN INTERMOLECULAR INTERACTIONS Chapter 2: Structure and Properties of Organic Molecules

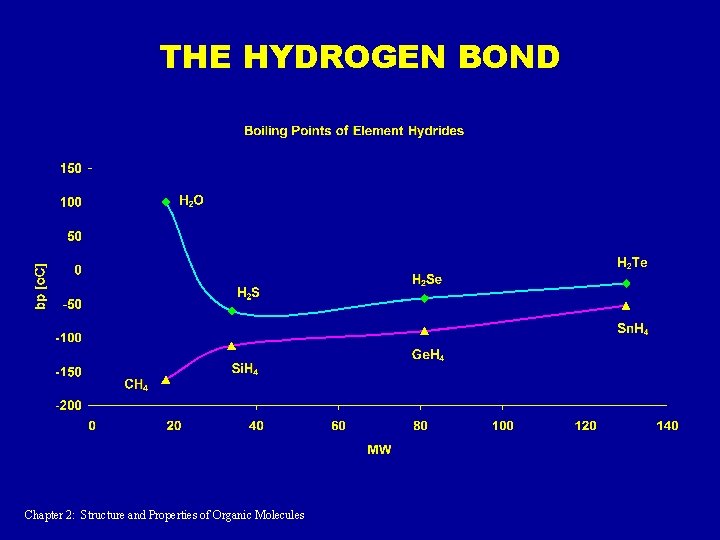
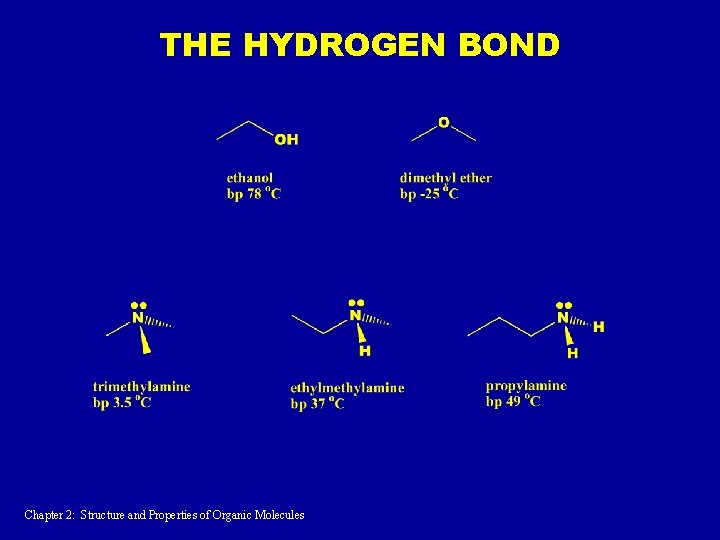
THE HYDROGEN BOND Chapter 2: Structure and Properties of Organic Molecules

THE HYDROGEN BOND Chapter 2: Structure and Properties of Organic Molecules