Chapter 2 Structure and Properties of Organic Molecules



- Slides: 17

Chapter 2: Structure and Properties of Organic Molecules n Previously: ¨ Atomic/electronic structure ¨ Lewis structures ¨ Bonding n Now: ¨ How do atoms form covalent bonds? ¨ Which orbitals are involved? ¨ What are the shapes of organic molecules? ¨ How do bonding and shape affect properties?

n Which electrons are involved in bonding? Valence electrons n Where are valence electrons? In atomic orbitals n Bonds are formed by the combination of atomic orbitals n Linear combination of atomic orbitals (LCAO)

Linear Combination of Atomic Orbitals Two theories n Molecular Orbital Theory n ¨ Atomic orbitals of two atoms interact ¨ Bonding and antibonding MO’s formed ¨ Skip this stuff n Valence Bond Theory (Hybridization) ¨ Atomic orbitals of the same atom interact ¨ Hybrid orbitals formed ¨ Bonds formed between hybrid orbitals

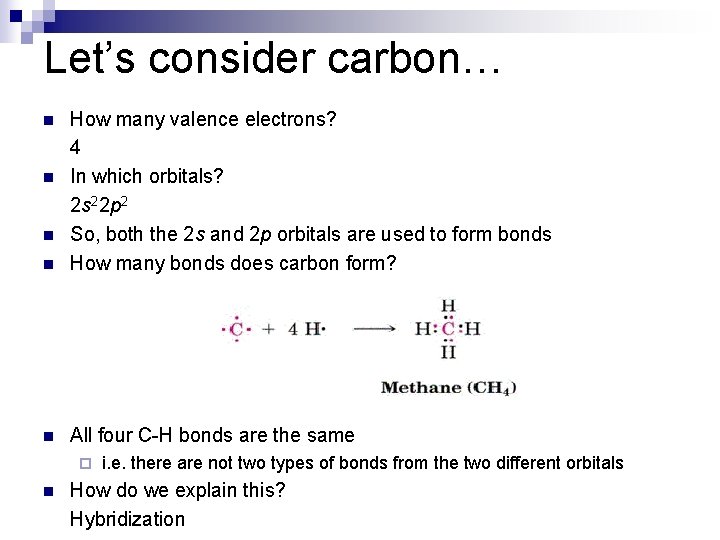
Let’s consider carbon… n How many valence electrons? 4 In which orbitals? 2 s 22 p 2 So, both the 2 s and 2 p orbitals are used to form bonds How many bonds does carbon form? n All four C-H bonds are the same n n n ¨ n i. e. there are not two types of bonds from the two different orbitals How do we explain this? Hybridization

Hybridization n The s and p orbitals of the C atom combine with each other to form hybrid orbitals before they combine with orbitals of another atom to form a covalent bond

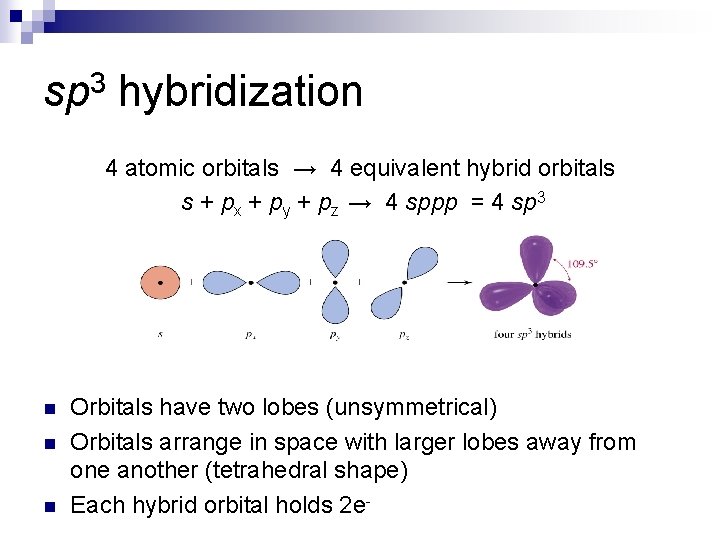
sp 3 hybridization 4 atomic orbitals → 4 equivalent hybrid orbitals s + px + py + pz → 4 sppp = 4 sp 3 n n n Orbitals have two lobes (unsymmetrical) Orbitals arrange in space with larger lobes away from one another (tetrahedral shape) Each hybrid orbital holds 2 e-

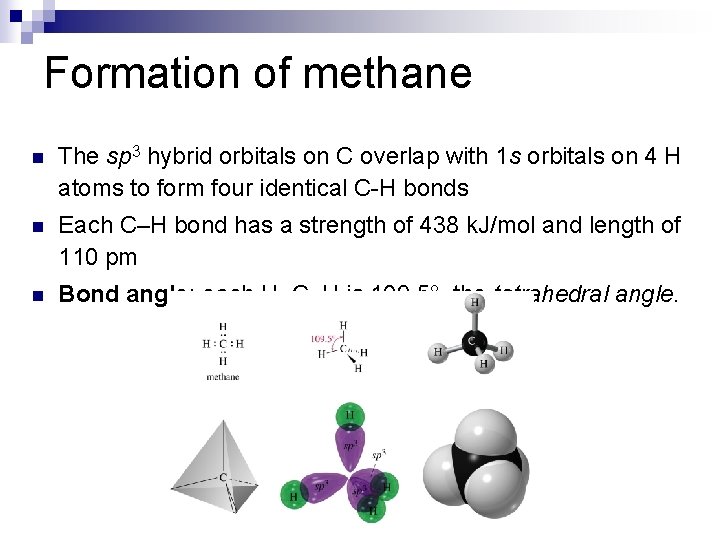
Formation of methane n The sp 3 hybrid orbitals on C overlap with 1 s orbitals on 4 H atoms to form four identical C-H bonds n Each C–H bond has a strength of 438 k. J/mol and length of 110 pm n Bond angle: each H–C–H is 109. 5°, the tetrahedral angle.

Motivation for hybridization? Better orbital overlap with larger lobe of sp 3 hybrid orbital then with unhybridized p orbital n Stronger bond n Electron pairs farther apart in hybrid orbitals n Lower energy n

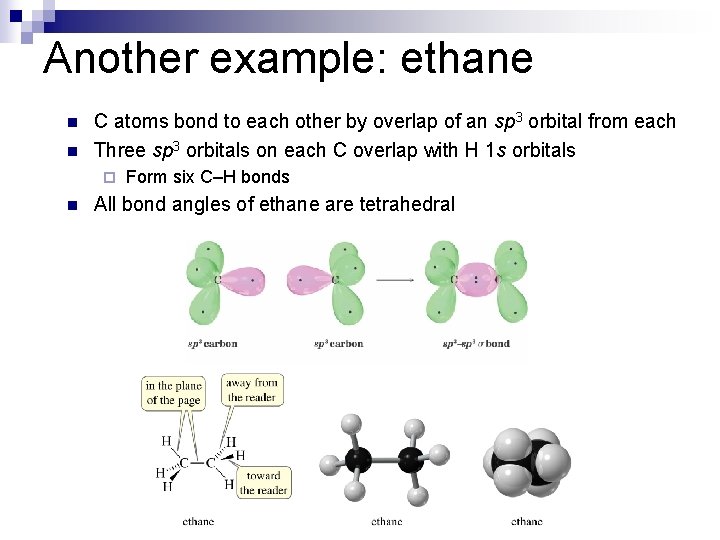
Another example: ethane n n C atoms bond to each other by overlap of an sp 3 orbital from each Three sp 3 orbitals on each C overlap with H 1 s orbitals ¨ n Form six C–H bonds All bond angles of ethane are tetrahedral

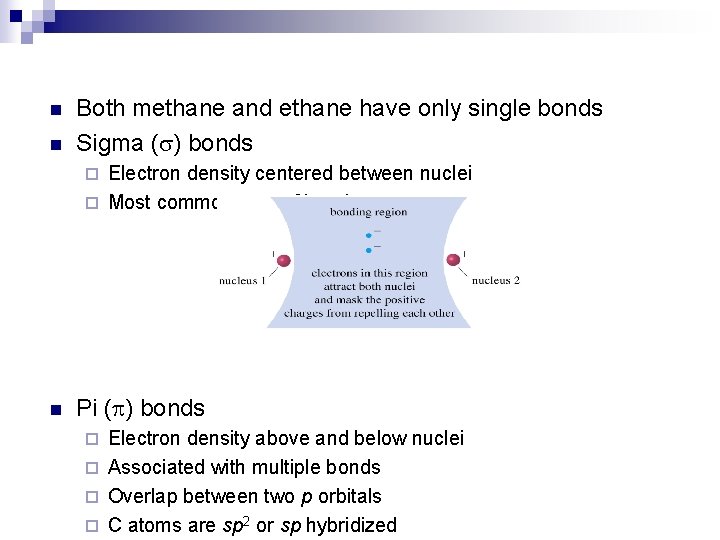
n n Both methane and ethane have only single bonds Sigma (s) bonds Electron density centered between nuclei ¨ Most common type of bond ¨ n Pi ( ) bonds Electron density above and below nuclei ¨ Associated with multiple bonds ¨ Overlap between two p orbitals ¨ C atoms are sp 2 or sp hybridized ¨

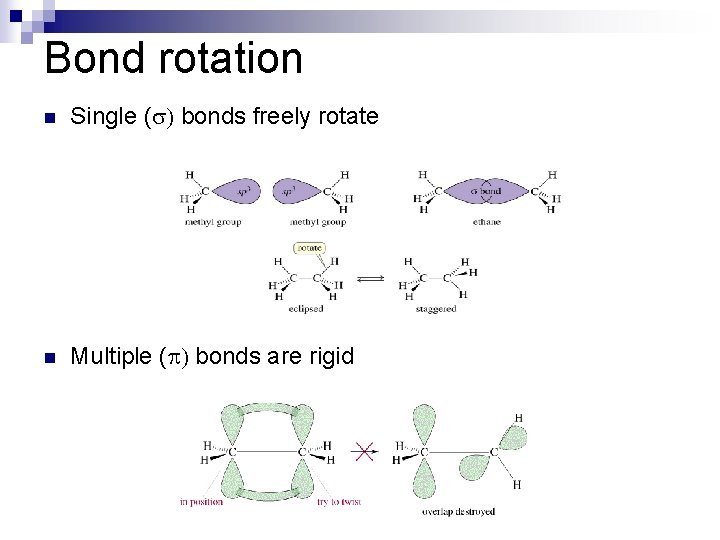
Bond rotation n Single (s) bonds freely rotate n Multiple ( ) bonds are rigid

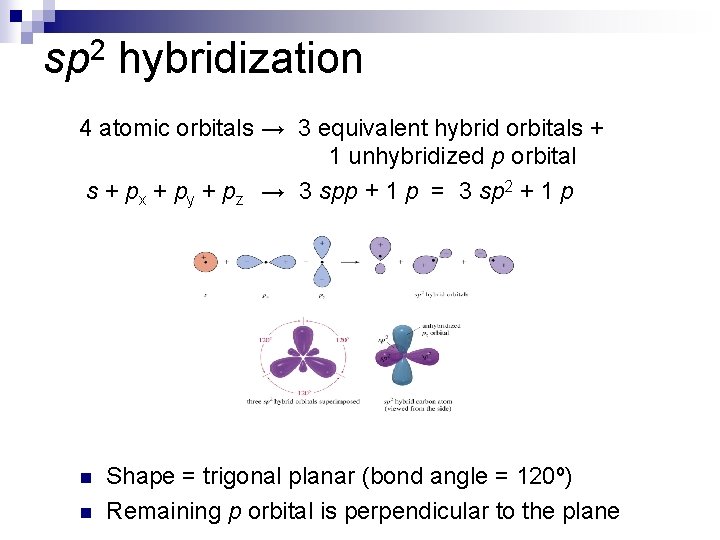
sp 2 hybridization 4 atomic orbitals → 3 equivalent hybrid orbitals + 1 unhybridized p orbital s + px + py + pz → 3 spp + 1 p = 3 sp 2 + 1 p n n Shape = trigonal planar (bond angle = 120º) Remaining p orbital is perpendicular to the plane

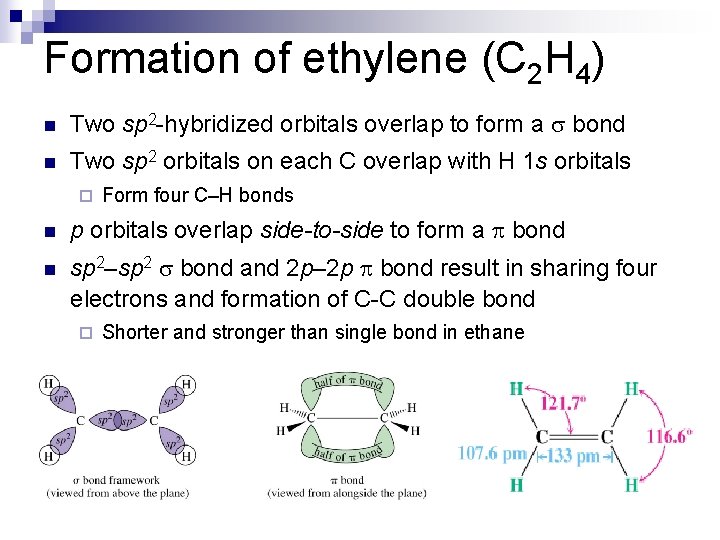
Formation of ethylene (C 2 H 4) n Two sp 2 -hybridized orbitals overlap to form a s bond n Two sp 2 orbitals on each C overlap with H 1 s orbitals ¨ Form four C–H bonds n p orbitals overlap side-to-side to form a bond n sp 2–sp 2 s bond and 2 p– 2 p bond result in sharing four electrons and formation of C-C double bond ¨ Shorter and stronger than single bond in ethane

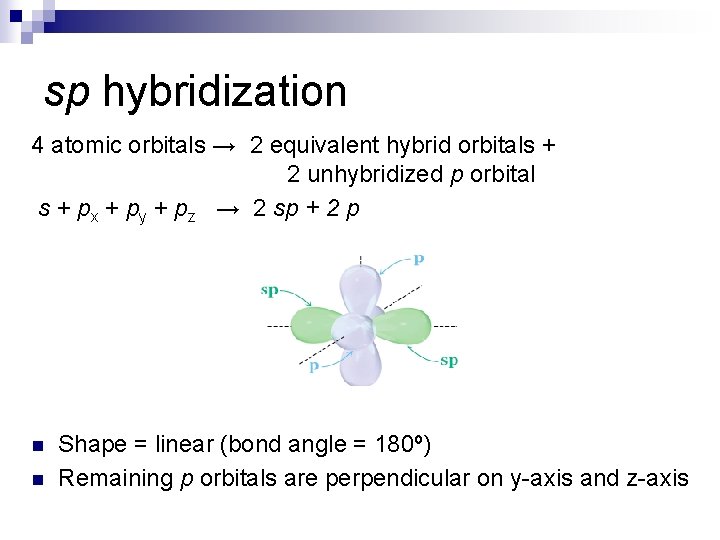
sp hybridization 4 atomic orbitals → 2 equivalent hybrid orbitals + 2 unhybridized p orbital s + px + py + pz → 2 sp + 2 p n n Shape = linear (bond angle = 180º) Remaining p orbitals are perpendicular on y-axis and z-axis

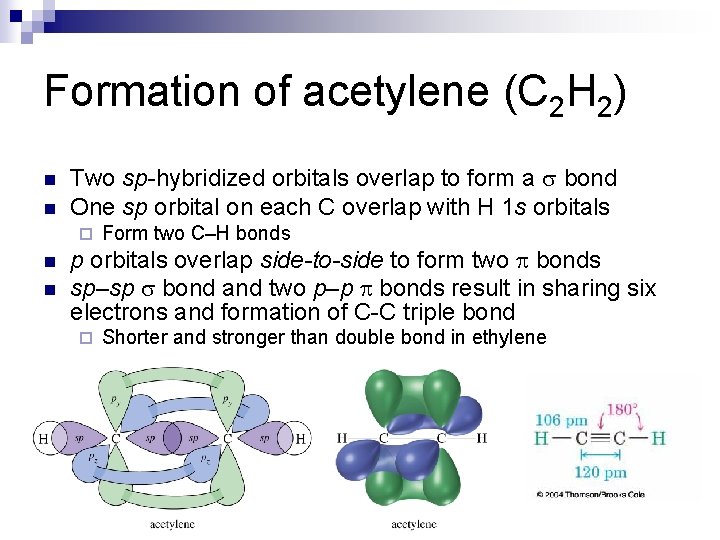
Formation of acetylene (C 2 H 2) n n Two sp-hybridized orbitals overlap to form a s bond One sp orbital on each C overlap with H 1 s orbitals ¨ n n Form two C–H bonds p orbitals overlap side-to-side to form two bonds sp–sp s bond and two p–p bonds result in sharing six electrons and formation of C-C triple bond ¨ Shorter and stronger than double bond in ethylene

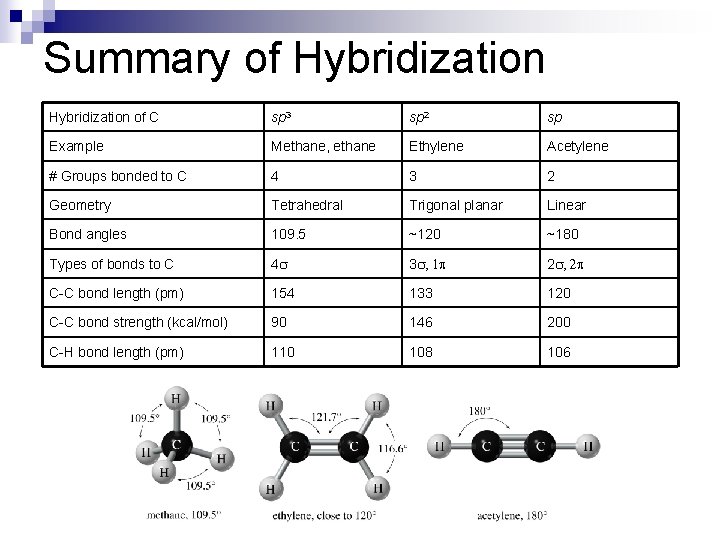
Summary of Hybridization of C sp 3 sp 2 sp Example Methane, ethane Ethylene Acetylene # Groups bonded to C 4 3 2 Geometry Tetrahedral Trigonal planar Linear Bond angles 109. 5 ~120 ~180 Types of bonds to C 4 s 3 s, 1 2 s, 2 C-C bond length (pm) 154 133 120 C-C bond strength (kcal/mol) 90 146 200 C-H bond length (pm) 110 108 106

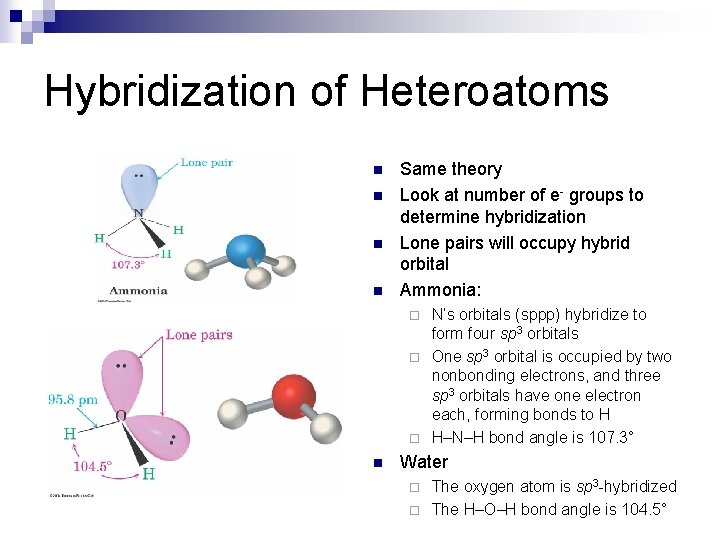
Hybridization of Heteroatoms n n Same theory Look at number of e- groups to determine hybridization Lone pairs will occupy hybrid orbital Ammonia: N’s orbitals (sppp) hybridize to form four sp 3 orbitals ¨ One sp 3 orbital is occupied by two nonbonding electrons, and three sp 3 orbitals have one electron each, forming bonds to H ¨ H–N–H bond angle is 107. 3° ¨ n Water The oxygen atom is sp 3 -hybridized ¨ The H–O–H bond angle is 104. 5° ¨