Chapter 2 Section 3 Using Scientific Measurements Objectives

Chapter 2 Section 3 Using Scientific Measurements Objectives- after viewing this lesson you should be able to do the following: • Distinguish between accuracy and precision. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 3 Using Scientific Measurements Accuracy and Precision • Accuracy refers to the closeness of measurements to the correct/true or accepted value of the quantity measured. • Comparison of taken measurement to the true measurement • Precision refers to the closeness of a set of measurements of the same quantity made in the same way. • Comparison of taken measurements to each other Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
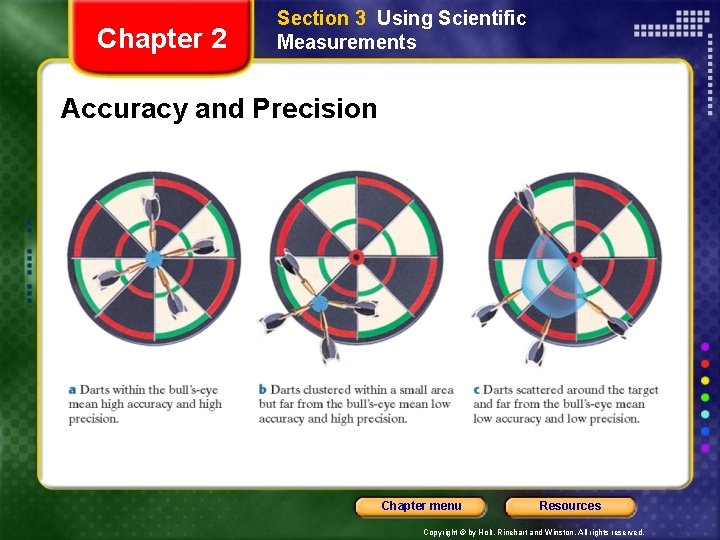
Chapter 2 Section 3 Using Scientific Measurements Accuracy and Precision Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Visual Concepts Accuracy and Precision Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 3 Using Scientific Measurements Accuracy and Precision, continued Percentage Error • Percentage error is calculated by subtracting the accepted value from the experimental value, dividing the difference by the accepted value, and then multiplying by 100. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 3 Using Scientific Measurements Accuracy and Precision, continued Sample Problem C A student measures the mass and volume of a substance and calculates its density as 1. 40 g/m. L. The correct, or accepted, value of the density is 1. 30 g/m. L. What is the percentage error of the student’s measurement? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
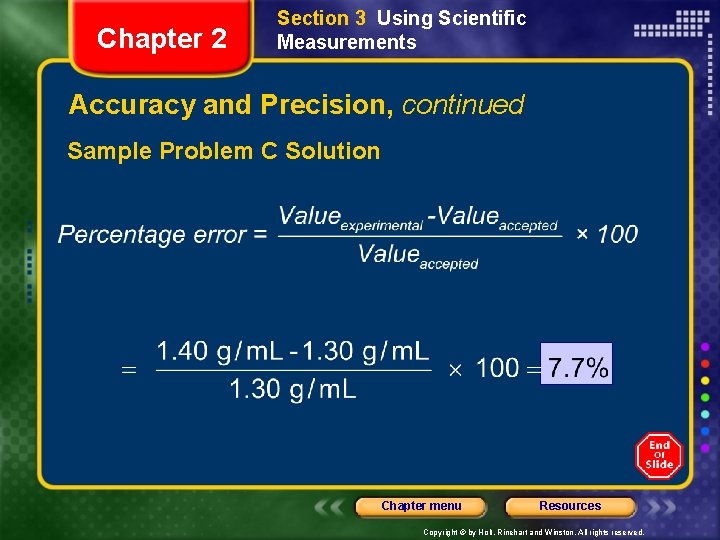
Chapter 2 Section 3 Using Scientific Measurements Accuracy and Precision, continued Sample Problem C Solution Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 3 Using Scientific Measurements Accuracy and Precision, continued Error in Measurement • Some error or uncertainty always exists in any measurement. • skill of the measurer (human error) • conditions of measurement • measuring instruments (instrumental error) Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Standardized Test Preparation Multiple Choice 4. All measurements in science A. must be expressed in scientific notation. B. have some degree of uncertainty. C. are both accurate and precise. D. must include only those digits that are known with certainty. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Standardized Test Preparation Multiple Choice 4. All measurements in science A. must be expressed in scientific notation. B. have some degree of uncertainty. C. are both accurate and precise. D. must include only those digits that are known with certainty. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Standardized Test Preparation Multiple Choice 7. The accuracy of a measurement A. is how close it is to the true value. B. does not depend on the instrument used to measure the object. C. indicates that the measurement is also precise. D. is something that scientists rarely achieve. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Standardized Test Preparation Multiple Choice 7. The accuracy of a measurement A. is how close it is to the true value. B. does not depend on the instrument used to measure the object. C. indicates that the measurement is also precise. D. is something that scientists rarely achieve. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
- Slides: 12