Chapter 2 Section 2 Units of Measurement Objectives

Chapter 2 Section 2 Units of Measurement Objectives- after viewing this lesson you should be able to do the following: • Distinguish between a quantity, a unit, and a measurement standard. • Name and use SI base units. • Name and use SI derived units. • Name and use metric prefixes. • Perform metric conversions. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Objectives • Distinguish between mass and weight. • Perform density calculations. • Transform a statement of equality into a conversion factor. • Use factor-label method, aka dimensional analysis, to solve problems. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Units of Measurement • Measurements represent quantities. • A quantity is something that has magnitude, size, or amount. • the liter is a unit of measurement used to represent volume • volume is a quantity • The choice of unit depends on the quantity being measured. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Units of Measurement SI Measurement • Scientists all over the world have agreed on a single measurement system called Le Système International d’Unités, abbreviated SI. • SI has seven base units • most other units are derived from these seven • A base unit is independent of other units • A base unit is a defined unit in a system of measurement that is based on an object or event in the physical world. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
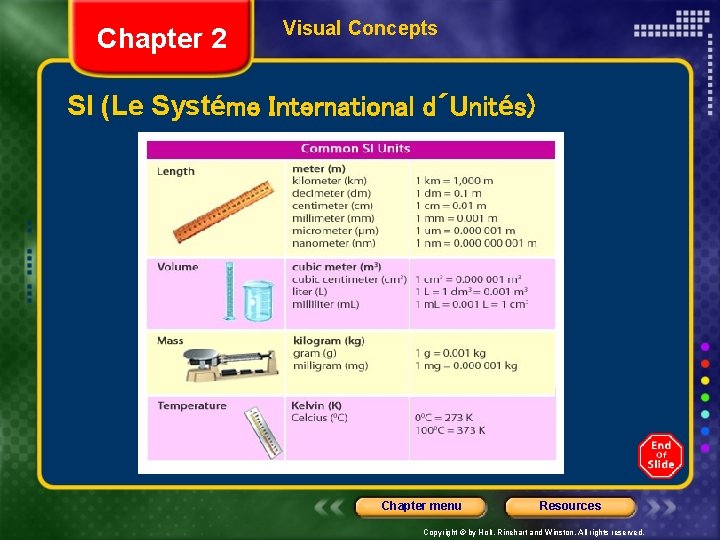
Chapter 2 Visual Concepts SI (Le Systéme International d´Unités) Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Units of Measurement SI Base Units Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
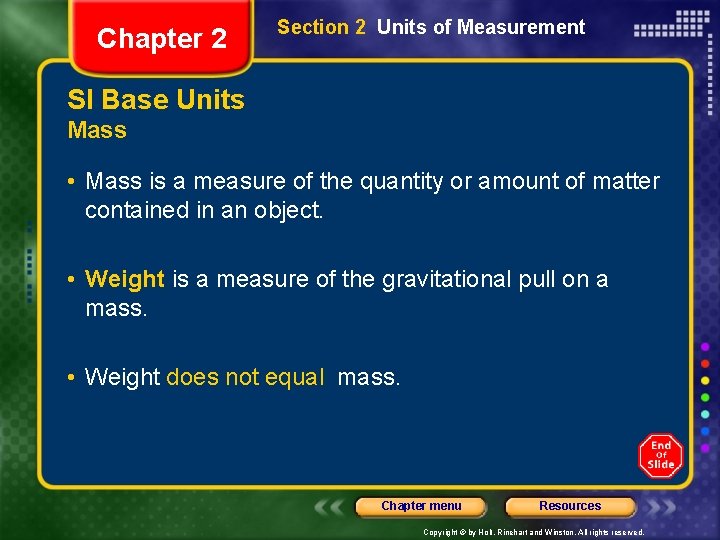
Chapter 2 Section 2 Units of Measurement SI Base Units Mass • Mass is a measure of the quantity or amount of matter contained in an object. • Weight is a measure of the gravitational pull on a mass. • Weight does not equal mass. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Units of Measurement SI Base Units Length, Temperature, Amt. of Substance • Length is a measure of distance. • Temperature is a measure of the average kinetic energy in a sample of matter. • The Amount of Substance measures the total number of individual particles of matter contained in a sample of matter. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
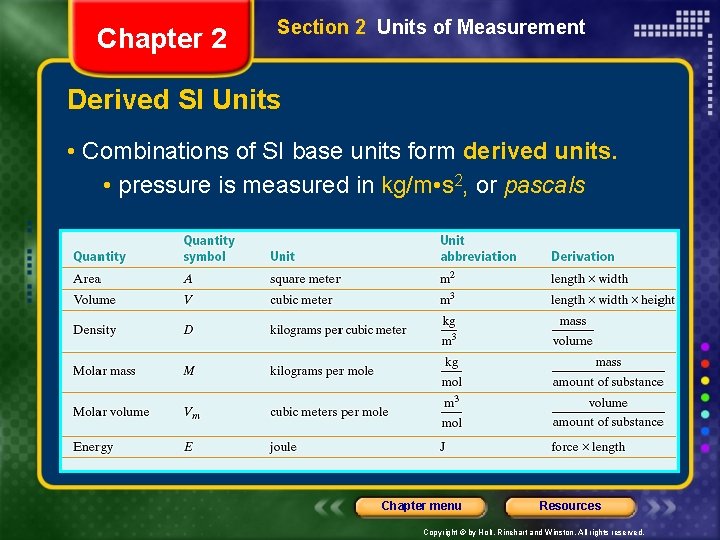
Chapter 2 Section 2 Units of Measurement Derived SI Units • Combinations of SI base units form derived units. • pressure is measured in kg/m • s 2, or pascals Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Units of Measurement Derived SI Units, continued Volume • Volume is the amount of space occupied by an object. • The derived SI unit is cubic meters, m 3 • The cubic centimeter, cm 3, is often used • The liter, L, is a non-SI unit • 1 L = 1000 cm 3 • 1 m. L = 1 cm 3 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Visual Concepts Volume Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Visual Concepts Measuring the Volume of Liquids Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
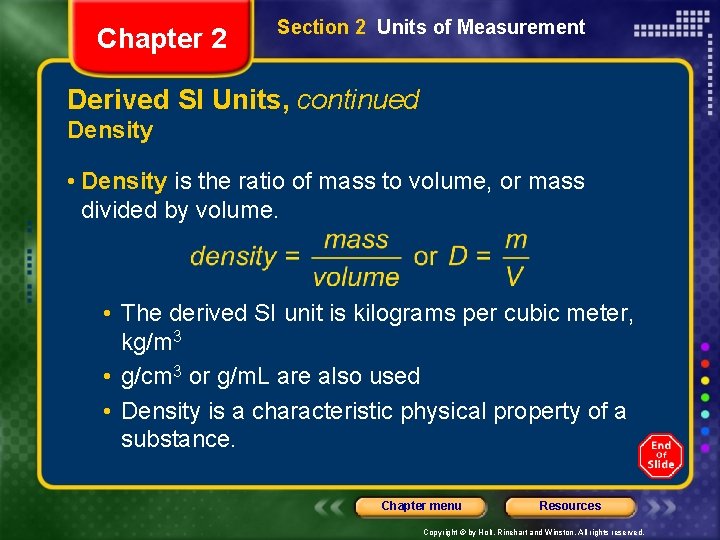
Chapter 2 Section 2 Units of Measurement Derived SI Units, continued Density • Density is the ratio of mass to volume, or mass divided by volume. • The derived SI unit is kilograms per cubic meter, kg/m 3 • g/cm 3 or g/m. L are also used • Density is a characteristic physical property of a substance. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Units of Measurement Derived SI Units, continued Density • Density can be used as one property to help identify a substance Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Visual Concepts Equation for Density Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Units of Measurement Derived SI Units, continued Sample Problem A A sample of aluminum metal has a mass of 8. 4 g. The volume of the sample is 3. 1 cm 3. Calculate the density of aluminum. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Units of Measurement Derived SI Units, continued Sample Problem A Solution Given: mass (m) = 8. 4 g volume (V) = 3. 1 cm 3 Unknown: density (D) Solution: Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
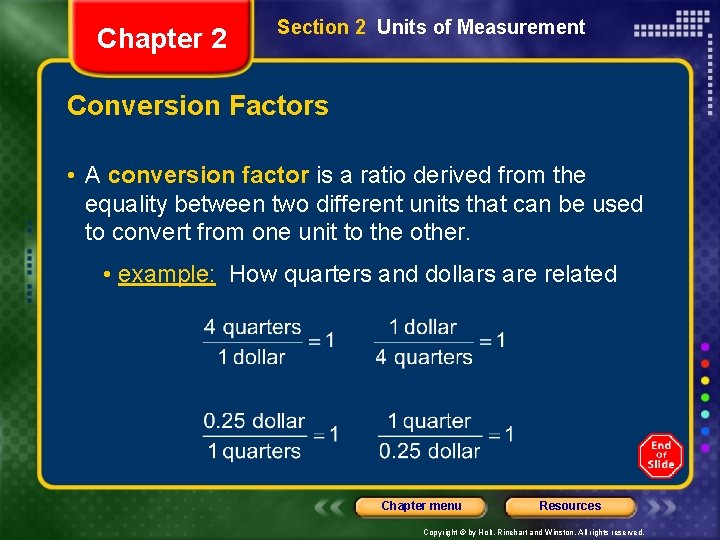
Chapter 2 Section 2 Units of Measurement Conversion Factors • A conversion factor is a ratio derived from the equality between two different units that can be used to convert from one unit to the other. • example: How quarters and dollars are related Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Visual Concepts Conversion Factor Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Units of Measurement Conversion Factors, continued • Dimensional analysis is a mathematical technique that allows you to use units to solve problems involving measurements. • quantity sought = quantity given × conversion factor • example: the number of quarters in 12 dollars number of quarters = 12 dollars × conversion factor Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
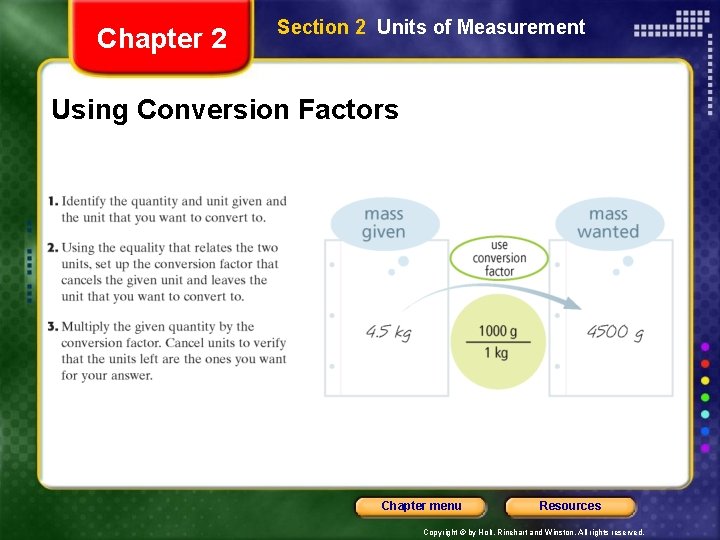
Chapter 2 Section 2 Units of Measurement Using Conversion Factors Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

Chapter 2 Section 2 Units of Measurement Conversion Factors, continued Deriving Conversion Factors • You can derive conversion factors if you know the relationship between the unit you have and the unit you want. • example: conversion factors for meters and decimeters Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.

• Chapter 2 • Base units can be too large or too small for some measurements, so the base units may be modified by attaching prefixes. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
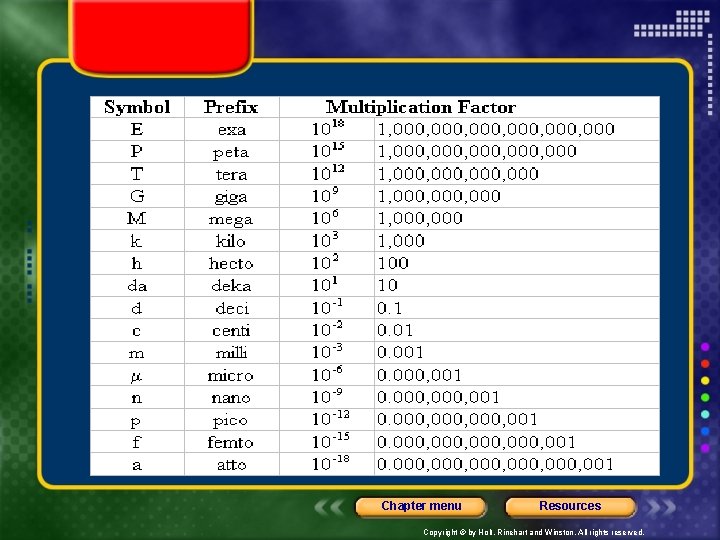
Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
Chapter 2 Section 2 Units of Measurement Conversion Factors, continued Sample Problem B Express a mass of 5. 712 grams in milligrams and in kilograms. Keep in mind that all “base” units such as the gram in this example are what the metric prefixes are affixed to in order to change the value on the unit. For conversion purposes all of these “base “ units are assigned a value of 100 (100=1) Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
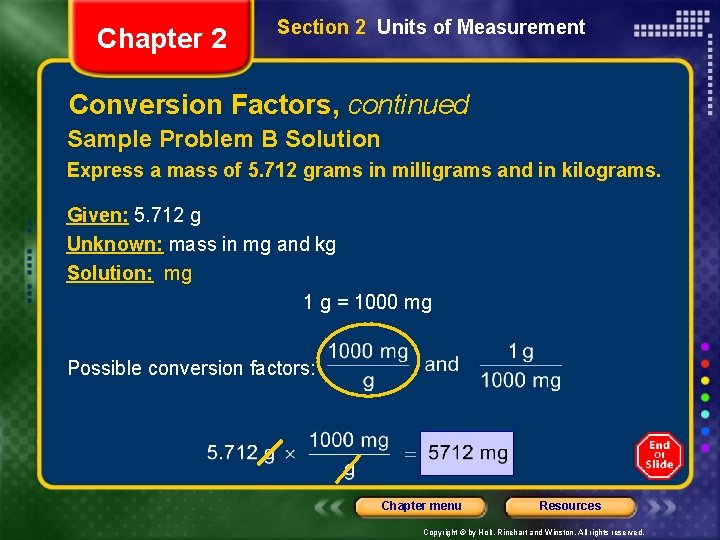
Chapter 2 Section 2 Units of Measurement Conversion Factors, continued Sample Problem B Solution Express a mass of 5. 712 grams in milligrams and in kilograms. Given: 5. 712 g Unknown: mass in mg and kg Solution: mg 1 g = 1000 mg Possible conversion factors: Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
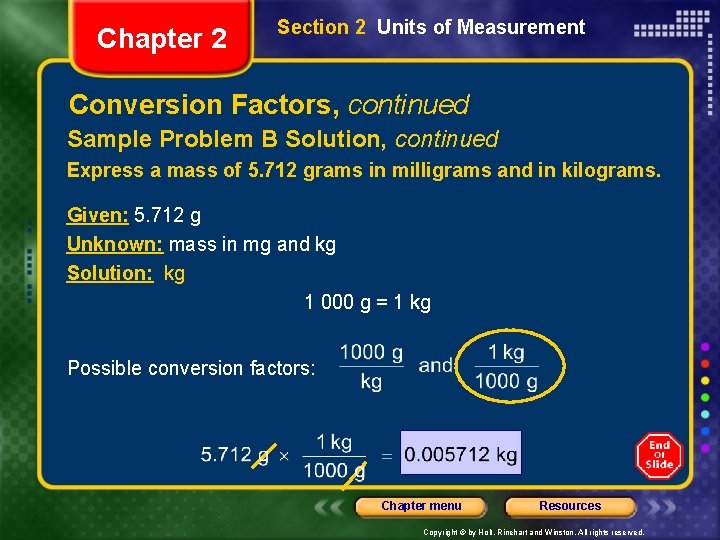
Chapter 2 Section 2 Units of Measurement Conversion Factors, continued Sample Problem B Solution, continued Express a mass of 5. 712 grams in milligrams and in kilograms. Given: 5. 712 g Unknown: mass in mg and kg Solution: kg 1 000 g = 1 kg Possible conversion factors: Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
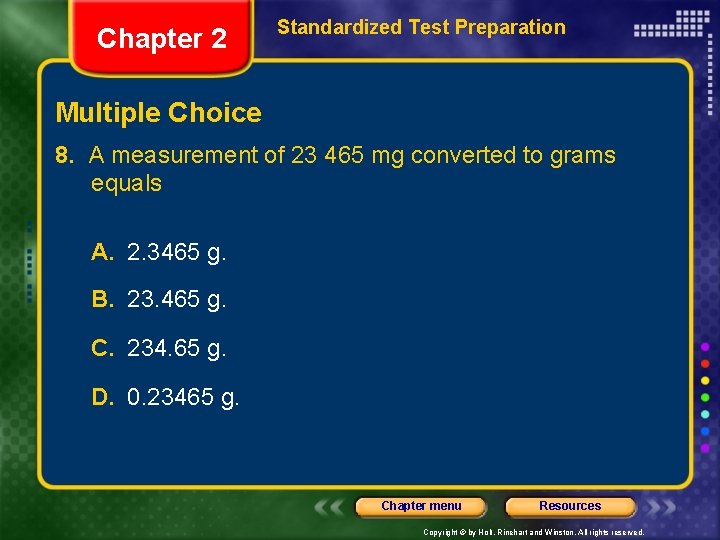
Chapter 2 Standardized Test Preparation Multiple Choice 8. A measurement of 23 465 mg converted to grams equals A. 2. 3465 g. B. 23. 465 g. C. 234. 65 g. D. 0. 23465 g. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
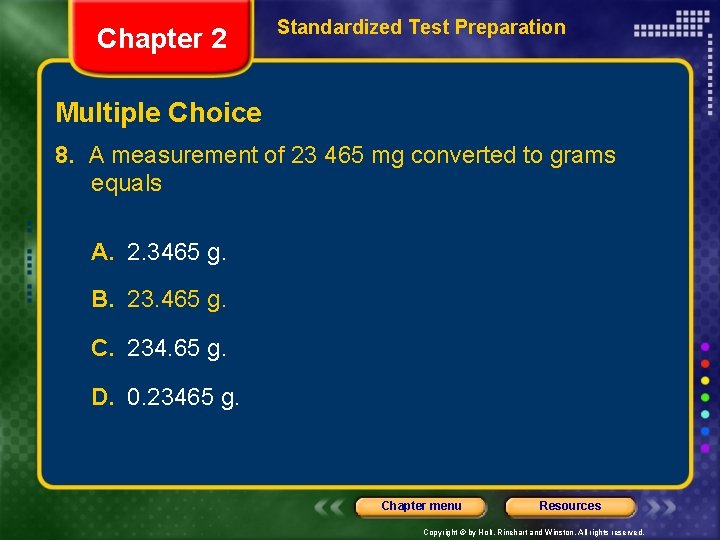
Chapter 2 Standardized Test Preparation Multiple Choice 8. A measurement of 23 465 mg converted to grams equals A. 2. 3465 g. B. 23. 465 g. C. 234. 65 g. D. 0. 23465 g. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.
- Slides: 29