CHAPTER 2 SECTION 1 Matter is described by

CHAPTER 2

SECTION 1 • Matter is described by its properties and may undergo changes. • Everything in the universe that you can see is made up of matter.

MATTER AND VOLUME • All matter takes up space, therefore all matter has volume. • Objects with volume cannot occupy the same space at the same time.

VOLUME OF LIQUIDS • The volume of a liquid can be measured using a graduated cylinder. • To determine the volume of a liquid, take a reading at the bottom of the meniscus. The reading will usually be in milliliters. • The meniscus is the curve at the surface of the liquid.
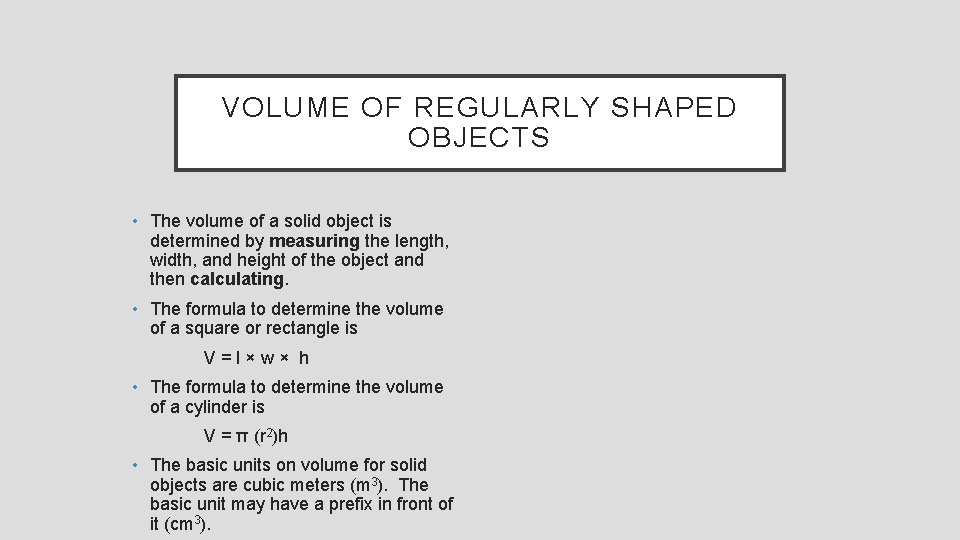
VOLUME OF REGULARLY SHAPED OBJECTS • The volume of a solid object is determined by measuring the length, width, and height of the object and then calculating. • The formula to determine the volume of a square or rectangle is V=l×w× h • The formula to determine the volume of a cylinder is V = π (r 2)h • The basic units on volume for solid objects are cubic meters (m 3). The basic unit may have a prefix in front of it (cm 3).

VOLUME OF AN IRREGULARLY SHAPED OBJECT • To determine the volume of an irregularly shaped object, use the water displacement method. • Put enough water in the graduated cylinder to cover the object. • Measure the amount of water in the cylinder. Take a reading at the bottom of the meniscus. The units will usually be in milliliters. Record this as your start volume. • Place the object in the graduated cylinder gently. Avoid splashing. • Find the new volume. Record this as the final volume • The amount of water displaced by the object is equal to its volume. • Volume of object = final volume – start volume • The volume of liquids can be expressed in liters or cubic units because of the following mathematical relationship: 1 ml = 1 cm 3

MATTER AND MASS • All matter has mass. • The mass of an object is the same, no matter where in the universe it is located. • An object’s mass only changes if the amount of matter making it up changes. • Weight is a measure of the force with which gravity pulls on an object’s mass. • Since weight is a force, the unit on weight is a Newton (N). • All objects with mass exert a gravitational pull.

MATTER AND MASS • The strength of the gravitational pull on an object is determined by 2 factors: • by the mass of the objects involved (bigger mass = bigger gravity) • by the distance between the objects involved (bigger distance =smaller gravity) • The greater an object’s mass, the stronger the pull of gravity will be on the object and the greater the object’s weight will be. • For every 1 kg of s an object has, gravity pulls on that object with a force of 9. 8 N. (on earth) • Weight = mass (in kg) x gravity W=m×g • Acceleration due to gravity (on earth) = 9. 8 m/s 2 = g • An object’s weight changes depending on where it is in the universe because gravity’s pull is not the same everywhere in the universe. • More massive planets such as Jupiter have stronger gravitational pulls, so your weight would be greater if you
INERTIA • Inertia is defined as the tendency of an object to resist a change in its motion. • Mass is a measure of inertia because the greater an object’s mass, the greater its inertia. • The greater an object’s mass the harder it is to stop or start moving.

SECTION 2: PHYSICAL PROPERTIES Knowing the properties of an unknown object can help you determine the identity of the object.
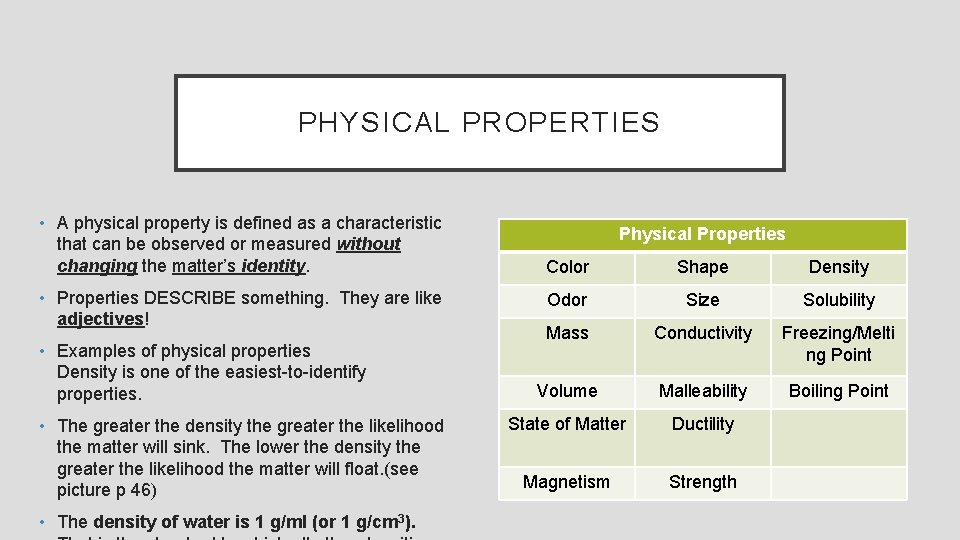
PHYSICAL PROPERTIES • A physical property is defined as a characteristic that can be observed or measured without changing the matter’s identity. • Properties DESCRIBE something. They are like adjectives! • Examples of physical properties Density is one of the easiest-to-identify properties. • The greater the density the greater the likelihood the matter will sink. The lower the density the greater the likelihood the matter will float. (see picture p 46) • The density of water is 1 g/ml (or 1 g/cm 3). Physical Properties Color Shape Density Odor Size Solubility Mass Conductivity Freezing/Melti ng Point Volume Malleability Boiling Point State of Matter Ductility Magnetism Strength

PHYSICAL CHANGES: DO NOT FORM NEW SUBSTANCES • A physical change is defined as a change that affects only the physical properties of a substance. No change in identity occurs. Examples of physical changes: • change in size • change in shape • change in state • dissolving • anything that does NOT change the identity

SECTION 3 Besides physical properties, matter also has chemical properties.

CHEMICAL PROPERTIES • Chemical properties are defined as characteristics of matter that are based on its ability to change into new matter that has different properties. Examples of chemical properties: • Flammability/combustibility – ability to burn • reactivity – ability to combine with other substances • acidity (p. H) – measure of if it is an acid or a base • Chemical properties are not as easily observed as physical properties.
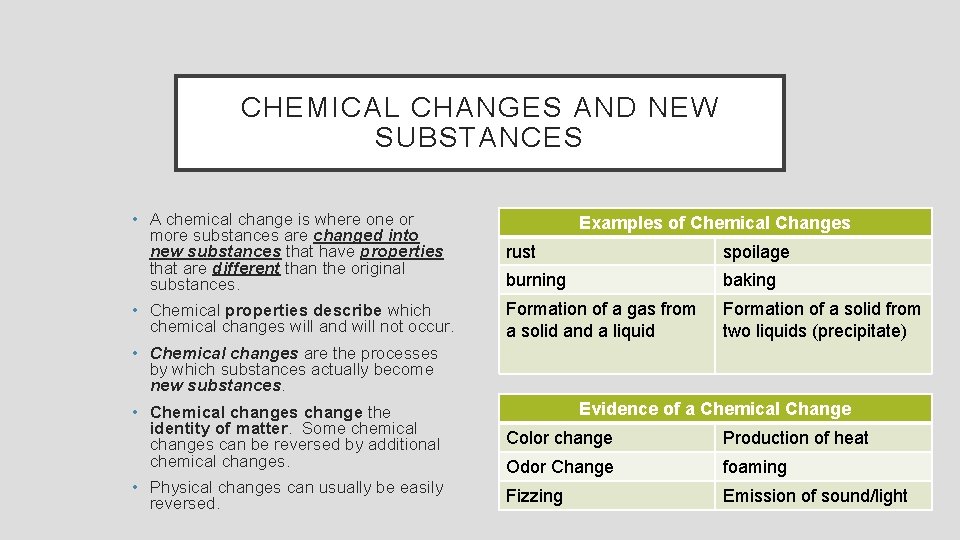
CHEMICAL CHANGES AND NEW SUBSTANCES • A chemical change is where one or more substances are changed into new substances that have properties that are different than the original substances. rust spoilage burning baking • Chemical properties describe which chemical changes will and will not occur. Formation of a gas from a solid and a liquid Formation of a solid from two liquids (precipitate) Examples of Chemical Changes • Chemical changes are the processes by which substances actually become new substances. Evidence of a Chemical Change • Chemical changes change the identity of matter. Some chemical changes can be reversed by additional chemical changes. Color change Production of heat Odor Change foaming • Physical changes can usually be easily reversed. Fizzing Emission of sound/light
- Slides: 15