Chapter 2 RadicalChain Polymerization 1 1 ChainRadical Polymerization






![Polymerization rate with different initiations Initiator ln([M]0/[M]) t: low conversion; [I] and f are Polymerization rate with different initiations Initiator ln([M]0/[M]) t: low conversion; [I] and f are](https://slidetodoc.com/presentation_image/340171e4d6457776aed20f8ba4edef20/image-54.jpg)


![kinetic chain length: [M][I]-0. 5 66 kinetic chain length: [M][I]-0. 5 66](https://slidetodoc.com/presentation_image/340171e4d6457776aed20f8ba4edef20/image-66.jpg)





![Ceiling Temperature (聚合上限温度) [M]=1. 0 115 Ceiling Temperature (聚合上限温度) [M]=1. 0 115](https://slidetodoc.com/presentation_image/340171e4d6457776aed20f8ba4edef20/image-115.jpg)
![Equilibrium monomer concentration equil. monomer concentration For example: MMA Styrene -Methylstyrene ΔH° ΔS° [M] Equilibrium monomer concentration equil. monomer concentration For example: MMA Styrene -Methylstyrene ΔH° ΔS° [M]](https://slidetodoc.com/presentation_image/340171e4d6457776aed20f8ba4edef20/image-117.jpg)

- Slides: 127

Chapter 2 Radical/Chain Polymerization 1

1. Chain/Radical Polymerization 2

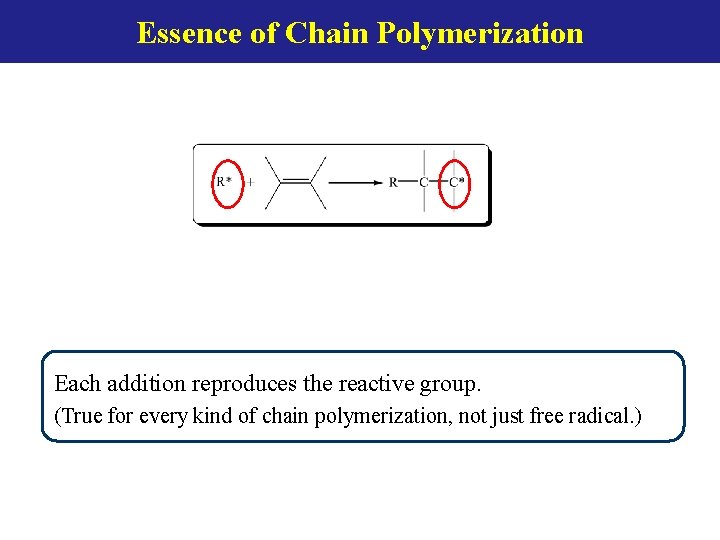
Essence of Chain Polymerization Each addition reproduces the reactive group. (True for every kind of chain polymerization, not just free radical. )

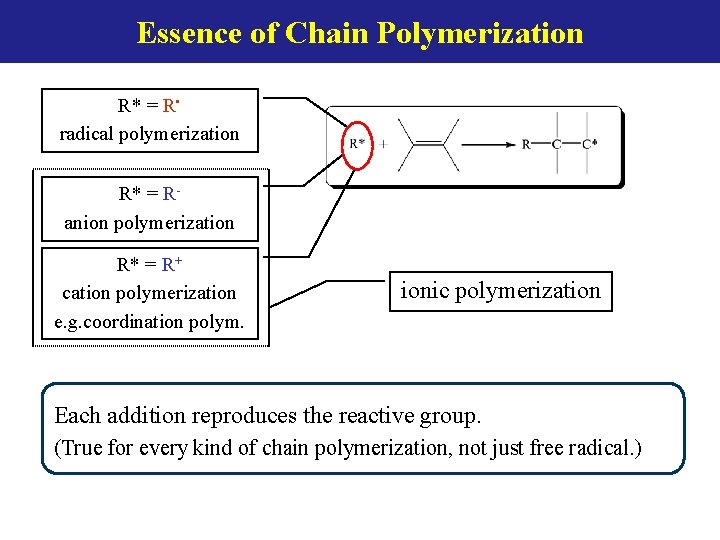
Essence of Chain Polymerization R* = R • radical polymerization R* = Ranion polymerization R* = R+ cation polymerization e. g. coordination polym. ionic polymerization Each addition reproduces the reactive group. (True for every kind of chain polymerization, not just free radical. )

2. Monomers for Chain Polymerization 5

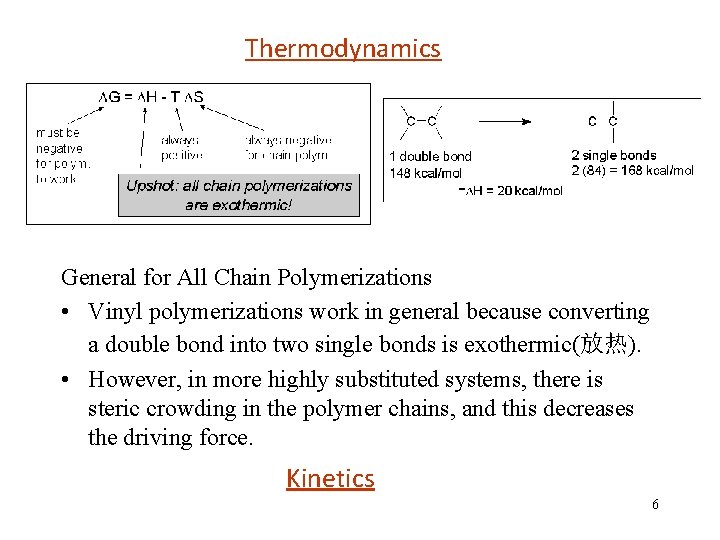
Thermodynamics - General for All Chain Polymerizations • Vinyl polymerizations work in general because converting a double bond into two single bonds is exothermic(放热). • However, in more highly substituted systems, there is steric crowding in the polymer chains, and this decreases the driving force. Kinetics 6

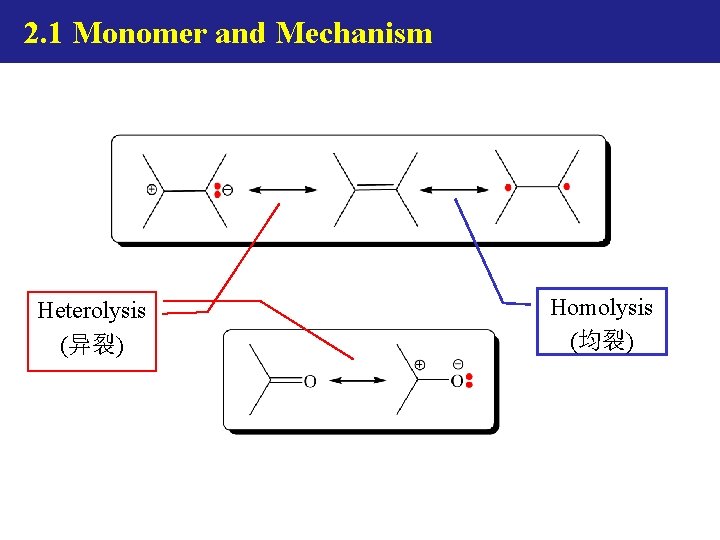
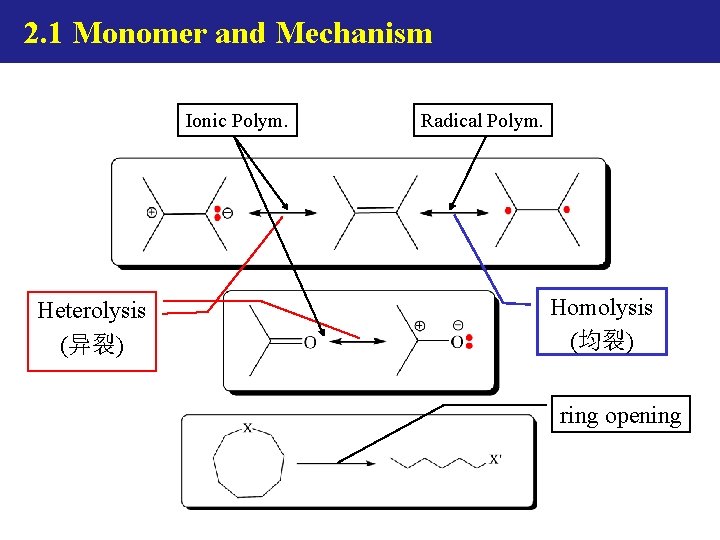
2. 1 Monomer and Mechanism Heterolysis (异裂) Homolysis (均裂)

2. 1 Monomer and Mechanism Ionic Polym. Heterolysis (异裂) Radical Polym. Homolysis (均裂) ring opening

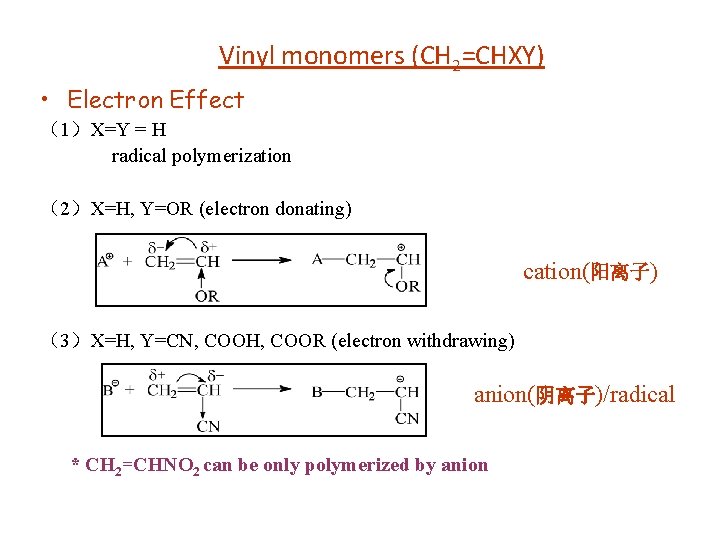
Vinyl monomers (CH 2=CHXY) • Electron Effect (1)X=Y = H radical polymerization (2)X=H, Y=OR (electron donating) cation(阳离子) (3)X=H, Y=CN, COOH, COOR (electron withdrawing) anion(阴离子)/radical * CH 2=CHNO 2 can be only polymerized by anion

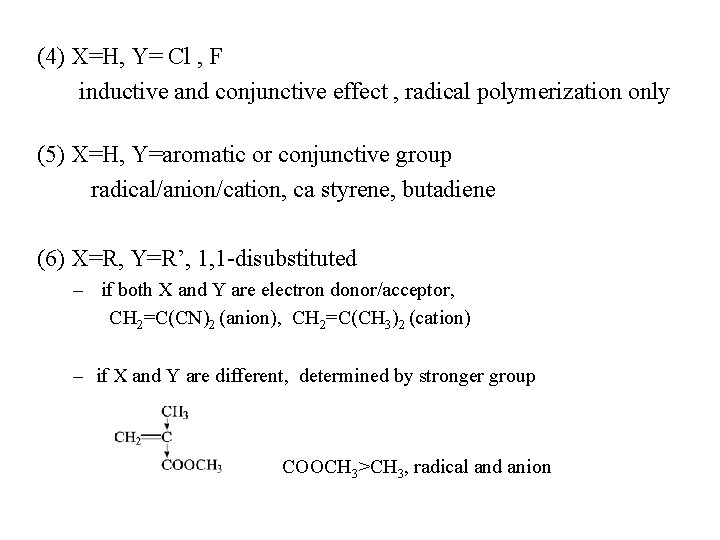
(4) X=H, Y= Cl , F inductive and conjunctive effect , radical polymerization only (5) X=H, Y=aromatic or conjunctive group radical/anion/cation, ca styrene, butadiene (6) X=R, Y=R’, 1, 1 -disubstituted – if both X and Y are electron donor/acceptor, CH 2=C(CN)2 (anion), CH 2=C(CH 3)2 (cation) – if X and Y are different, determined by stronger group COOCH 3>CH 3, radical and anion

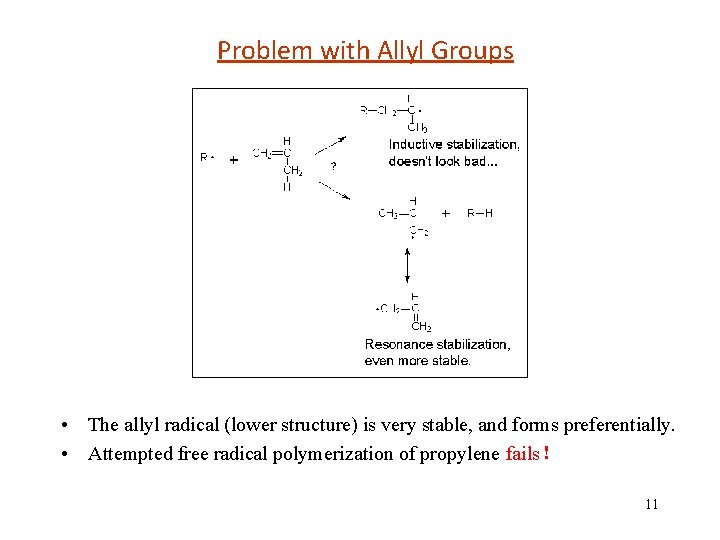
Problem with Allyl Groups • The allyl radical (lower structure) is very stable, and forms preferentially. • Attempted free radical polymerization of propylene fails! 11

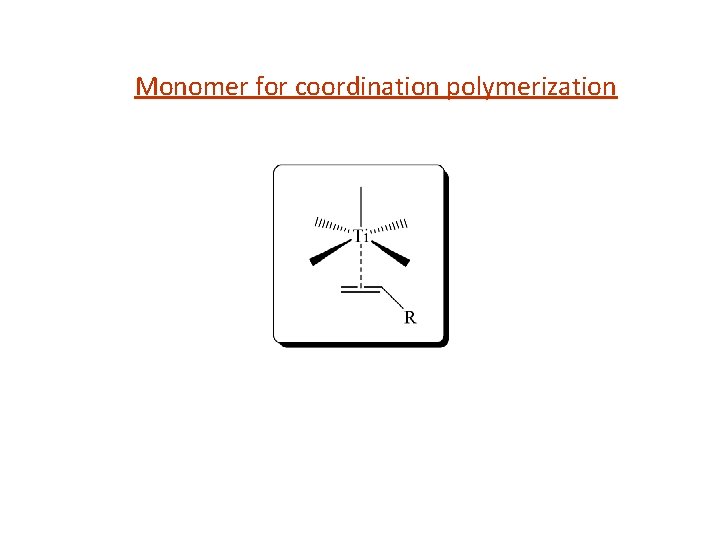
Monomer for coordination polymerization

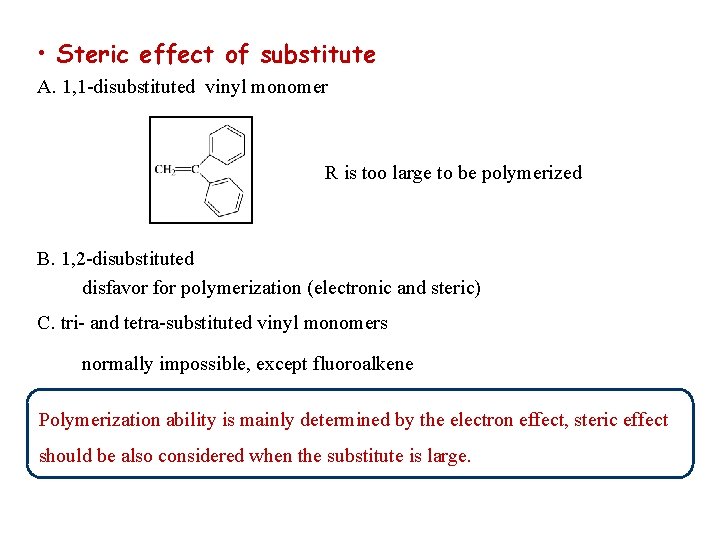
• Steric effect of substitute A. 1, 1 -disubstituted vinyl monomer R is too large to be polymerized B. 1, 2 -disubstituted disfavor for polymerization (electronic and steric) C. tri- and tetra-substituted vinyl monomers normally impossible, except fluoroalkene Polymerization ability is mainly determined by the electron effect, steric effect should be also considered when the substitute is large.

3. Mechanism of Radical Polymerization 14

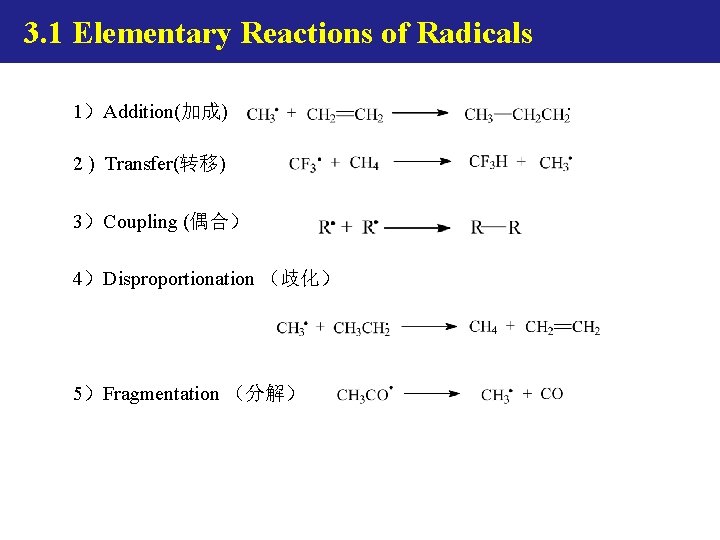
3. 1 Elementary (基元) Reactions of Radicals

3. 1 Elementary Reactions of Radicals. 1)Addition(加成) 2 ) Transfer(转移) 3)Coupling (偶合) 4)Disproportionation (歧化) . 5)Fragmentation (分解)

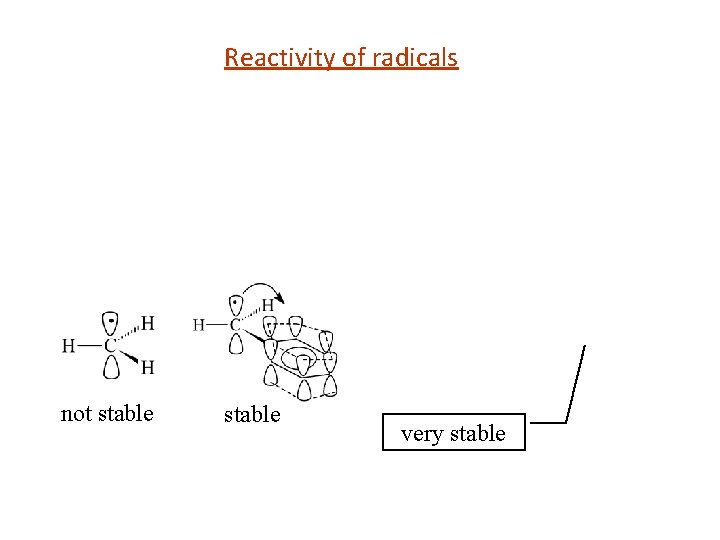
Reactivity of radicals not stable very stable

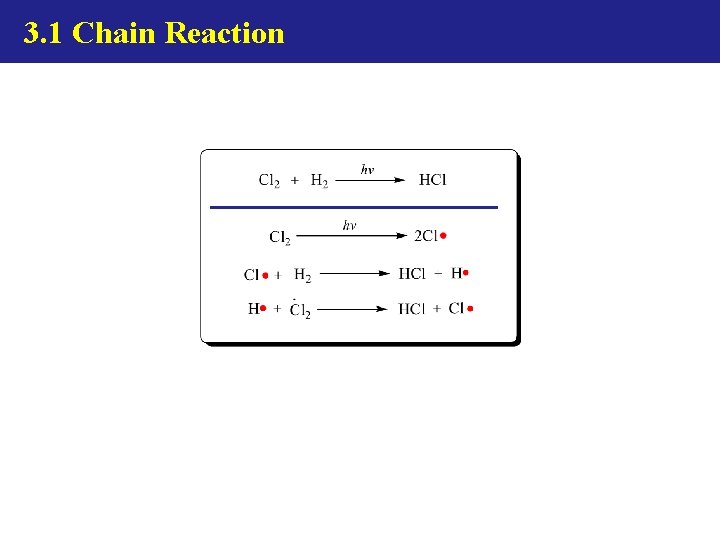
3. 1 Chain Reaction

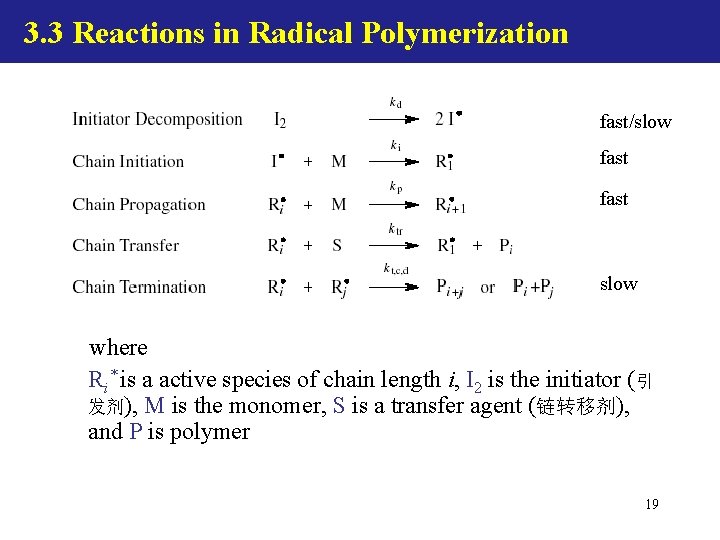
3. 3 Reactions in Radical Polymerization fast/slow fast slow where Ri* is a active species of chain length i, I 2 is the initiator (引 发剂), M is the monomer, S is a transfer agent (链转移剂), and P is polymer 19

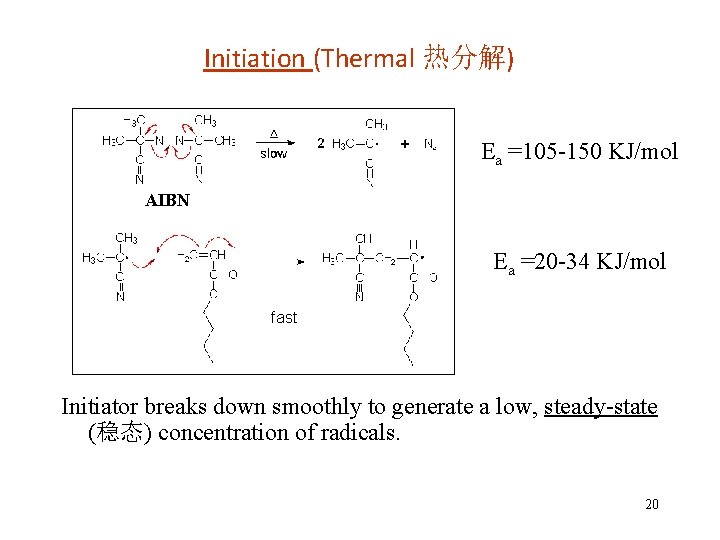
Initiation (Thermal 热分解) Ea =105 -150 KJ/mol AIBN Ea =20 -34 KJ/mol fast Initiator breaks down smoothly to generate a low, steady-state (稳态) concentration of radicals. 20

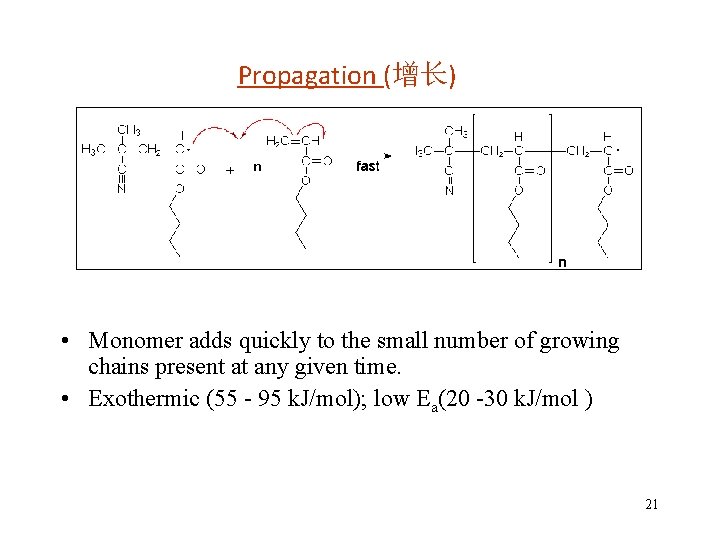
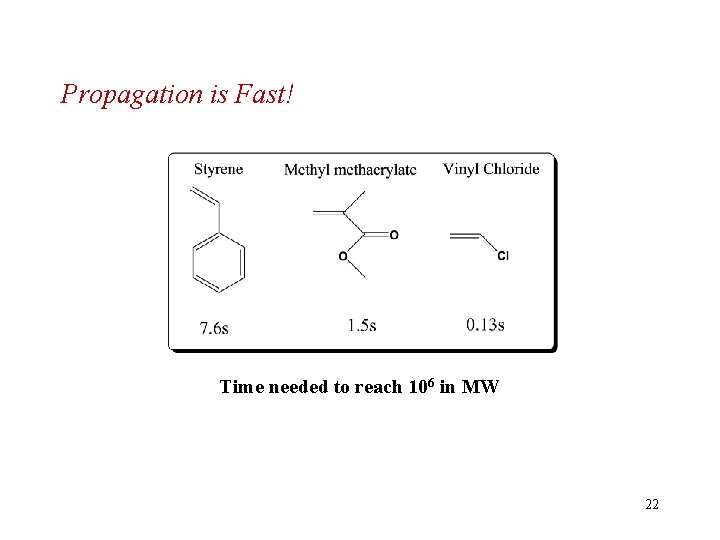
Propagation (增长) • Monomer adds quickly to the small number of growing chains present at any given time. • Exothermic (55 - 95 k. J/mol); low Ea(20 -30 k. J/mol ) 21

Propagation is Fast! Time needed to reach 106 in MW 22

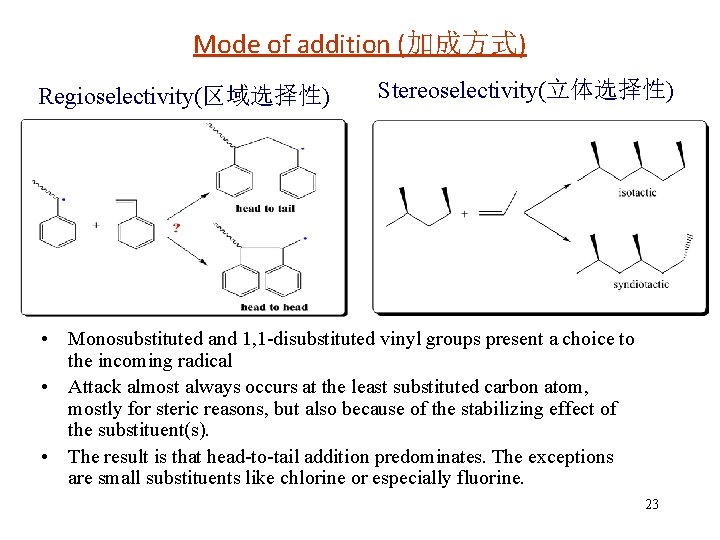
Mode of addition (加成方式) Regioselectivity(区域选择性) Stereoselectivity(立体选择性) • Monosubstituted and 1, 1 -disubstituted vinyl groups present a choice to the incoming radical • Attack almost always occurs at the least substituted carbon atom, mostly for steric reasons, but also because of the stabilizing effect of the substituent(s). • The result is that head-to-tail addition predominates. The exceptions are small substituents like chlorine or especially fluorine. 23

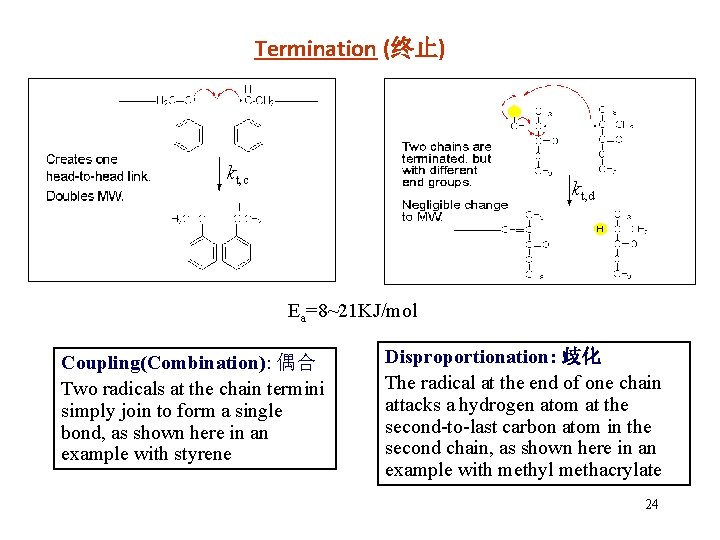
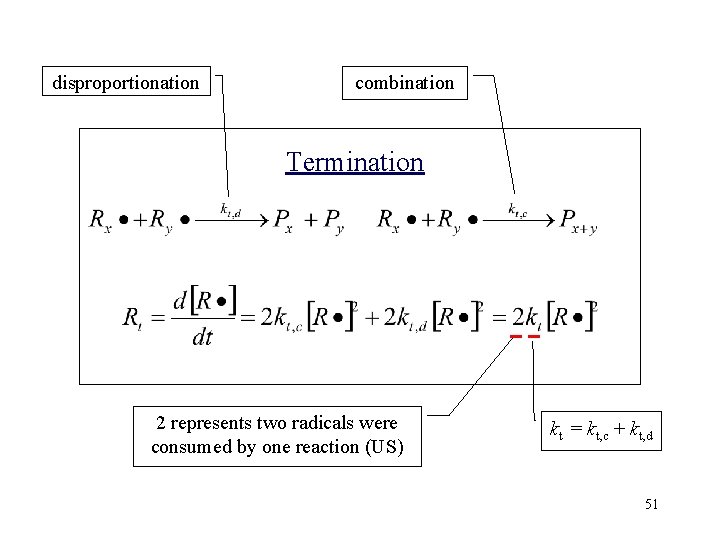
Termination (终止) kt, c kt, d Ea=8~21 KJ/mol Coupling(Combination): 偶合 Two radicals at the chain termini simply join to form a single bond, as shown here in an example with styrene Disproportionation: 歧化 The radical at the end of one chain attacks a hydrogen atom at the second-to-last carbon atom in the second chain, as shown here in an example with methyl methacrylate 24

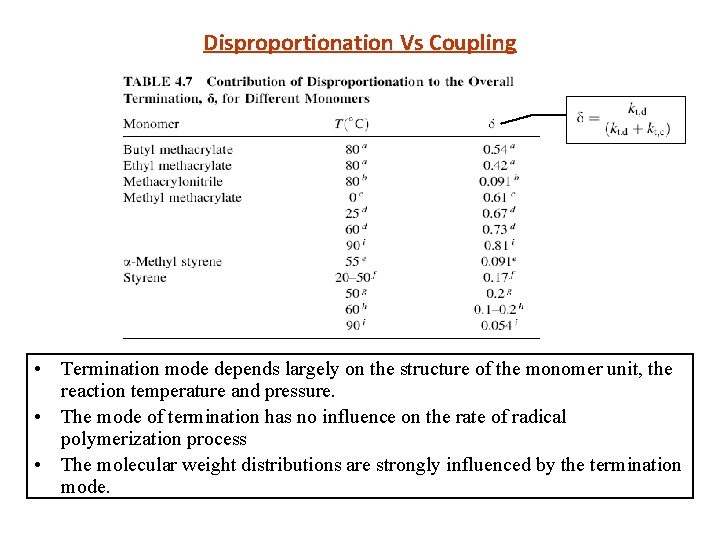
Disproportionation Vs Coupling • Termination mode depends largely on the structure of the monomer unit, the reaction temperature and pressure. • The mode of termination has no influence on the rate of radical polymerization process • The molecular weight distributions are strongly influenced by the termination mode.

The Steady State (稳态) • Initiation is relatively slow but continuous. • Termination speeds up as active radical concentration builds. • Termination removes (kills) active radicals. a steady-state concentration of radicals is established early in the reaction. The concentration of radicals is very small (ca. 10 -8 M) and nearly constant throughout. 26

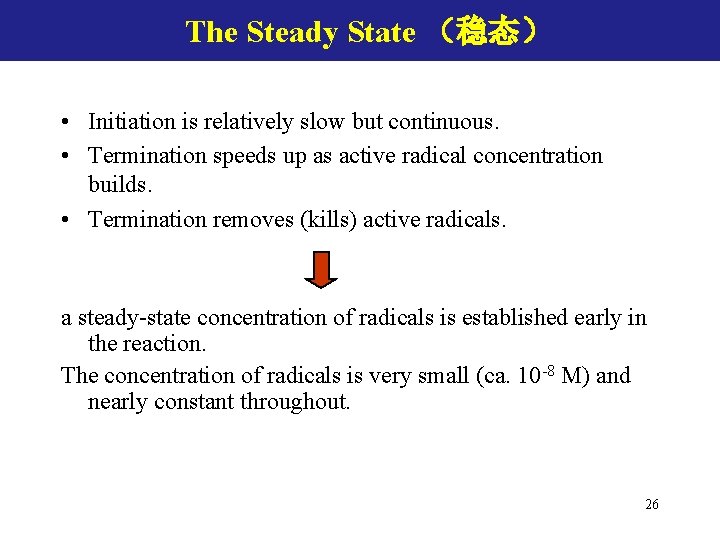
Chain Transfer (链转移) X-Y can be monomer; solvent; polymer; other chemicals Chain transfer occurs when a radical species reacts with a nonradical species. The result must be at least one radical species. Decrease the MW Impact on polymerization rate ? 27

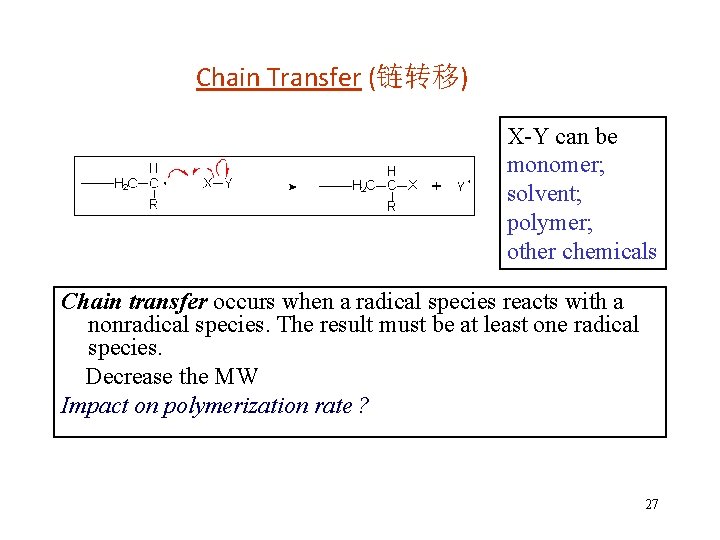
Types of Chain Transfer Chain transfer agent • In many cases, a chain transfer agent is added deliberately to the reaction mixture. Mercaptans (thiols) are the most general. • The sulfur-centered radical reinitiates very efficiently. The result is a diminution of the molecular weight without changing the overall rate of conversion of monomer to polymer. • Using more initiator is another way to decrease MW, but the reaction rate would increase proportionally, a possibly dangerous situation. 28

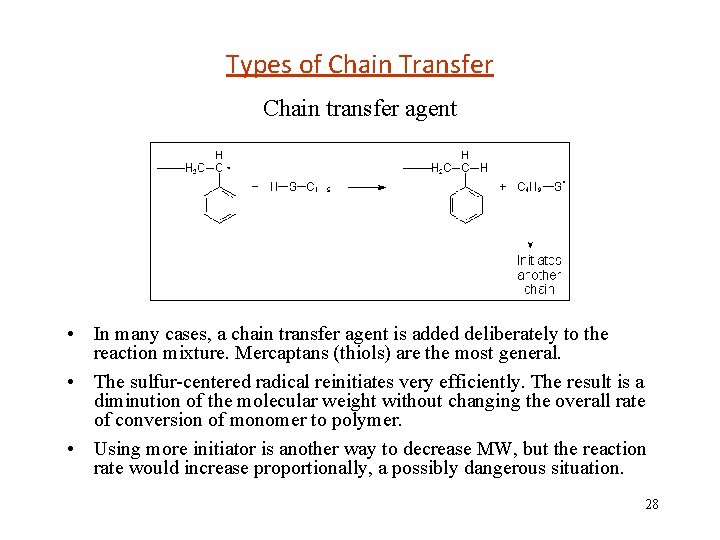
Chain transfer to others • Naturally, there are many even-electron species present in the reaction mixture (i. e. , monomer, initiator, solvents, etc. ), and all of these may participate in transfer reactions. • Here is an example of transfer to initiator featuring acrylonitrile and benzoyl peroxide (BPO) 29

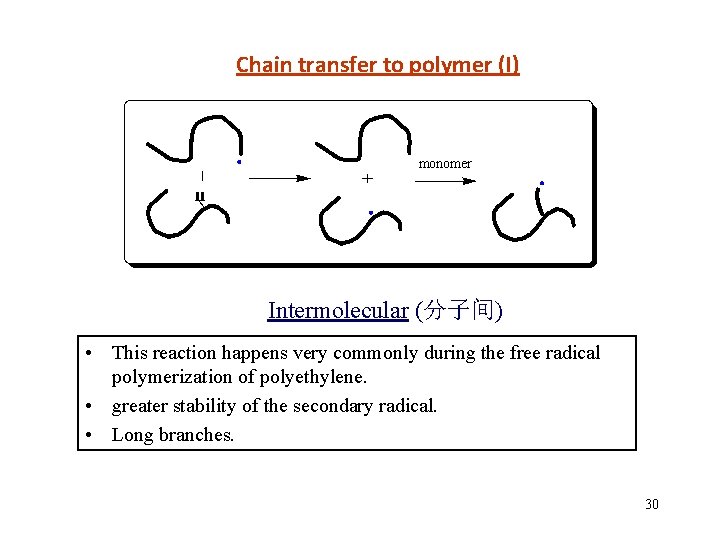
Chain transfer to polymer (I) monomer Intermolecular (分子间) • This reaction happens very commonly during the free radical polymerization of polyethylene. • greater stability of the secondary radical. • Long branches. 30

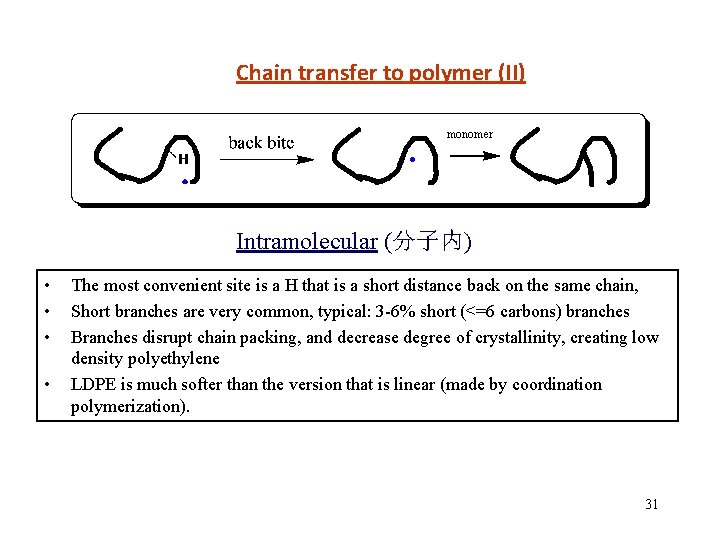
Chain transfer to polymer (II) monomer Intramolecular (分子内) • • The most convenient site is a H that is a short distance back on the same chain, Short branches are very common, typical: 3 -6% short (<=6 carbons) branches Branches disrupt chain packing, and decrease degree of crystallinity, creating low density polyethylene LDPE is much softer than the version that is linear (made by coordination polymerization). 31

Summary Elementary Reactions of radical polymerization initiation: propagation: termination: chain transfer: Ea = 80 ~ 120 k. J/mol; slow Ea = 20 ~ 34 k. J/mol; fast Ea = 8. 5 ~ 20 k. J/mol; fast Ea = 8 ~ 60 k. J/mol; fast

4 Initiation 33

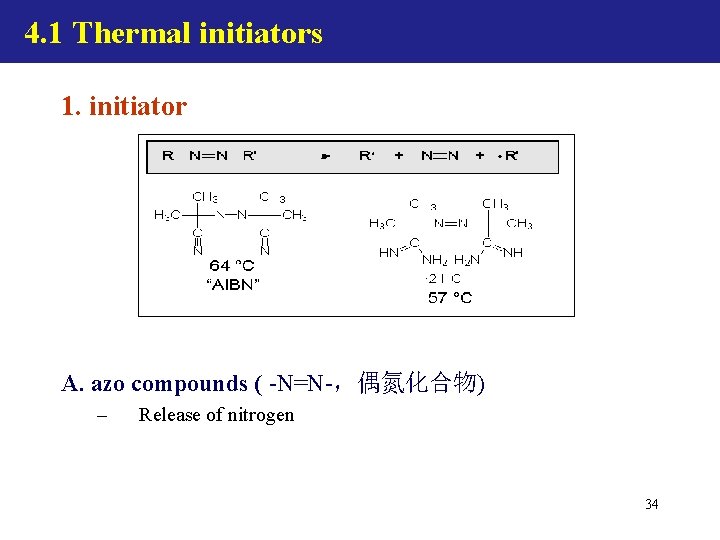
4. 1 Thermal initiators 1. initiator A. azo compounds ( -N=N-,偶氮化合物) – Release of nitrogen 34

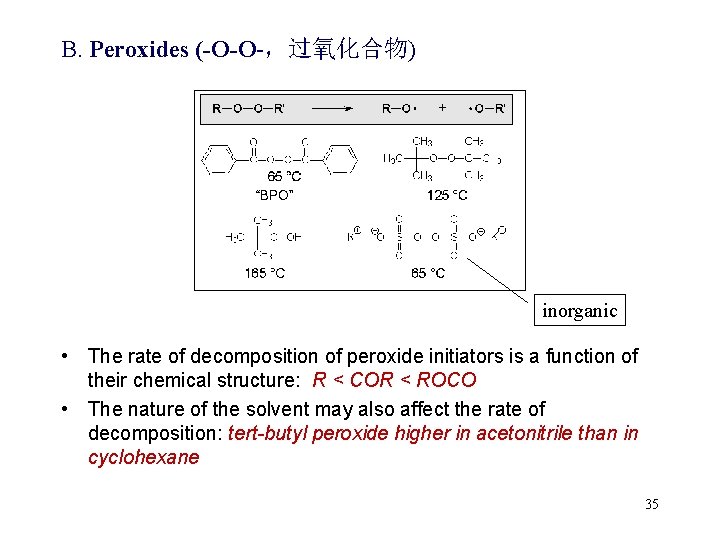
B. Peroxides (-O-O-,过氧化合物) inorganic • The rate of decomposition of peroxide initiators is a function of their chemical structure: R < COR < ROCO • The nature of the solvent may also affect the rate of decomposition: tert-butyl peroxide higher in acetonitrile than in cyclohexane 35

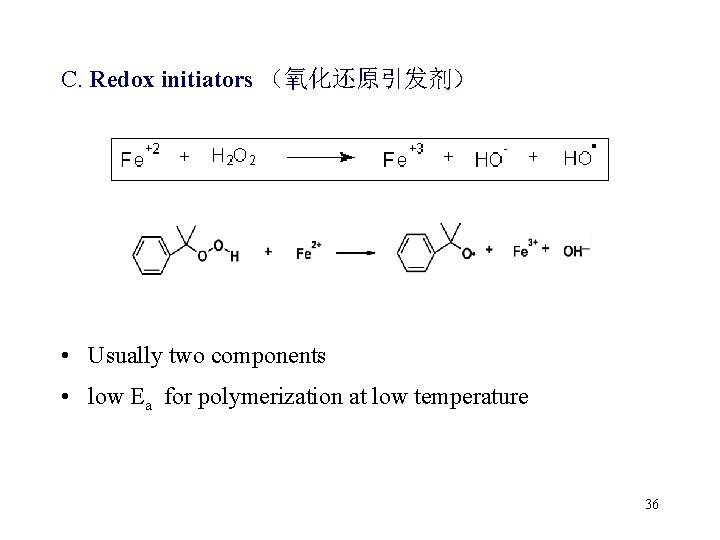
C. Redox initiators (氧化还原引发剂) • Usually two components • low Ea for polymerization at low temperature 36

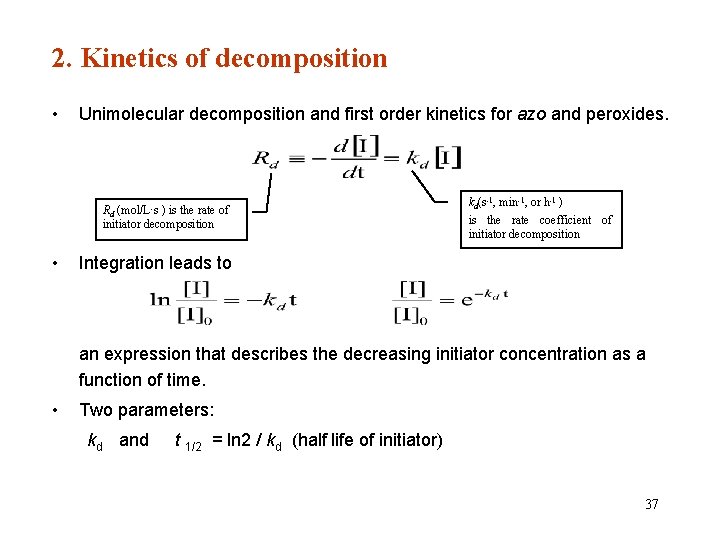
2. Kinetics of decomposition • Unimolecular decomposition and first order kinetics for azo and peroxides. Rd (mol/L·s ) is the rate of initiator decomposition • kd(s-1, min-1, or h-1 ) is the rate coefficient of initiator decomposition Integration leads to an expression that describes the decreasing initiator concentration as a function of time. • Two parameters: kd and t 1/2 = ln 2 / kd (half life of initiator) 37

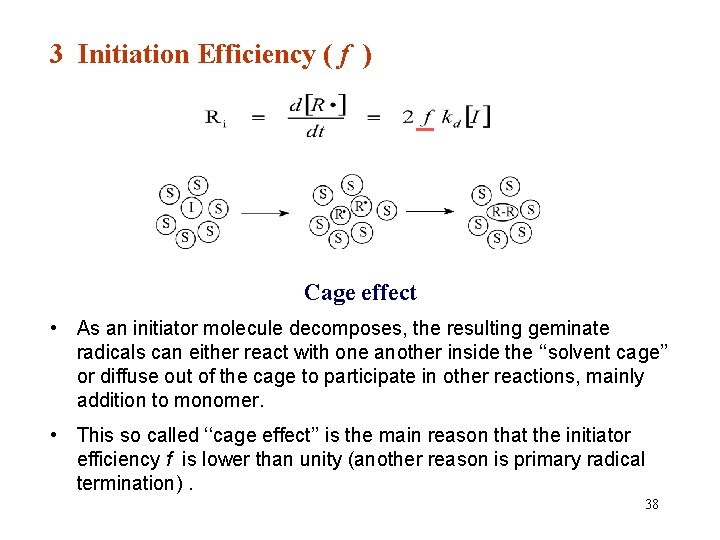
3 Initiation Efficiency ( f ) Cage effect • As an initiator molecule decomposes, the resulting geminate radicals can either react with one another inside the ‘‘solvent cage’’ or diffuse out of the cage to participate in other reactions, mainly addition to monomer. • This so called ‘‘cage effect’’ is the main reason that the initiator efficiency f is lower than unity (another reason is primary radical termination). 38

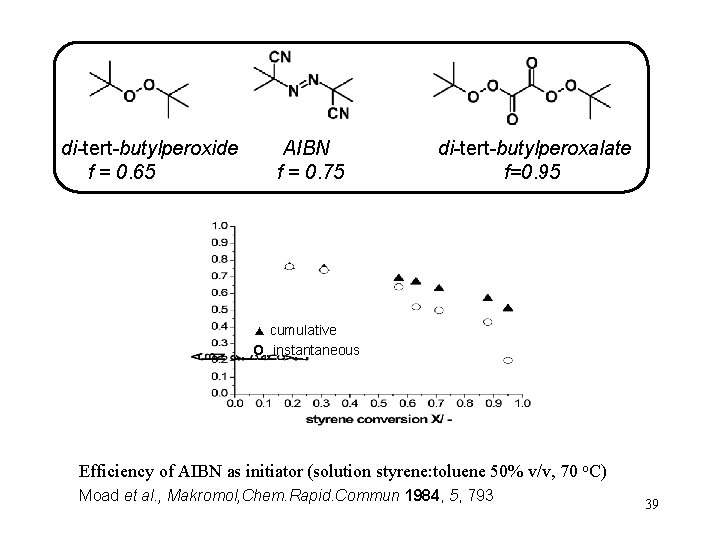
di-tert-butylperoxide f = 0. 65 AIBN f = 0. 75 di-tert-butylperoxalate f=0. 95 ▲ cumulative O instantaneous Efficiency of AIBN as initiator (solution styrene: toluene 50% v/v, 70 o. C) Moad et al. , Makromol, Chem. Rapid. Commun 1984, 5, 793 39

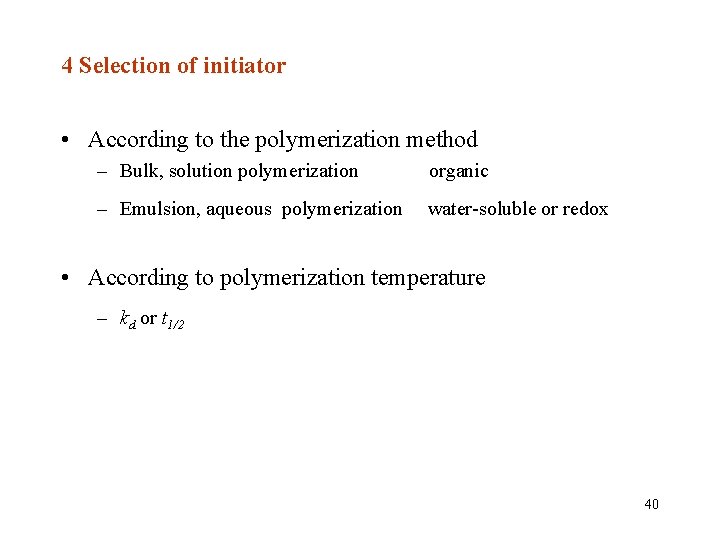
4 Selection of initiator • According to the polymerization method – Bulk, solution polymerization organic – Emulsion, aqueous polymerization water-soluble or redox • According to polymerization temperature – kd or t 1/2 40

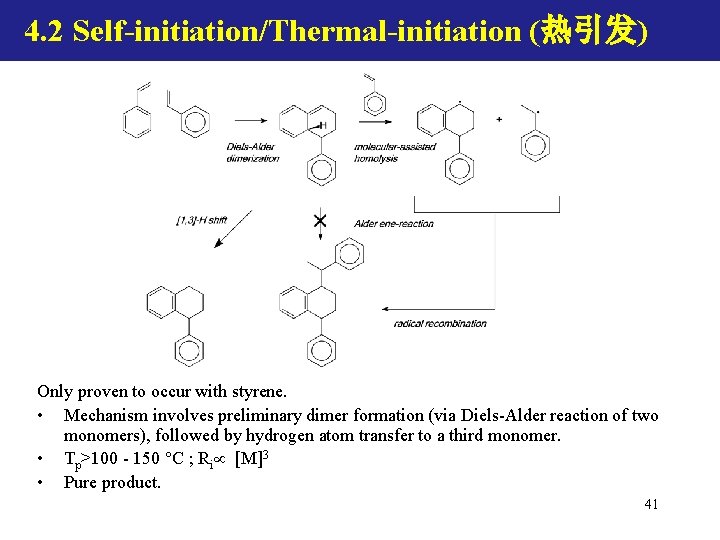
4. 2 Self-initiation/Thermal-initiation (热引发) Only proven to occur with styrene. • Mechanism involves preliminary dimer formation (via Diels-Alder reaction of two monomers), followed by hydrogen atom transfer to a third monomer. • Tp>100 - 150 C ; Ri [M]3 • Pure product. 41

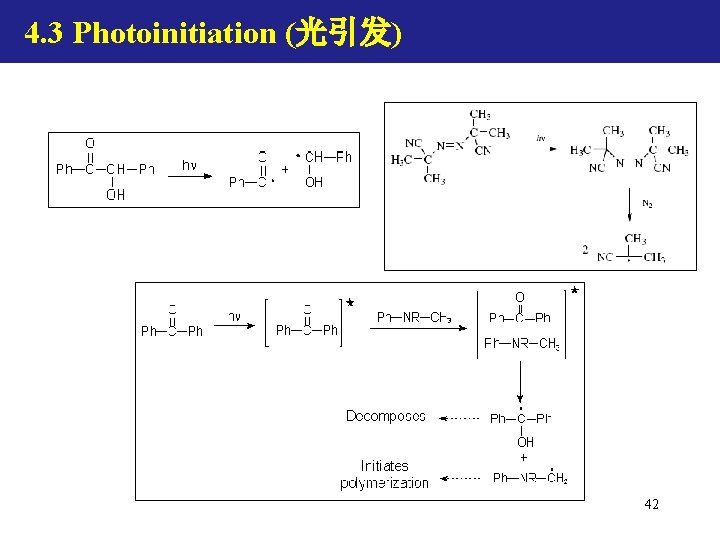
4. 3 Photoinitiation (光引发) 42

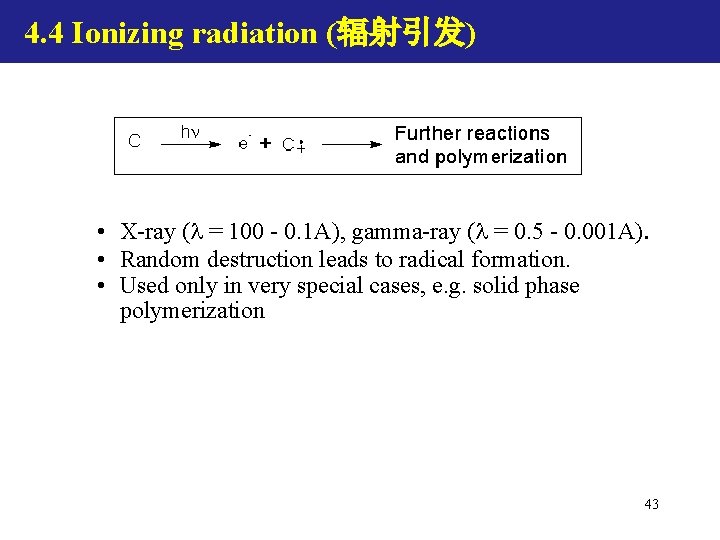
4. 4 Ionizing radiation (辐射引发) • X-ray ( = 100 - 0. 1 A), gamma-ray ( = 0. 5 - 0. 001 A). • Random destruction leads to radical formation. • Used only in very special cases, e. g. solid phase polymerization 43

4. 5 Plasma (等离子体引发) 4. 6 Microwave (微波引发) • = 1 m-1 mm • accelerate the polymerization rate 44

5. Polymerization Rate 45

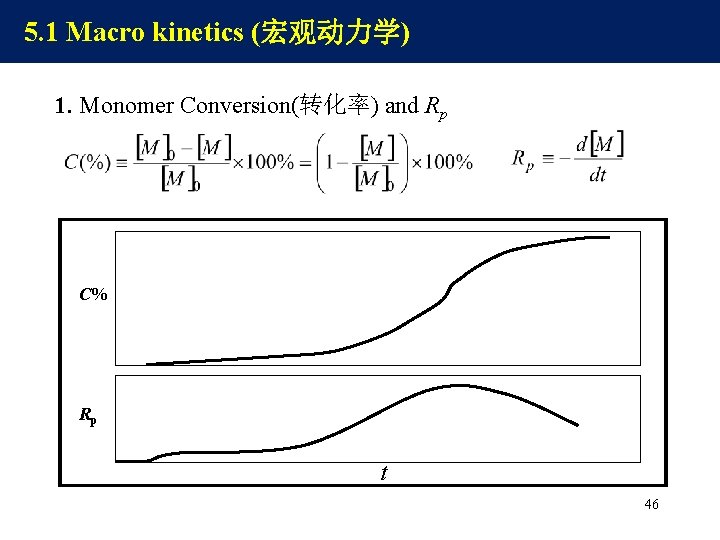
5. 1 Macro kinetics (宏观动力学) 1. Monomer Conversion(转化率) and Rp C% Rp t 46

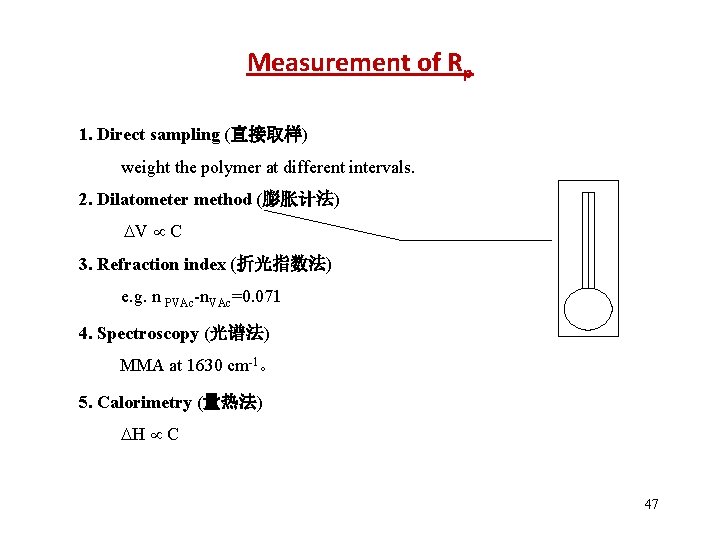
Measurement of Rp 1. Direct sampling (直接取样) weight the polymer at different intervals. 2. Dilatometer method (膨胀计法) V C 3. Refraction index (折光指数法) e. g. n PVAc-n. VAc=0. 071 4. Spectroscopy (光谱法) MMA at 1630 cm-1。 5. Calorimetry (量热法) H C 47

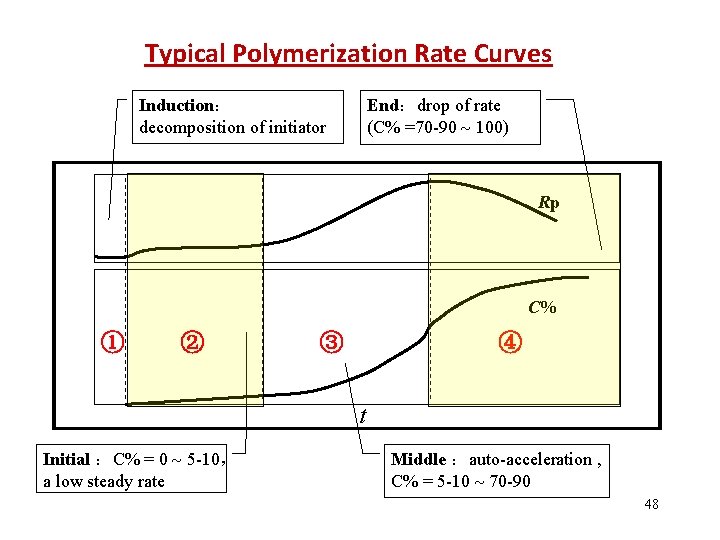
Typical Polymerization Rate Curves Induction: decomposition of initiator End:drop of rate (C% =70 -90 100) Rp C% ① ② ③ ④ t Initial :C% = 0 5 -10, a low steady rate Middle :auto-acceleration , C% = 5 -10 70 -90 48

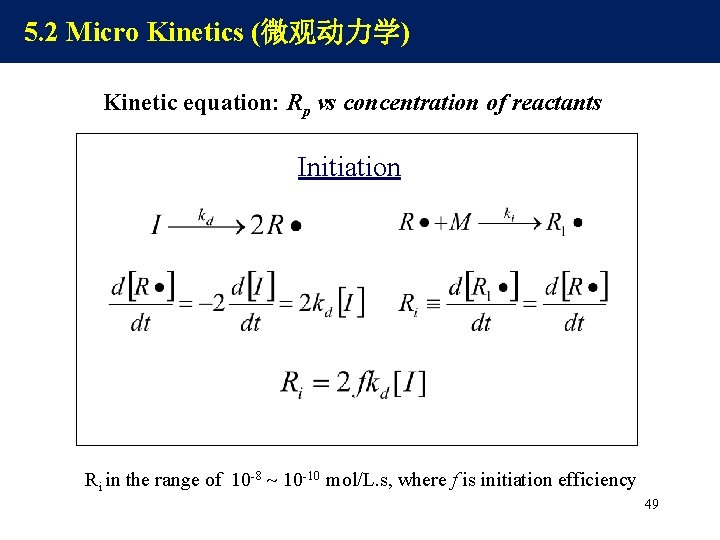
5. 2 Micro Kinetics (微观动力学) Kinetic equation: Rp vs concentration of reactants Initiation Ri in the range of 10 -8 10 -10 mol/L. s, where f is initiation efficiency 49

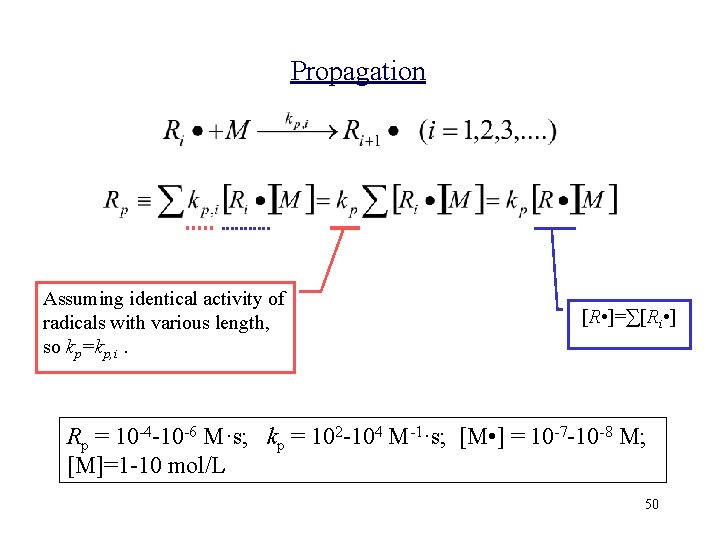
Propagation Assuming identical activity of radicals with various length, so kp=kp, i. [R • ]=∑[Ri • ] Rp = 10 -4 -10 -6 M·s; kp = 102 -104 M-1·s; [M • ] = 10 -7 -10 -8 M; [M]=1 -10 mol/L 50

disproportionation combination Termination 2 represents two radicals were consumed by one reaction (US) kt = kt, c + kt, d 51

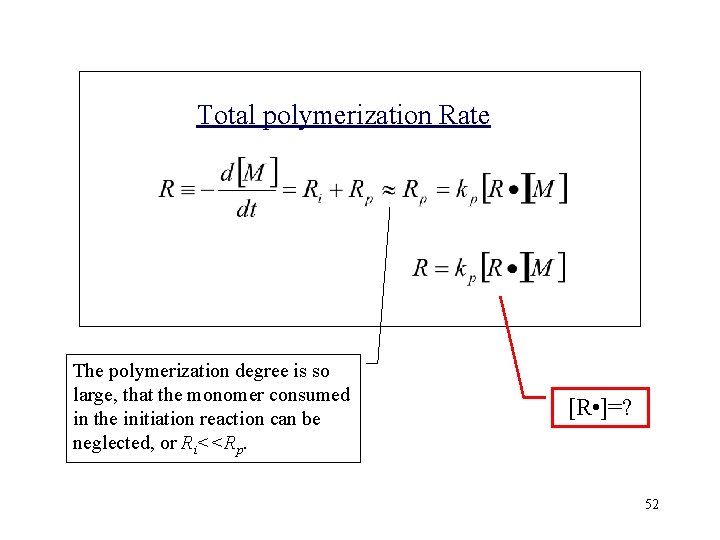
Total polymerization Rate The polymerization degree is so large, that the monomer consumed in the initiation reaction can be neglected, or Ri<<Rp. [R • ]=? 52

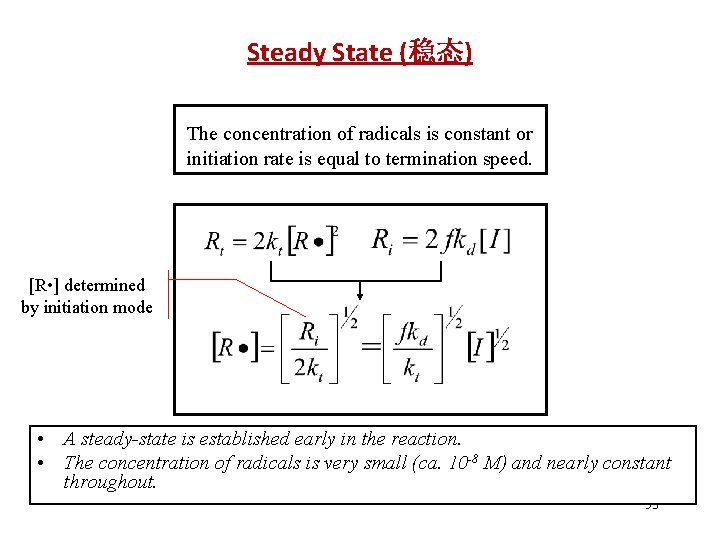
Steady State (稳态) The concentration of radicals is constant or initiation rate is equal to termination speed. [R • ] determined by initiation mode • A steady-state is established early in the reaction. • The concentration of radicals is very small (ca. 10 -8 M) and nearly constant throughout. 53
![Polymerization rate with different initiations Initiator lnM0M t low conversion I and f are Polymerization rate with different initiations Initiator ln([M]0/[M]) t: low conversion; [I] and f are](https://slidetodoc.com/presentation_image/340171e4d6457776aed20f8ba4edef20/image-54.jpg)
Polymerization rate with different initiations Initiator ln([M]0/[M]) t: low conversion; [I] and f are constant Self-initiation of Styrene 54

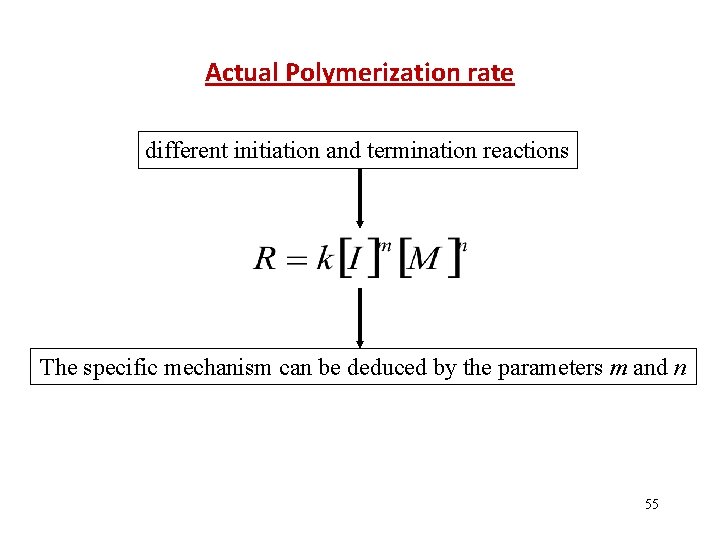
Actual Polymerization rate different initiation and termination reactions The specific mechanism can be deduced by the parameters m and n 55

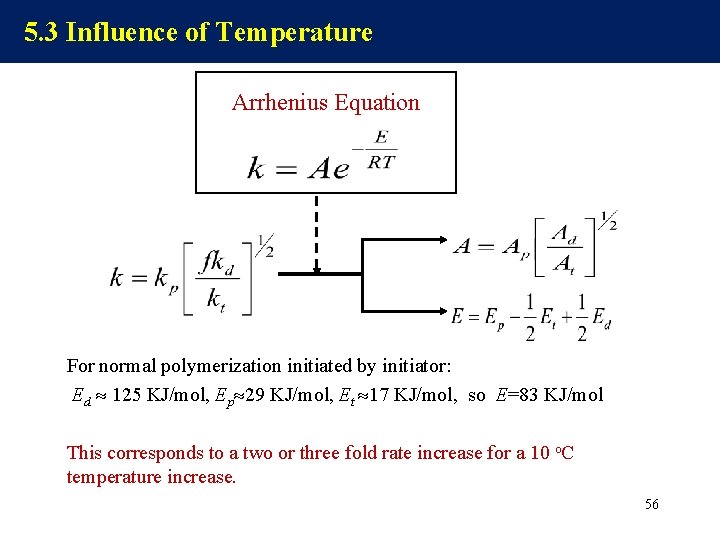
5. 3 Influence of Temperature Arrhenius Equation For normal polymerization initiated by initiator: Ed 125 KJ/mol, Ep 29 KJ/mol, Et 17 KJ/mol, so E=83 KJ/mol This corresponds to a two or three fold rate increase for a 10 o. C temperature increase. 56

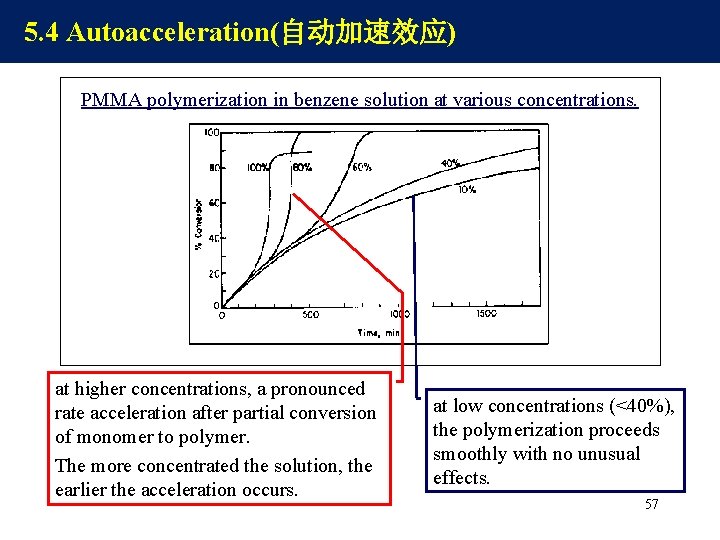
5. 4 Autoacceleration(自动加速效应) PMMA polymerization in benzene solution at various concentrations. at higher concentrations, a pronounced rate acceleration after partial conversion of monomer to polymer. The more concentrated the solution, the earlier the acceleration occurs. at low concentrations (<40%), the polymerization proceeds smoothly with no unusual effects. 57

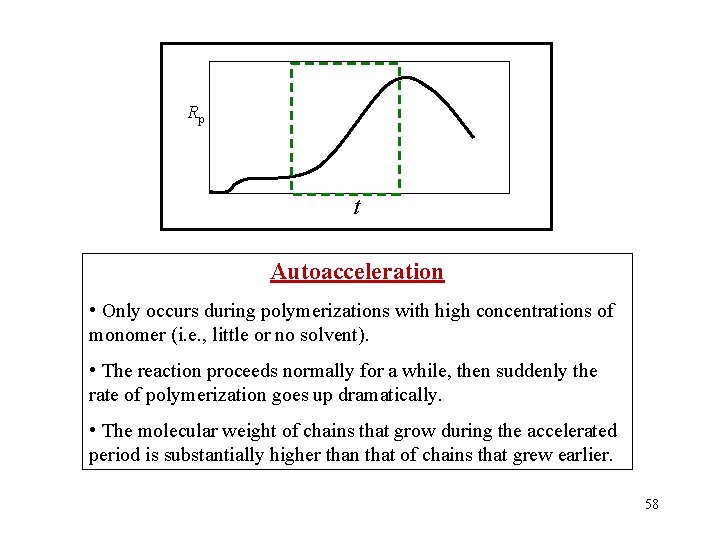
Rp t Autoacceleration • Only occurs during polymerizations with high concentrations of monomer (i. e. , little or no solvent). • The reaction proceeds normally for a while, then suddenly the rate of polymerization goes up dramatically. • The molecular weight of chains that grow during the accelerated period is substantially higher than that of chains that grew earlier. 58

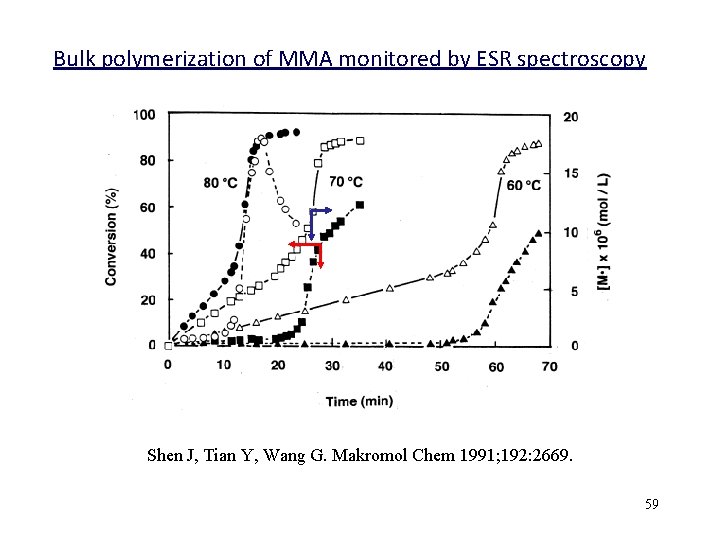
Bulk polymerization of MMA monitored by ESR spectroscopy Shen J, Tian Y, Wang G. Makromol Chem 1991; 192: 2669. 59

Reasons and Explanation • Termination involves the reaction between two chain ends. The high viscosity hinders the diffusion of chains because of entanglements, so the rate of termination slows considerably. • The initiation and diffusion of small molecular monomers is hardly affected by viscosity, so propagation proceeds as before. • Once a low, steady state concentration of radicals gives way to increasing concentration, chains grow without termination, so the conversion is rapid and the MW is high. • For neat monomer, often in cases where the polymer formed is a high Tg material, there can come a point at which even the diffusion of monomer is slow. The mixture has become a hard glass, and unreacted radicals become trapped inside. The reaction shuts down at less than 100% conversion. • Note that autoacceleration can be dangerous because the exotherm of polymerization can be released suddenly, leading to a runaway reaction. 60

Conditions affecting autoacceleration • Temperature the higher the Tp, the lower the viscosity • Monomer concentration the content of polymer • Solvent good solvent in favor of the diffusion of polymer chain • Tg of polymer • The homogeneity of polymerization media homogeneous or heterogeneous 61

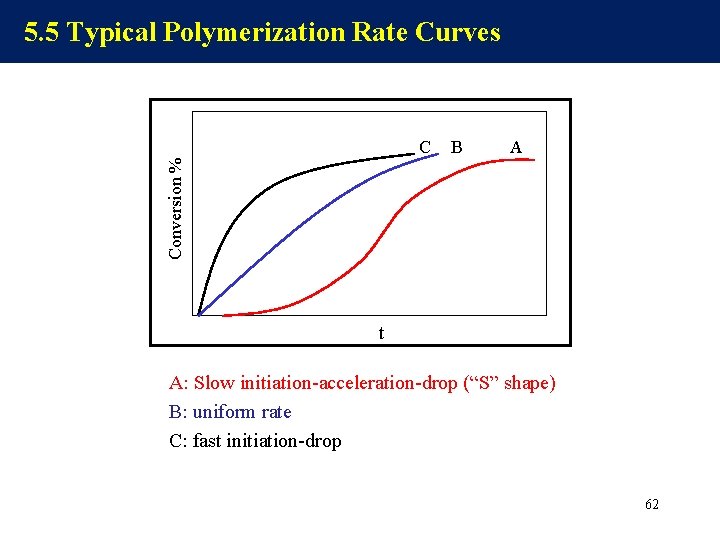
5. 5 Typical Polymerization Rate Curves B A Conversion % C t A: Slow initiation-acceleration-drop (“S” shape) B: uniform rate C: fast initiation-drop 62

6. Average Molecular Weight 63

Average Molecular Weight • Polymer consists of chains with a variable number of monomer units. • Of the key parameters influencing the physical properties of polymers, the average molecular weight usually is the dominant factor, often influencing the other parameters. • Control on the molecular weight is one of the most important topics of polymer synthesis 64

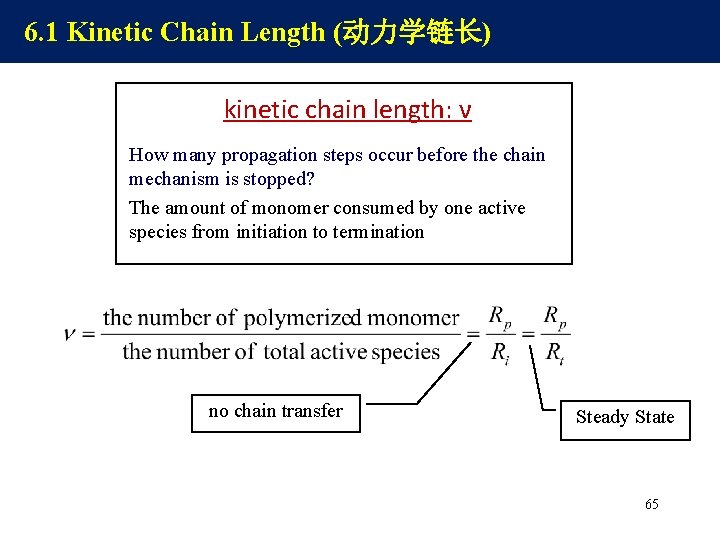
6. 1 Kinetic Chain Length (动力学链长) kinetic chain length: ν How many propagation steps occur before the chain mechanism is stopped? The amount of monomer consumed by one active species from initiation to termination no chain transfer Steady State 65
![kinetic chain length MI0 5 66 kinetic chain length: [M][I]-0. 5 66](https://slidetodoc.com/presentation_image/340171e4d6457776aed20f8ba4edef20/image-66.jpg)
kinetic chain length: [M][I]-0. 5 66

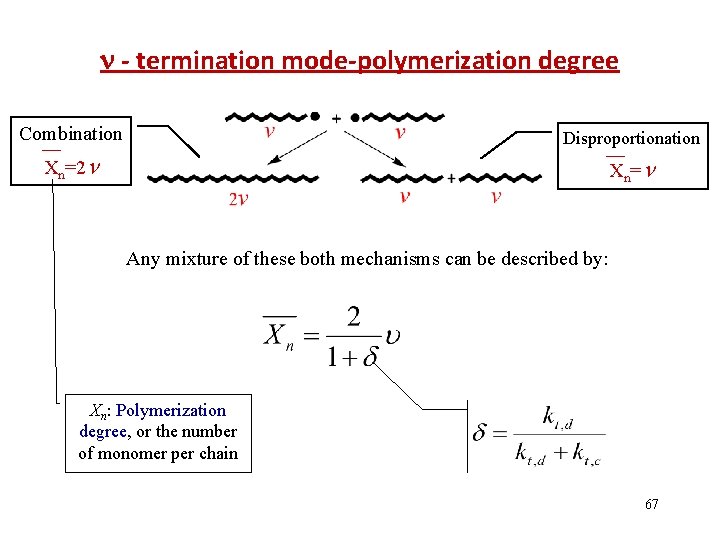
- termination mode-polymerization degree Combination Disproportionation Xn=2 Xn = Any mixture of these both mechanisms can be described by: Xn: Polymerization degree, or the number of monomer per chain 67

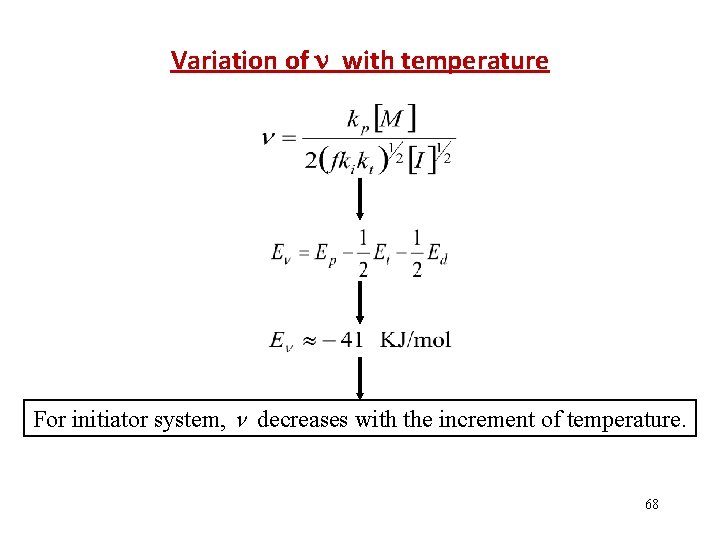
Variation of with temperature For initiator system, decreases with the increment of temperature. 68

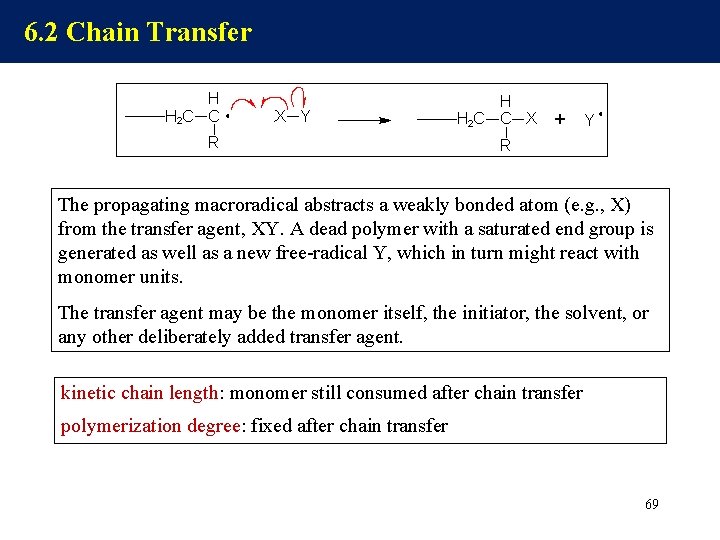
6. 2 Chain Transfer The propagating macroradical abstracts a weakly bonded atom (e. g. , X) from the transfer agent, XY. A dead polymer with a saturated end group is generated as well as a new free-radical Y, which in turn might react with monomer units. The transfer agent may be the monomer itself, the initiator, the solvent, or any other deliberately added transfer agent. kinetic chain length: monomer still consumed after chain transfer polymerization degree: fixed after chain transfer 69

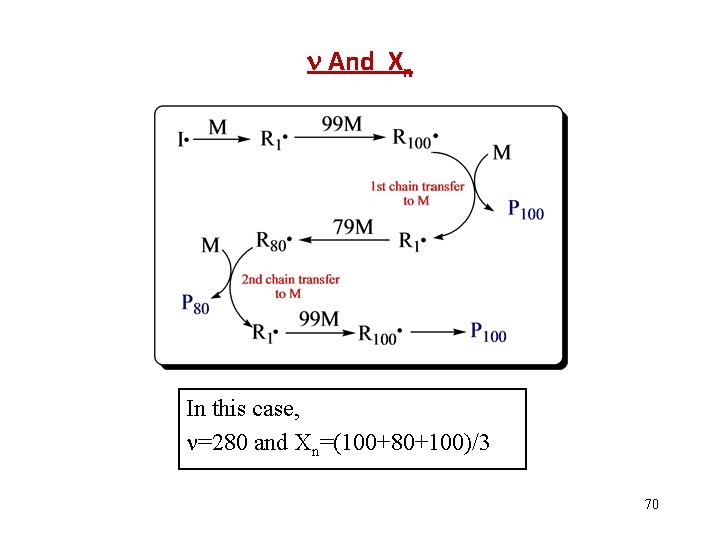
And Xn In this case, =280 and Xn=(100+80+100)/3 70

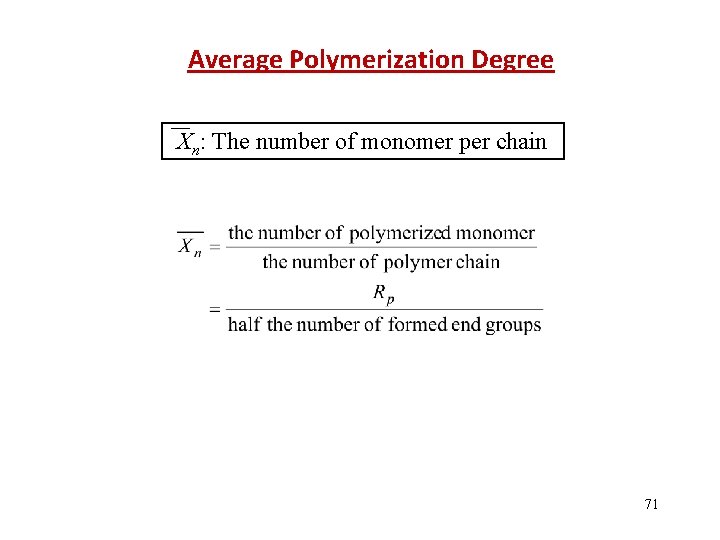
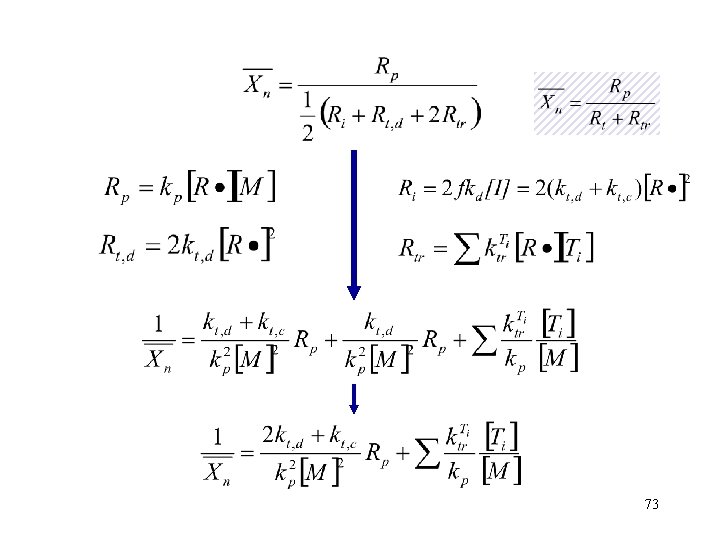
Average Polymerization Degree Xn: The number of monomer per chain 71

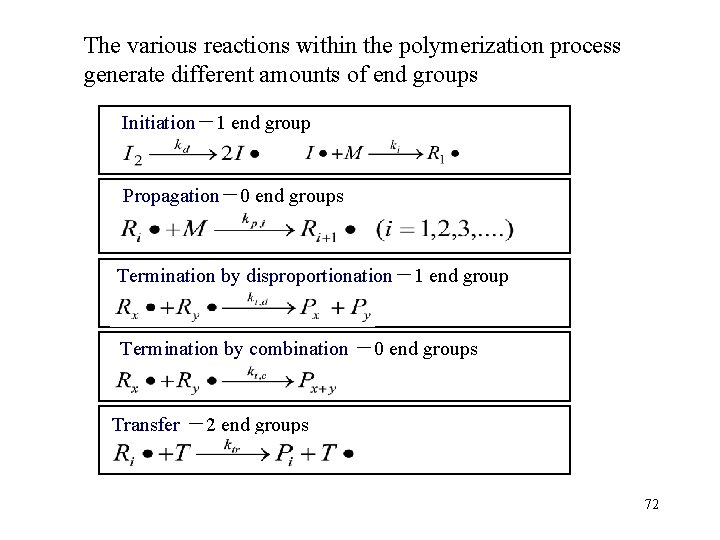
The various reactions within the polymerization process generate different amounts of end groups Initiation-1 end group Propagation-0 end groups Termination by disproportionation-1 end group Termination by combination -0 end groups Transfer -2 end groups 72

73

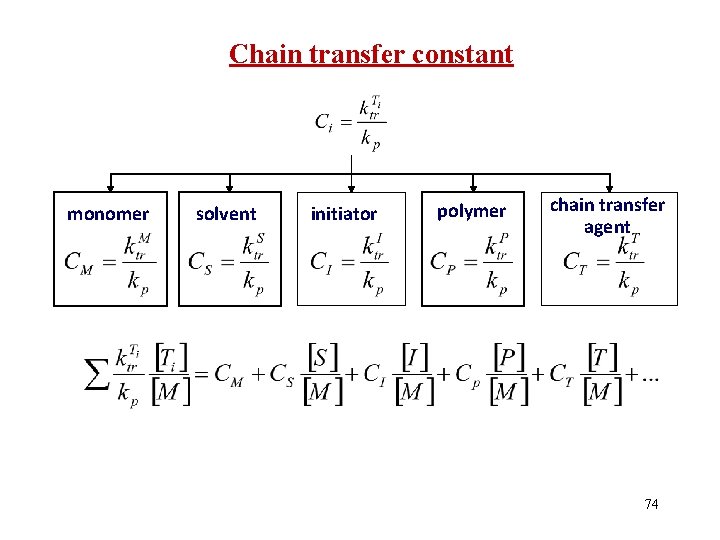
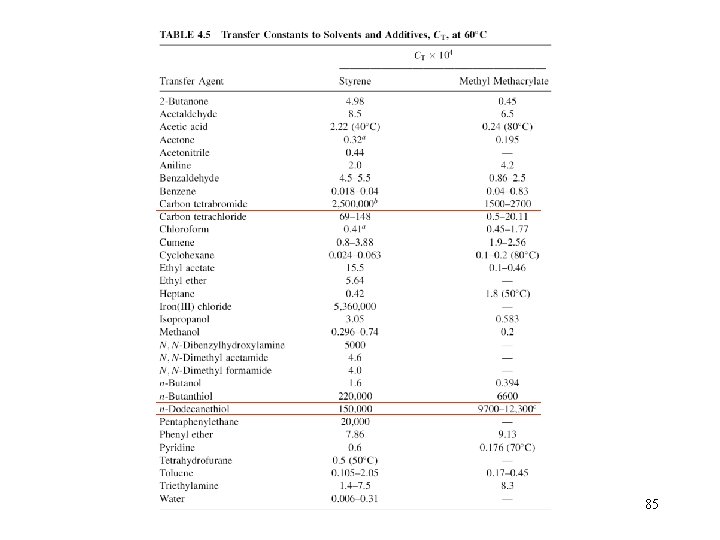
Chain transfer constant monomer solvent initiator polymer chain transfer agent 74

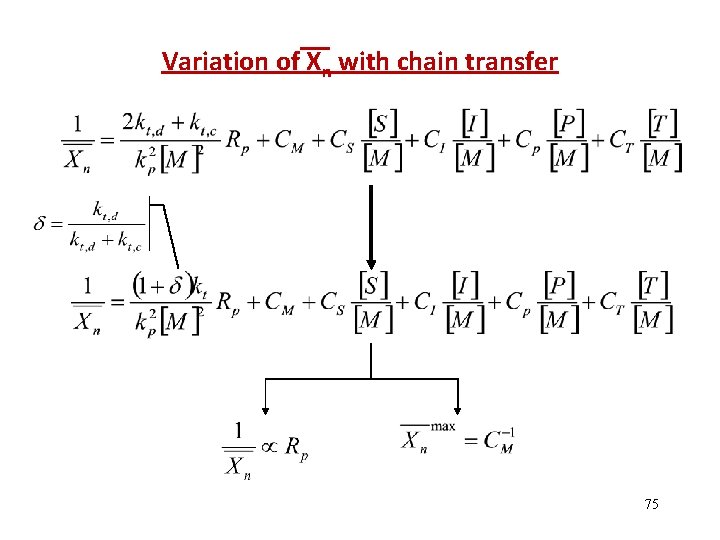
Variation of Xn with chain transfer 75

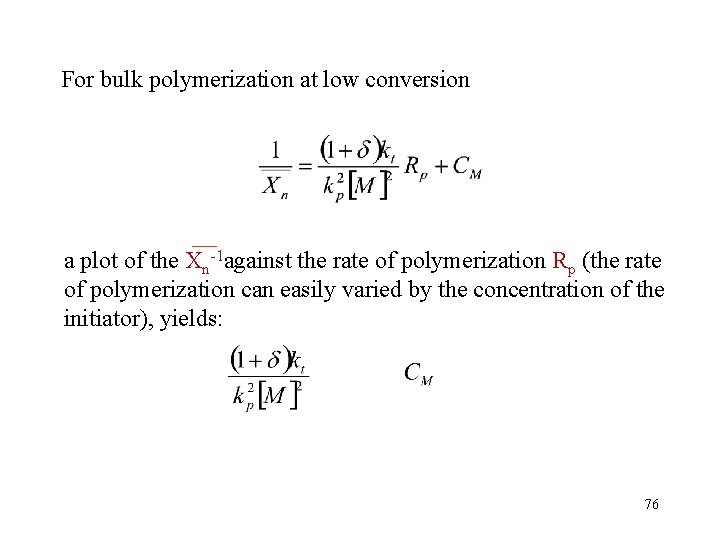
For bulk polymerization at low conversion a plot of the Xn-1 against the rate of polymerization Rp (the rate of polymerization can easily varied by the concentration of the initiator), yields: 76

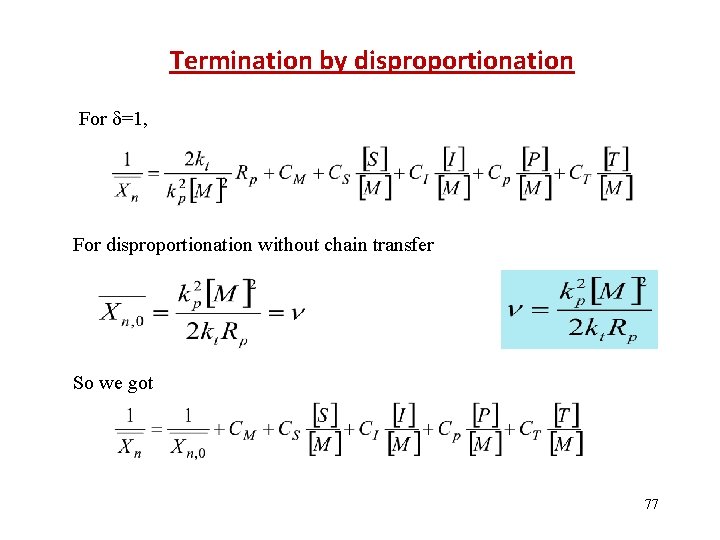
Termination by disproportionation For =1, For disproportionation without chain transfer So we got 77

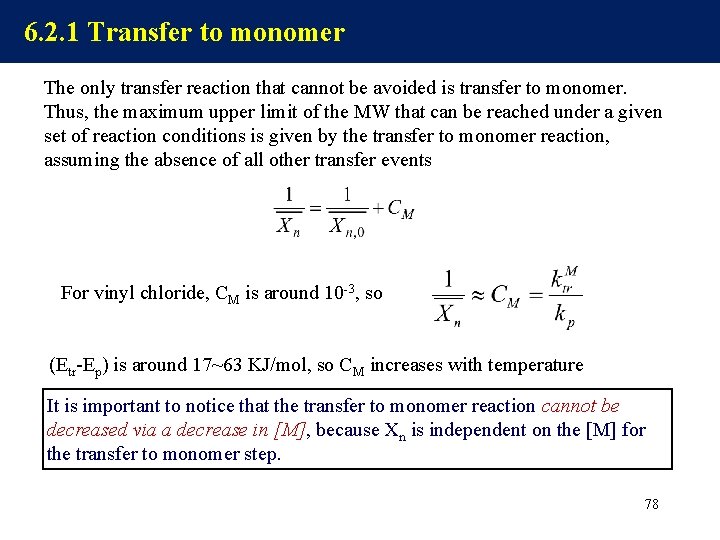
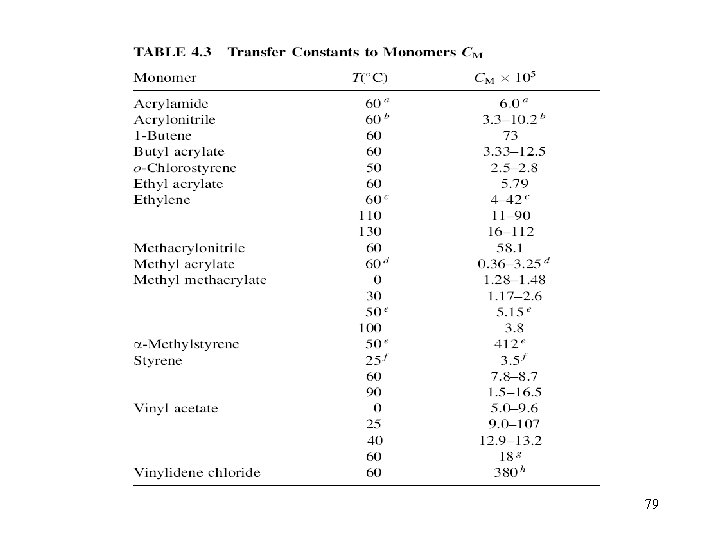
6. 2. 1 Transfer to monomer The only transfer reaction that cannot be avoided is transfer to monomer. Thus, the maximum upper limit of the MW that can be reached under a given set of reaction conditions is given by the transfer to monomer reaction, assuming the absence of all other transfer events For vinyl chloride, CM is around 10 -3, so (Etr-Ep) is around 17~63 KJ/mol, so CM increases with temperature It is important to notice that the transfer to monomer reaction cannot be decreased via a decrease in [M], because Xn is independent on the [M] for the transfer to monomer step. 78

79

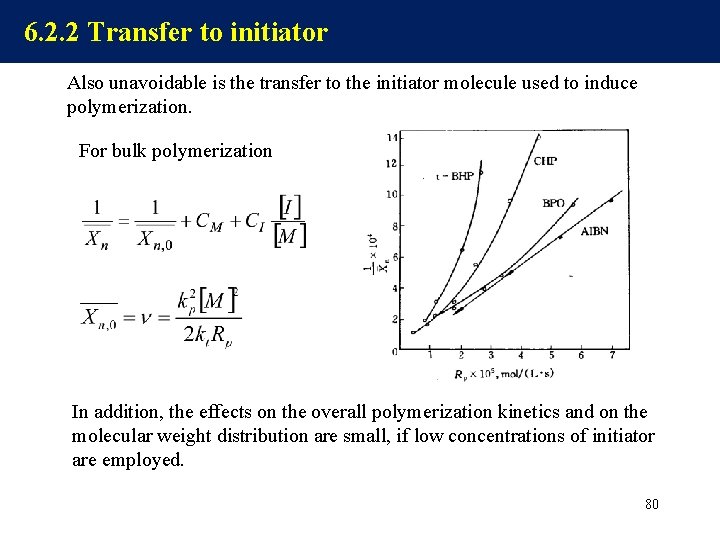
6. 2. 2 Transfer to initiator Also unavoidable is the transfer to the initiator molecule used to induce polymerization. For bulk polymerization In addition, the effects on the overall polymerization kinetics and on the molecular weight distribution are small, if low concentrations of initiator are employed. 80

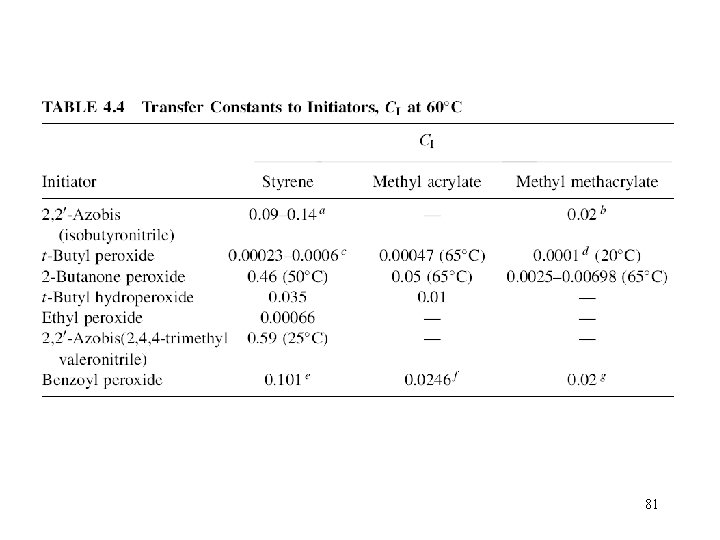
81

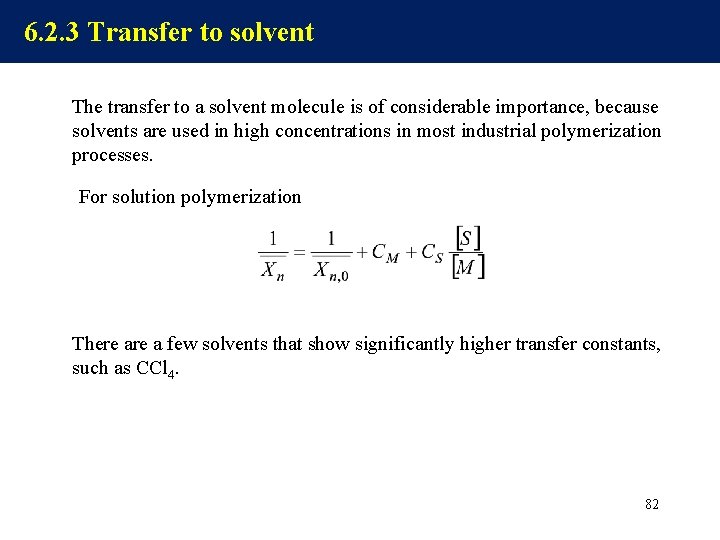
6. 2. 3 Transfer to solvent The transfer to a solvent molecule is of considerable importance, because solvents are used in high concentrations in most industrial polymerization processes. For solution polymerization There a few solvents that show significantly higher transfer constants, such as CCl 4. 82

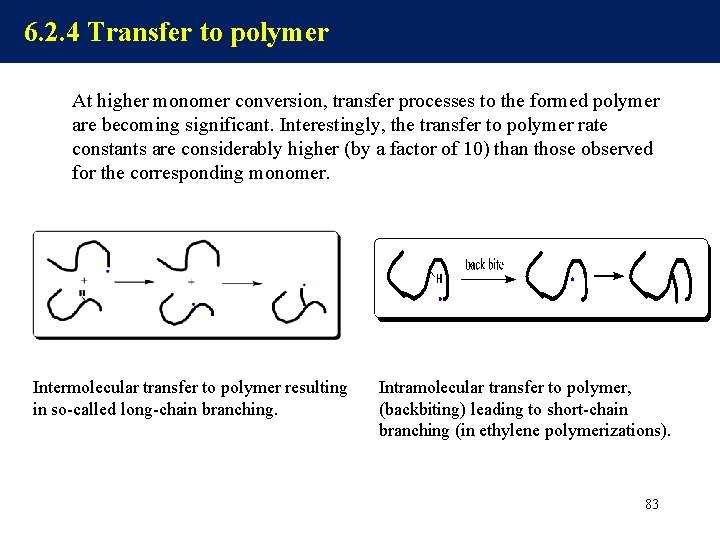
6. 2. 4 Transfer to polymer At higher monomer conversion, transfer processes to the formed polymer are becoming significant. Interestingly, the transfer to polymer rate constants are considerably higher (by a factor of 10) than those observed for the corresponding monomer. Intermolecular transfer to polymer resulting in so-called long-chain branching. Intramolecular transfer to polymer, (backbiting) leading to short-chain branching (in ethylene polymerizations). 83

6. 2. 5 Transfer to additives Typical agents with very high transfer constants are thiols and halogenated compounds such as CBr 4. • Chain transfer agents with chain transfer constants greater than one are very useful, as they can be employed in low concentrations. • These agents are important for industrial processes, because they allow for the regulation of the molecular weight of the generated polymer and thus significantly reducing the viscosity of the reaction medium and allowing for an optimum heat transfer. 84

85

7. Inhibition and Retardation 86

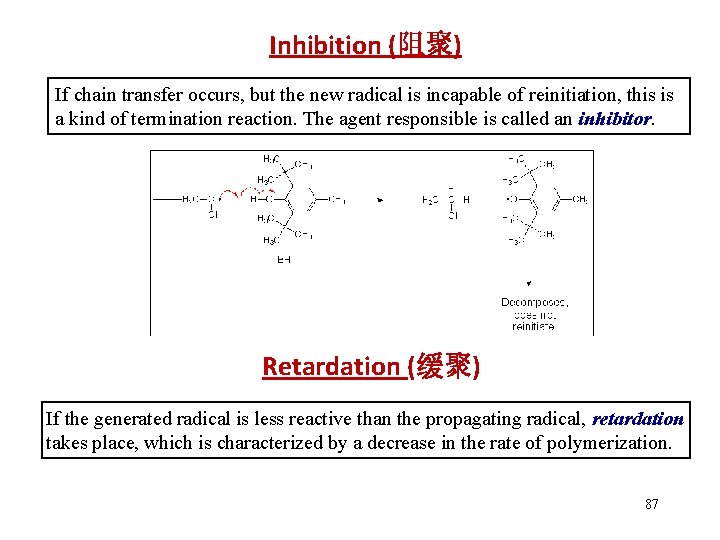
Inhibition (阻聚) If chain transfer occurs, but the new radical is incapable of reinitiation, this is a kind of termination reaction. The agent responsible is called an inhibitor. Retardation (缓聚) If the generated radical is less reactive than the propagating radical, retardation takes place, which is characterized by a decrease in the rate of polymerization. 87

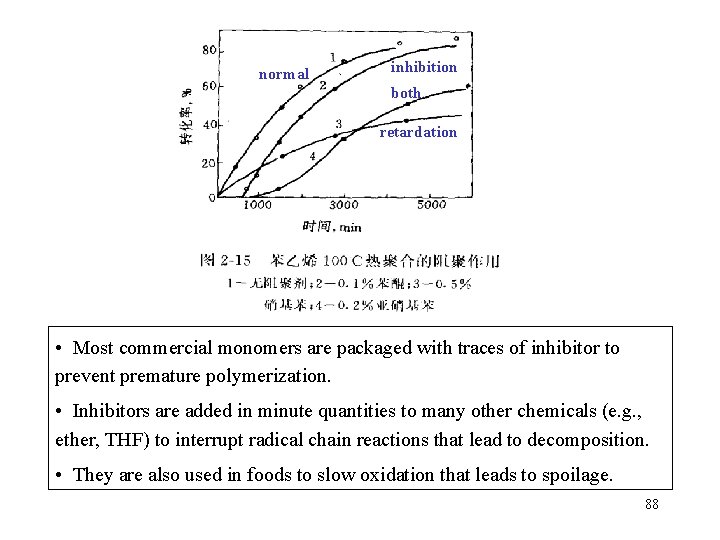
normal inhibition both retardation • Most commercial monomers are packaged with traces of inhibitor to prevent premature polymerization. • Inhibitors are added in minute quantities to many other chemicals (e. g. , ether, THF) to interrupt radical chain reactions that lead to decomposition. • They are also used in foods to slow oxidation that leads to spoilage. 88

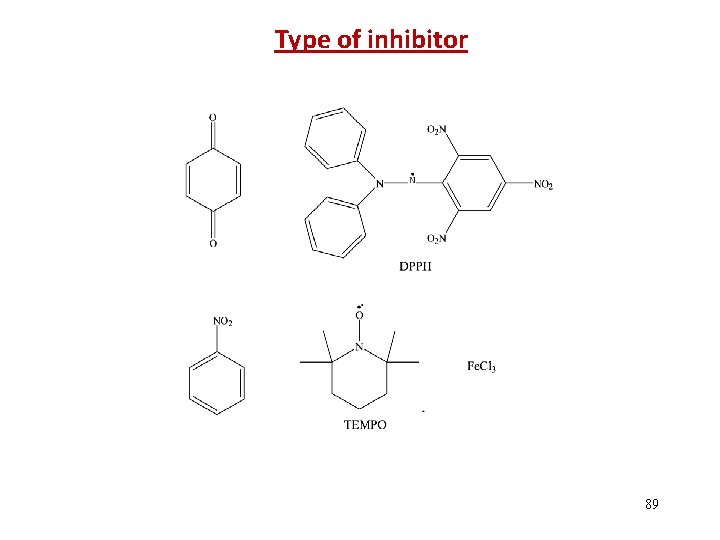
Type of inhibitor 89

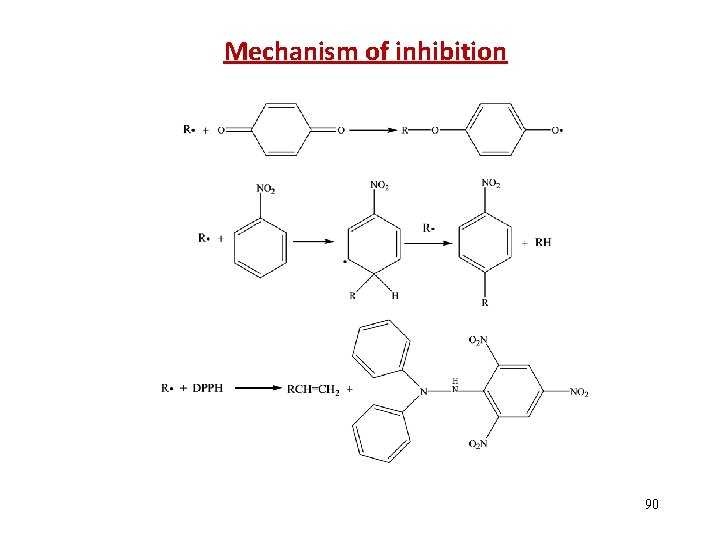
Mechanism of inhibition 90

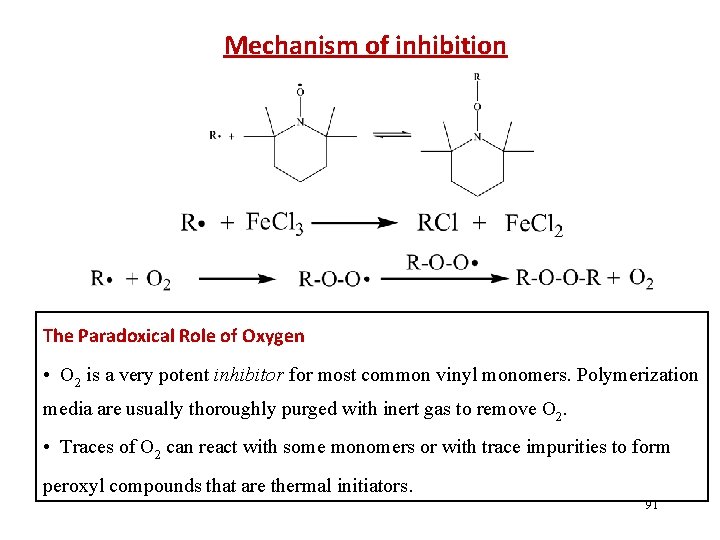
Mechanism of inhibition The Paradoxical Role of Oxygen • O 2 is a very potent inhibitor for most common vinyl monomers. Polymerization media are usually thoroughly purged with inert gas to remove O 2. • Traces of O 2 can react with some monomers or with trace impurities to form peroxyl compounds that are thermal initiators. 91

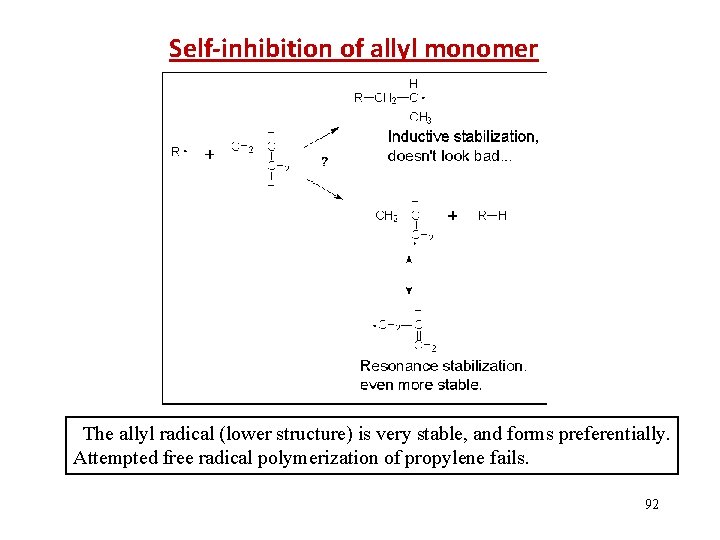
Self-inhibition of allyl monomer The allyl radical (lower structure) is very stable, and forms preferentially. Attempted free radical polymerization of propylene fails. 92

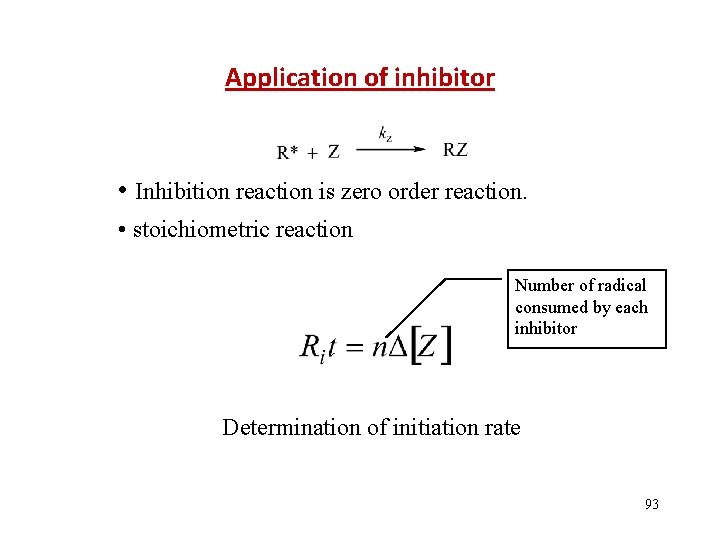
Application of inhibitor • Inhibition reaction is zero order reaction. • stoichiometric reaction Number of radical consumed by each inhibitor Determination of initiation rate 93

8. Measurement of rate coefficients 94

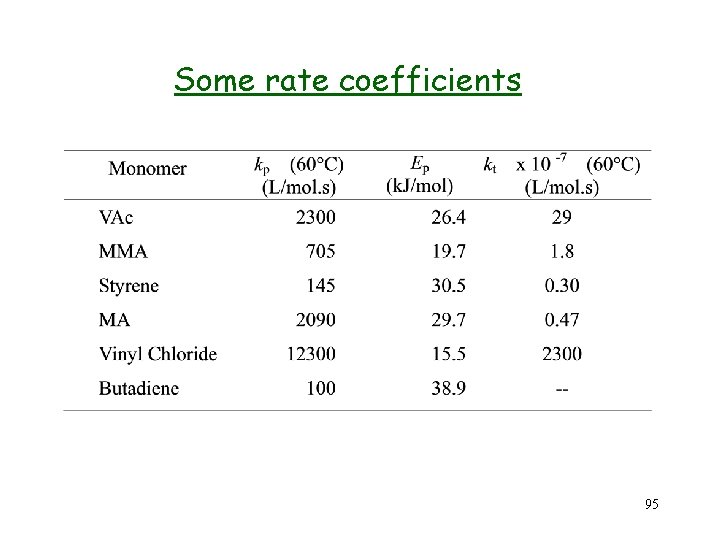
Some rate coefficients 95

9. Molecular Weight Distribution 96

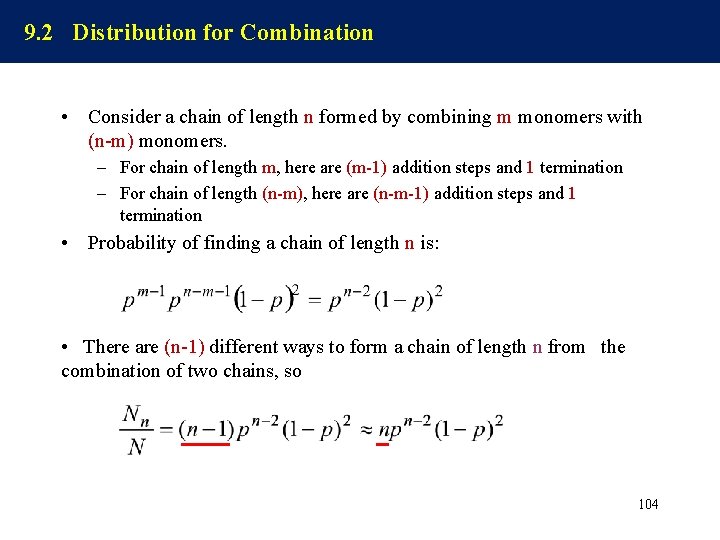
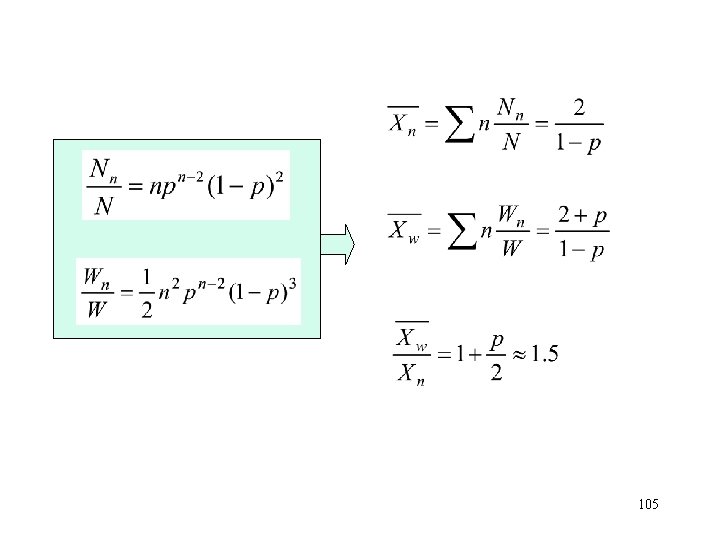
How to know MWD? • From the experimental measurement – Precipitation fractionation (沉淀分级) – Gel permeation chromatograph/Size exclusion chromatograph (GPC/SEC 凝胶渗透色谱/体积排 斥色谱) – MALDI-TOF • Form theoretical Analysis – Statistical approach (统计方法) – Kinetic approach (动力学法) 97

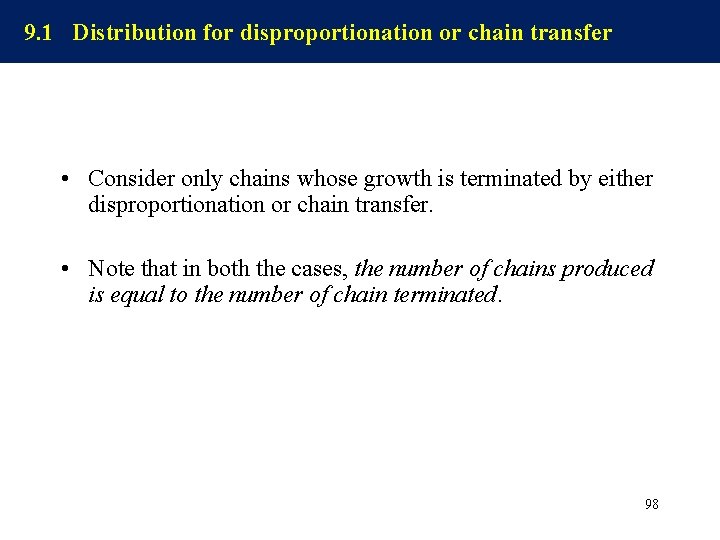
9. 1 Distribution for disproportionation or chain transfer • Consider only chains whose growth is terminated by either disproportionation or chain transfer. • Note that in both the cases, the number of chains produced is equal to the number of chain terminated. 98

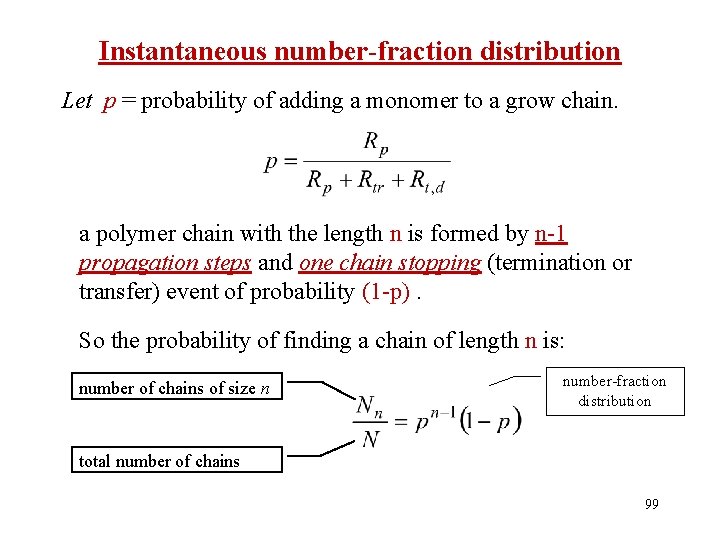
Instantaneous number-fraction distribution Let p = probability of adding a monomer to a grow chain. a polymer chain with the length n is formed by n-1 propagation steps and one chain stopping (termination or transfer) event of probability (1 -p). So the probability of finding a chain of length n is: number of chains of size n number-fraction distribution total number of chains 99

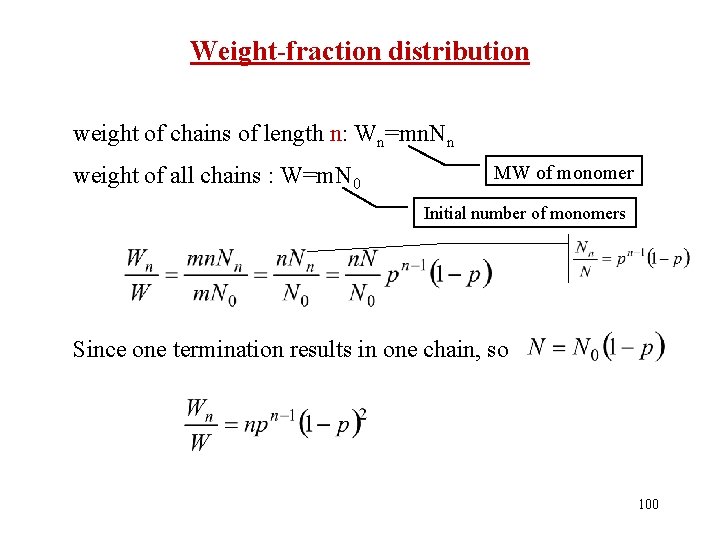
Weight-fraction distribution weight of chains of length n: Wn=mn. Nn weight of all chains : W=m. N 0 MW of monomer Initial number of monomers Since one termination results in one chain, so 100

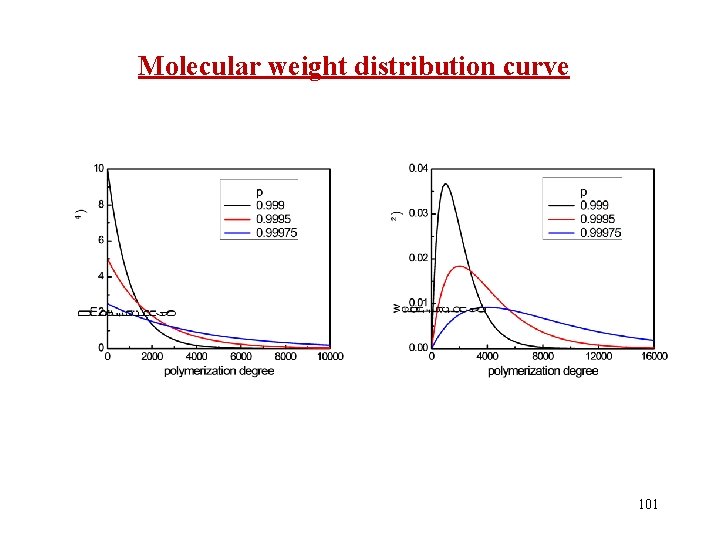
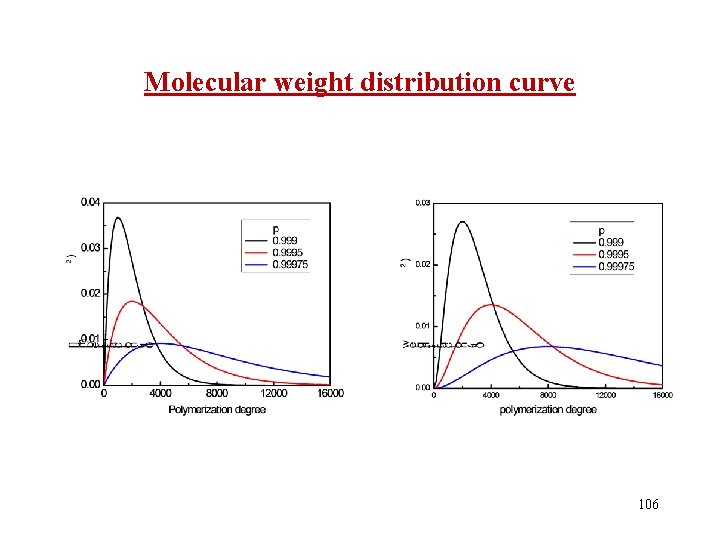
Molecular weight distribution curve 101

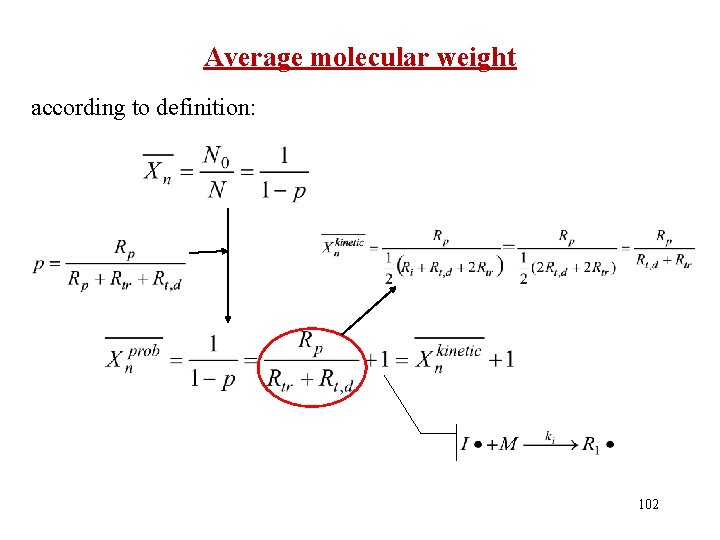
Average molecular weight according to definition: 102

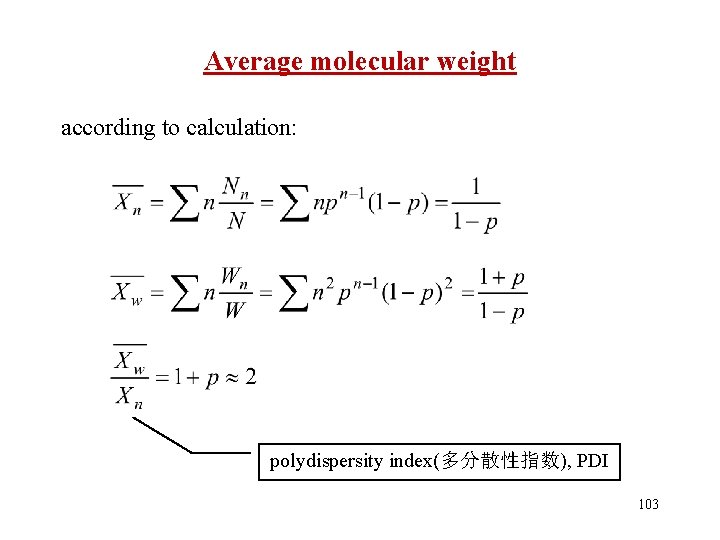
Average molecular weight according to calculation: polydispersity index(多分散性指数), PDI 103

9. 2 Distribution for Combination • Consider a chain of length n formed by combining m monomers with (n-m) monomers. – For chain of length m, here are (m-1) addition steps and 1 termination – For chain of length (n-m), here are (n-m-1) addition steps and 1 termination • Probability of finding a chain of length n is: • There are (n-1) different ways to form a chain of length n from the combination of two chains, so 104

105

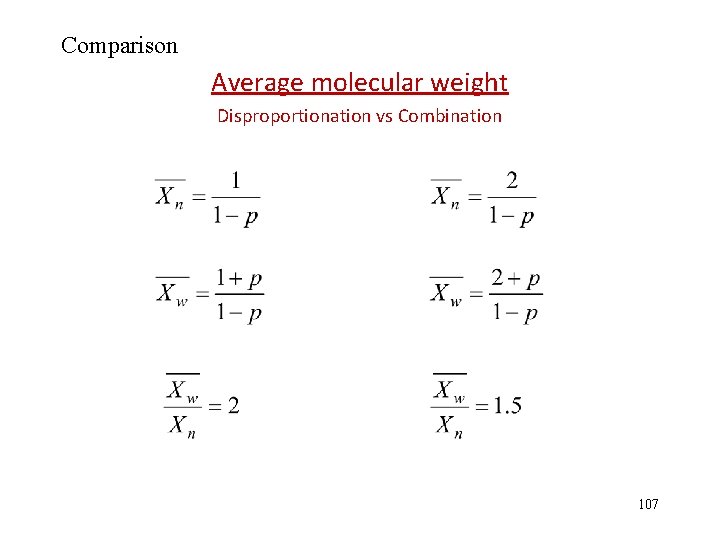
Molecular weight distribution curve 106

Comparison Average molecular weight Disproportionation vs Combination 107

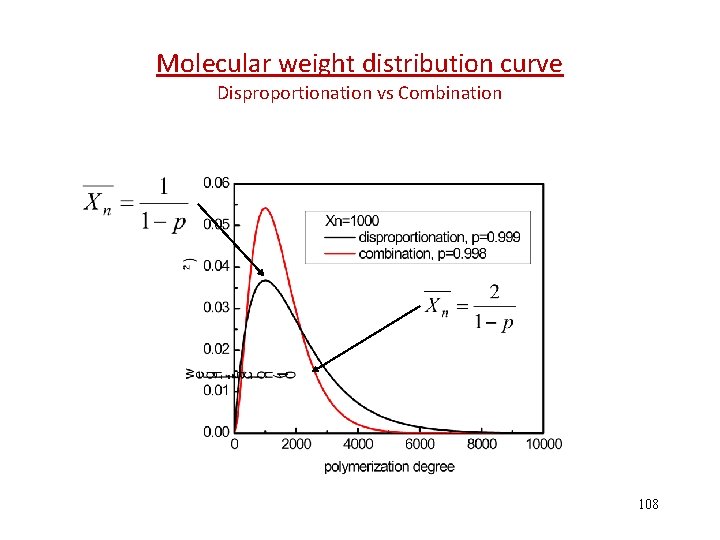
Molecular weight distribution curve Disproportionation vs Combination 108

10. Polymerization Thermodynamics 109

Thermodynamics • Polymerization possibility – Is the monomer polymerizable? • Polymerization equilibrium – Polymerization or depolymerization ? 110

• G<0 from monomer to polymer • G>0 from polymer to monomer • G=0 equilibrium between monomer and polymer 111

enthalpy contribution H • all chain polymerizations are exothermic. • most free-radical polymerizations are negative with typical values ranging from 30 to 80 k. J/mol. entropy contribution S • S is always negative, reflecting the loss of degrees of freedom of the monomer becoming a part of the polymer chain. • S is around -105~-125 J/(mol*K) • for polymerization from R. T. to 100 o. C, -T S is around 30~42 KJ Polymerization is mainly determined by H 112

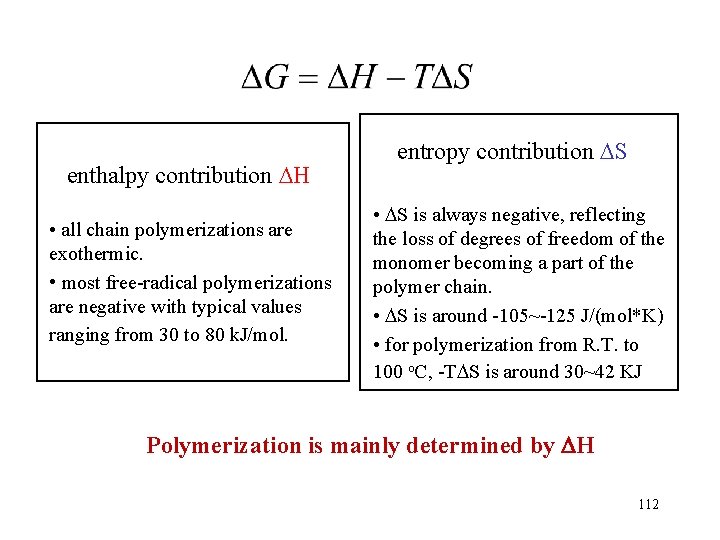
Polymerization heat Steric effect: - H Resonance effect: - H Styrene(69. 9) butadiene(72. 8) - Negative substitute: - H vinyl chloride(95. 8) vinylidene fluoride(129. 7) acrylonitrile (72. 4) H bond & solvent: - H acrylic acid(66. 9) methacrylic acid(42. 3) tetrafluoroethylene(154. 8) 113

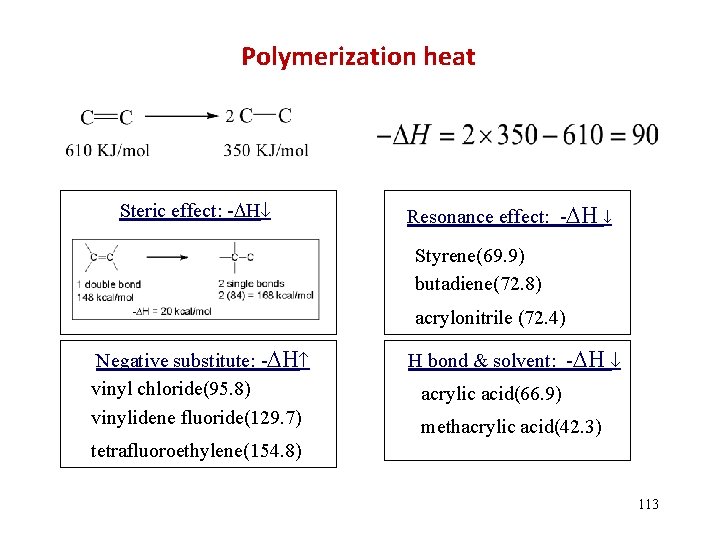
Data of H and S for some monomers (25 C) monomer ethylene propylene butylene-1 isobutylene isoprene butadiene styrene -methylstyrene tetrafluroethylene vinylchloride methly methacrylate acrylic acid acrylamide - H (k. J/mol) 95. 0 85. 8 79. 5 51. 5 72. 8 73 69. 9 35. 1 155. 6 95. 6 56. 5 66. 9 82. 0 - S (J/mol·K) 100. 4 116. 3 112. 1 119. 7 85. 8 89. 0 104. 6 103. 8 112. 1 117. 2 114
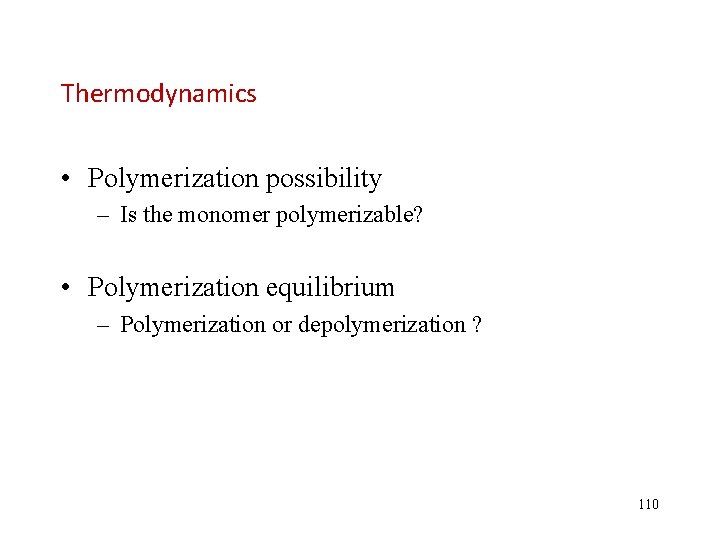
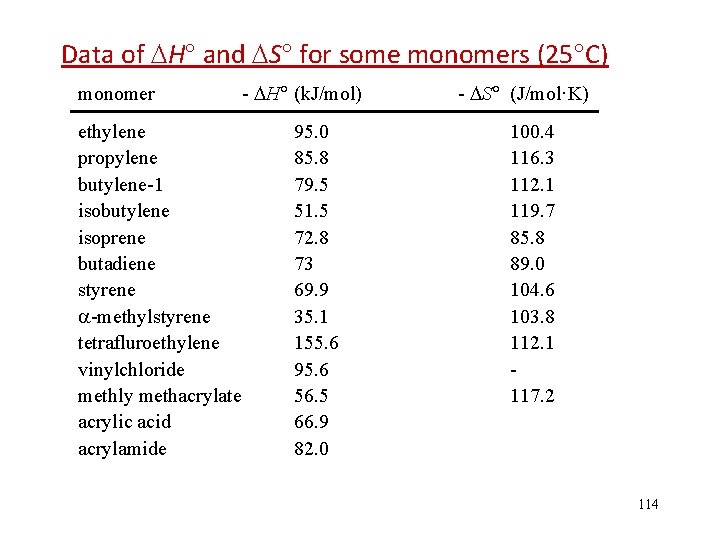
![Ceiling Temperature 聚合上限温度 M1 0 115 Ceiling Temperature (聚合上限温度) [M]=1. 0 115](https://slidetodoc.com/presentation_image/340171e4d6457776aed20f8ba4edef20/image-115.jpg)
Ceiling Temperature (聚合上限温度) [M]=1. 0 115

116
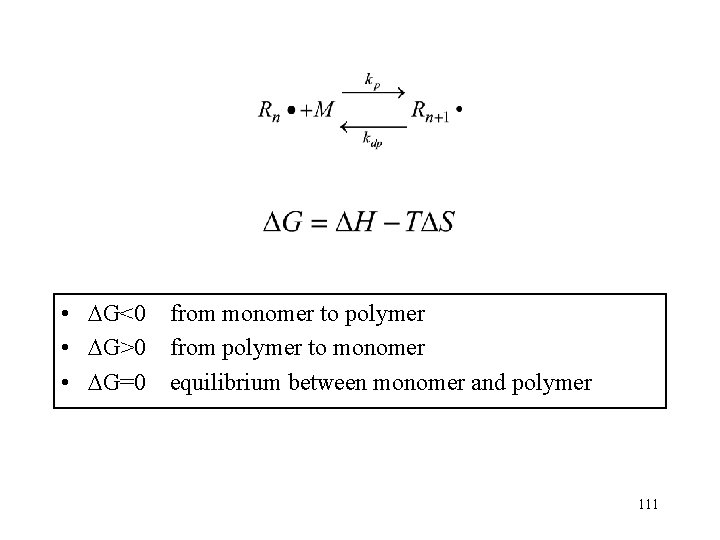
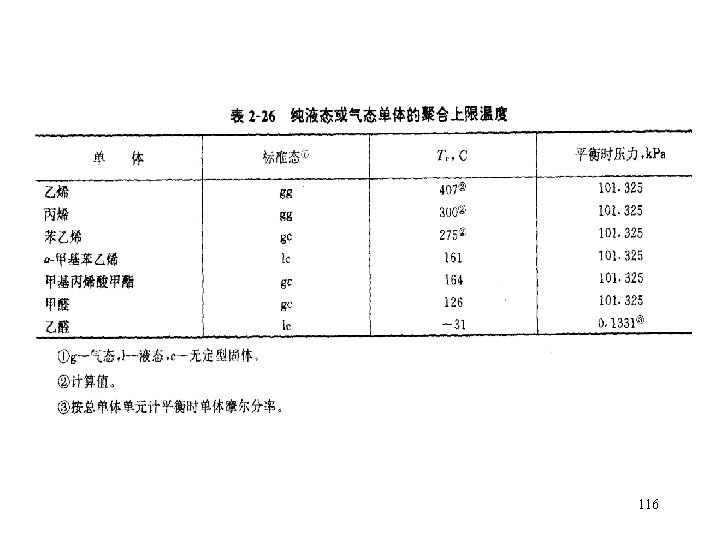
![Equilibrium monomer concentration equil monomer concentration For example MMA Styrene Methylstyrene ΔH ΔS M Equilibrium monomer concentration equil. monomer concentration For example: MMA Styrene -Methylstyrene ΔH° ΔS° [M]](https://slidetodoc.com/presentation_image/340171e4d6457776aed20f8ba4edef20/image-117.jpg)
Equilibrium monomer concentration equil. monomer concentration For example: MMA Styrene -Methylstyrene ΔH° ΔS° [M] at 70 o. C k. J/mol J/(mol. K) 56 73 45 118 104 148 4. 36× 10 -3 2. 09× 10 -6 7. 60 117

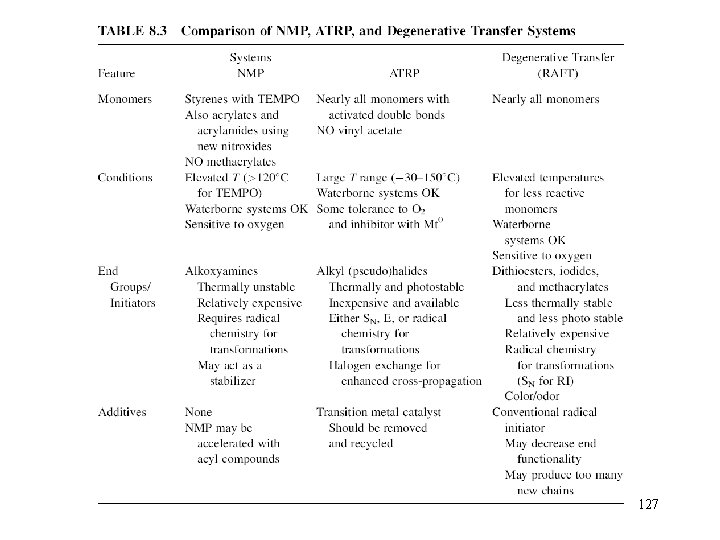
11. Controlled/Living Radical Polymerization 118

Disadvantages of tradition radical Polymerization • Slow and continuous initiation prevents synthesis of welldefined polymers with degrees of polymerization predetermined by the ratio of concentrations of the converted monomer to the introduced initiator, with controlled topologies (stars, combs) and compositions (blocks, grafts, gradients). • The typical lifetime of a propagating chain is very short, in the range of 1 s. • Molecular weight distribution is broad. • Poor control on the regio- and stereoselectivity 119

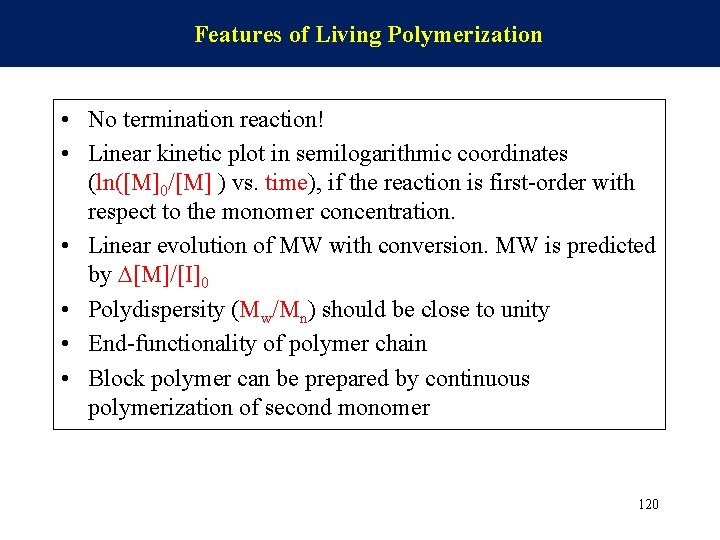
Features of Living Polymerization • No termination reaction! • Linear kinetic plot in semilogarithmic coordinates (ln([M]0/[M] ) vs. time), if the reaction is first-order with respect to the monomer concentration. • Linear evolution of MW with conversion. MW is predicted by [M]/[I]0 • Polydispersity (Mw/Mn) should be close to unity • End-functionality of polymer chain • Block polymer can be prepared by continuous polymerization of second monomer 120

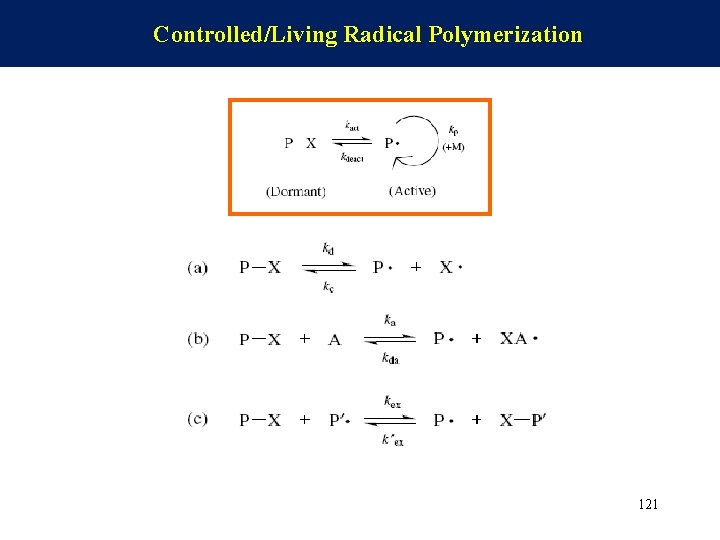
Controlled/Living Radical Polymerization 121

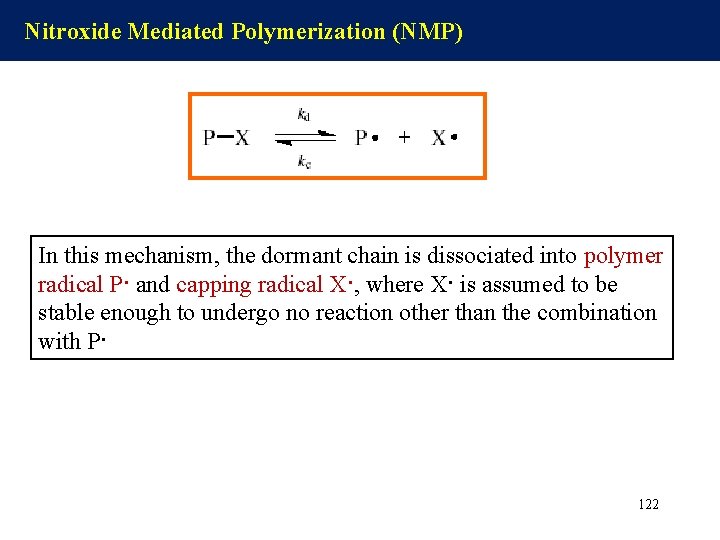
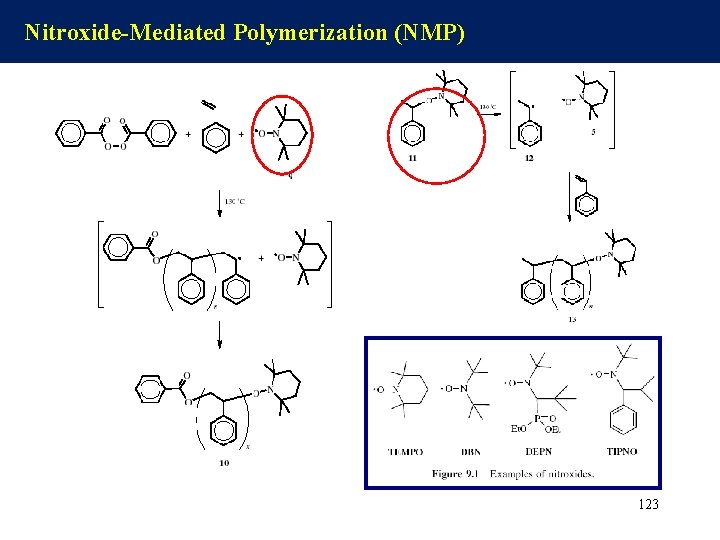
Nitroxide Mediated Polymerization (NMP) In this mechanism, the dormant chain is dissociated into polymer radical P· and capping radical X·, where X· is assumed to be stable enough to undergo no reaction other than the combination with P· 122

Nitroxide-Mediated Polymerization (NMP) 123

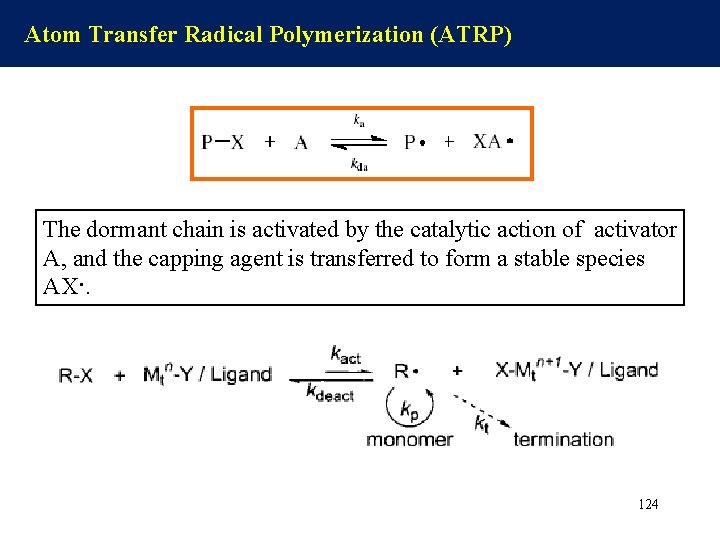
Atom Transfer Radical Polymerization (ATRP) The dormant chain is activated by the catalytic action of activator A, and the capping agent is transferred to form a stable species AX·. 124

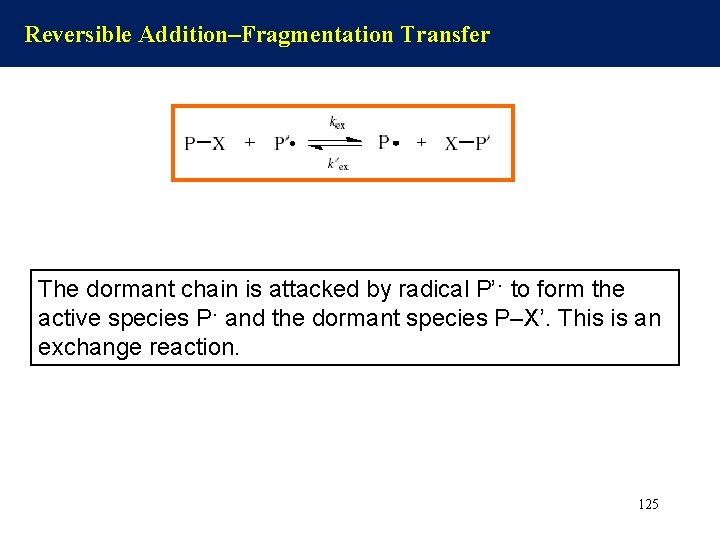
Reversible Addition–Fragmentation Transfer The dormant chain is attacked by radical P’· to form the active species P· and the dormant species P–X’. This is an exchange reaction. 125

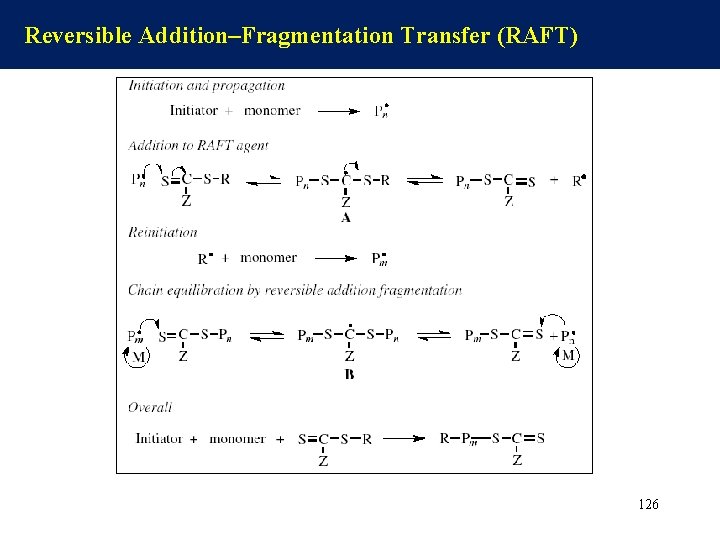
Reversible Addition–Fragmentation Transfer (RAFT) 126

127