Chapter 2 PROPERTIES OF MATTER Matter anything that




































- Slides: 50

Chapter 2 PROPERTIES OF MATTER

Matter anything that has mass and volume Mass: measure of the amount of matter an object contains Volume: measure of space occupied by the object 2

Matter Every sample of matter is Pure Substance ○ Element or Compound Mixture ○ Homogeneous or Heterogeneous

Matter

Elements A substance that cannot be broken down into simpler substances by chemical means Elements are made up of one type of atom Atom: smallest unit of matter that keeps its chemical properties

Elements are represented by an element symbol

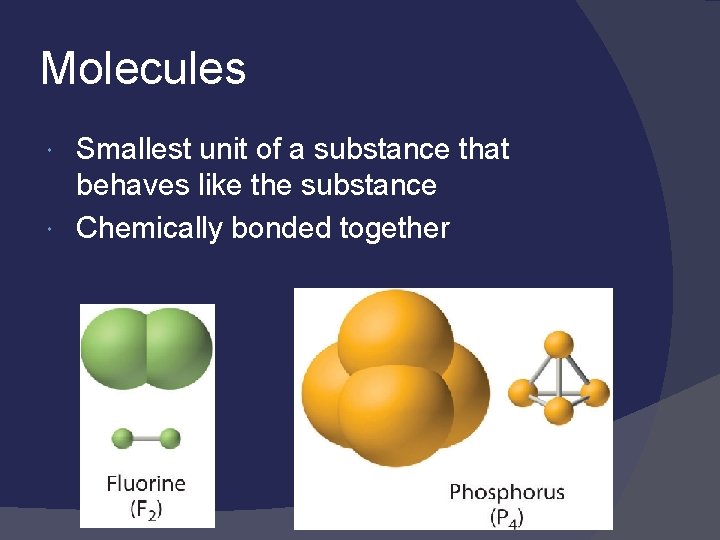
Molecules Smallest unit of a substance that behaves like the substance Chemically bonded together

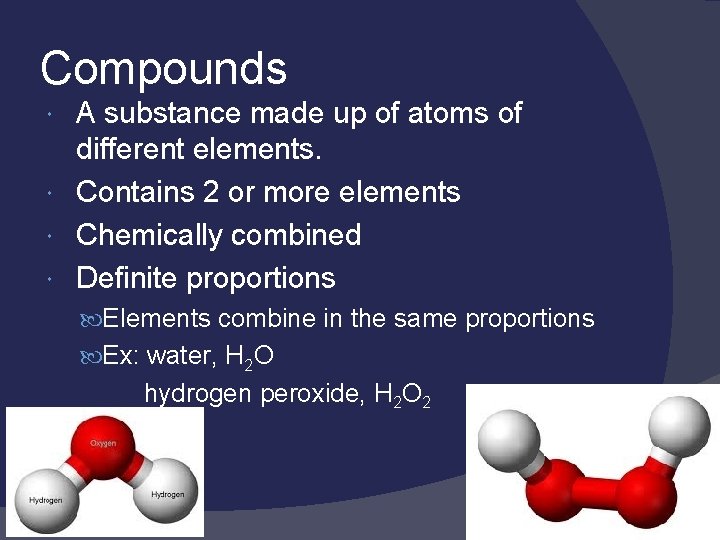
Compounds A substance made up of atoms of different elements. Contains 2 or more elements Chemically combined Definite proportions Elements combine in the same proportions Ex: water, H 2 O hydrogen peroxide, H 2 O 2

Chemical Formula Represent compounds C 12 H 22 O 11 table sugar 3 molecules of table sugar (3 C 12 H 22 O 11) are equivalent to 36 Carbons 66 Hydrogens 22 Oxygens

Pure substances and Mixtures All matter is either a pure substance or a mixture Pure substance Compound or Element Mixture Heterogeneous or Homogenous

Pure substance Element or Compound Matter that has fixed composition and definite properties Table Salt (Na. Cl – sodium chloride)

Mixtures Physical blend of 2 or more compounds Heterogeneous: composition is not uniform throughout (2 or more phases) Homogeneous: composition is uniform throughout (1 phase) aka: solution Phase: any part of a sample with uniform composition

Properties of Matter Extensive: depends on amount of matter Ex: volume, mass Intensive: depends on type of matter Ex: density, m. p. , hardness

Properties of Matter Physical property: can be observed or measured without changing substance’s composition Color, mass, etc. Chemical property: ability of substance to undergo a specific chemical change Flammability, reactivity, etc. Gold vs. Copper

Density is a physical property = Mass per Volume Grams/cubic centimeter (g/cm 3) Grams/ milliliter (g/ml)

Classification of Matter

END of DAY 1

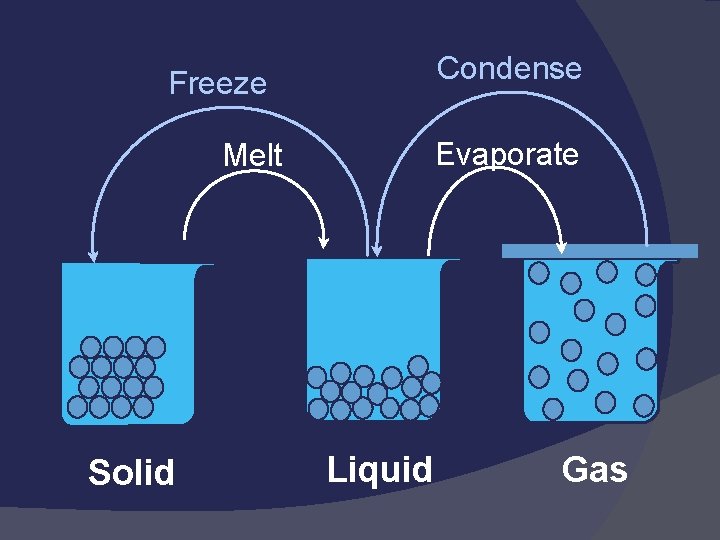
Physical Changes Some properties of a material change, but the composition of the material does not change Reversible: Boil, freeze, melt, condense Irreversible: Break, split, grind, cut, crush

Physical Changes Affects one or more physical properties – does not change the identity Composition of substance remains unchanged Reversible: melt, freeze, sublime, etc Irreversible: cut, crack, chip, etc

Chemical Changes A complete new substance is formed Reversible/Irreversible Burn, rot, rust, decompose, ferment, explode, corrode, grow

Chemical Change A change in which one or more substances are converted into different substances. Heat and light are often evidence of a chemical change.

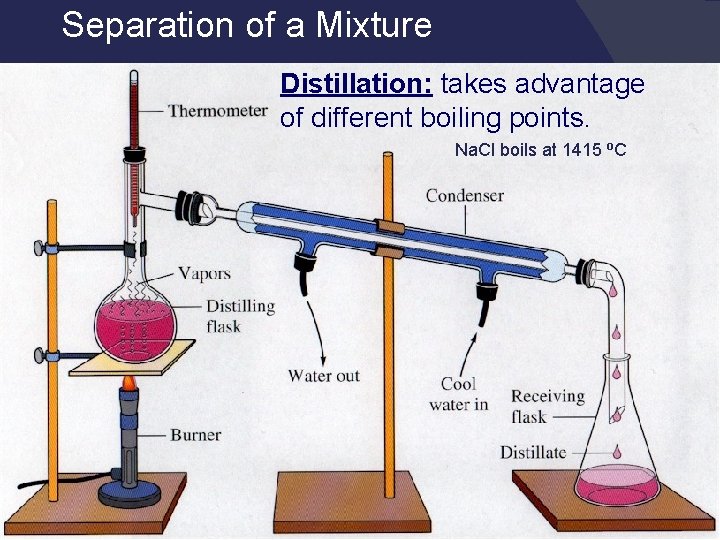
Separating Mixtures Physical means: rocks and marbles, iron filings and sulfur (use magnet) Differences in physical properties can be used to separate mixtures. Magnetic, temperature, etc. Filtration - separates a solid from the liquid in a heterogeneous mixture (by size) Distillation – liquid boiled to produce a vapor that is condensed into a liquid

Separation of a Mixture Distillation: takes advantage of different boiling points. Na. Cl boils at 1415 o. C

Separation of a Mixture Paper chromatography - Components of dyes such as ink may be separated

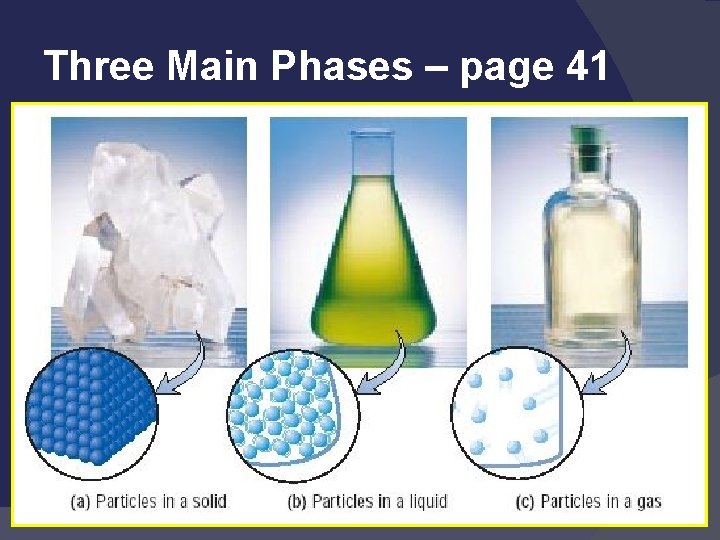
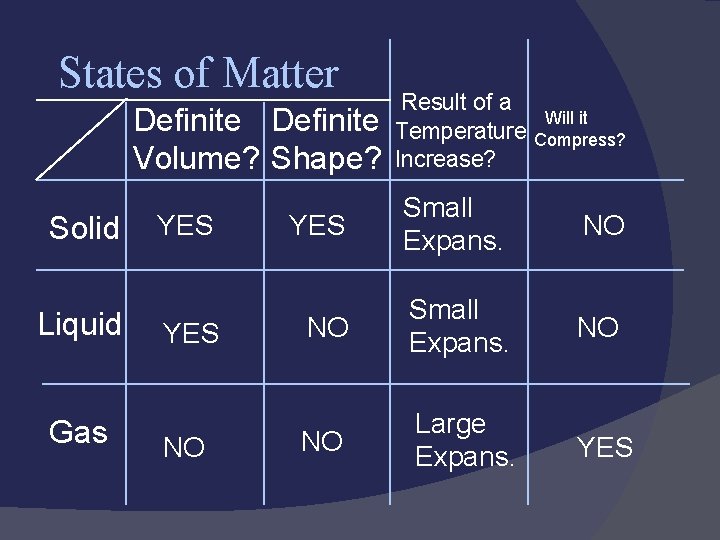
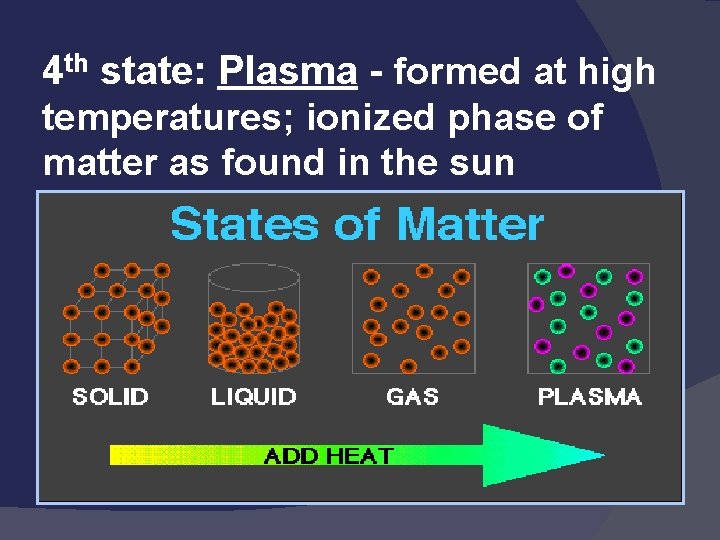
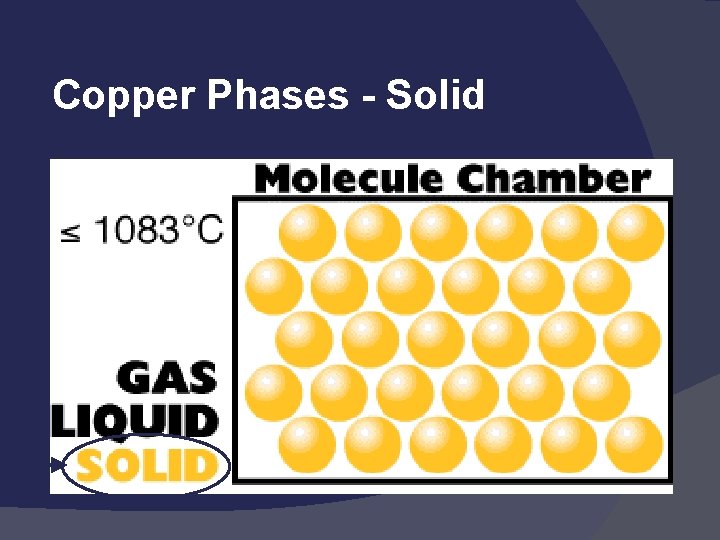
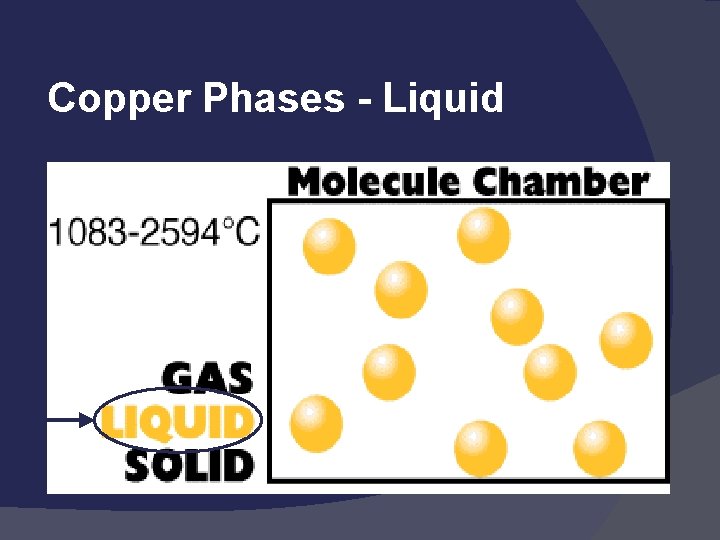
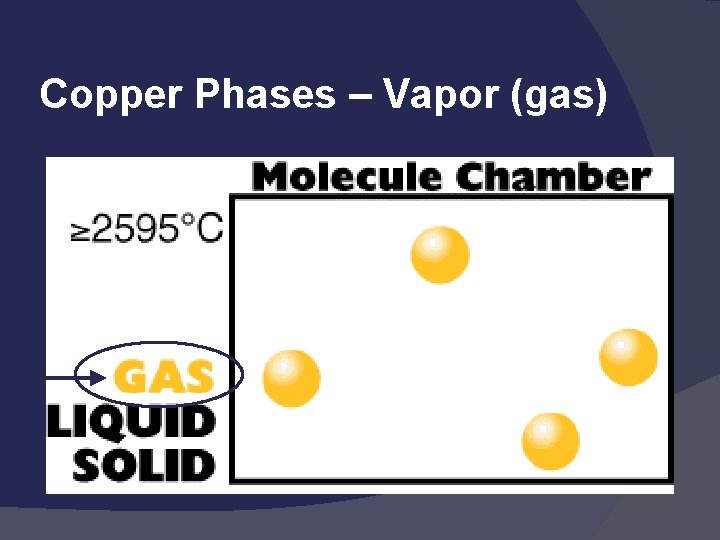
States of Matter Solid: def shape, def volume Liquid: indef shape, def volume Gas: indef shape, indef volume Easily compressed Vapor: the gaseous state of a substance that is generally a liquid

Three Main Phases – page 41

States of Matter Definite Volume? Shape? Solid Liquid Gas YES NO Result of a Temperature Increase? Will it Compress? YES Small Expans. NO NO Large Expans. YES

Condense Freeze Evaporate Melt Solid Liquid Gas

Substances: Element or Compound Elements- simplest kind of matter cannot be broken down any simpler and still have properties of that element! all one kind of atom. Compounds are substances that can be broken down only by chemical methods when broken down, the pieces have completely different properties than the original compound. made of two or more atoms, chemically combined (not just a physical blend!)

Elements vs. Compounds can be broken down into simpler substances by chemical means, but elements cannot. A “chemical change” is a change that produces matter with a different composition than the original matter.

Compound vs. Mixture Compound Mixture Made of one kind of material Made of more than one kind of material Made by a chemical change Made by a physical change Definite composition Variable composition

Which is it? Mixture Element Compound

Volume is: A measure of the space occupied by the object

Describing Matter Properties used to describe matter can be classified as: 1) Extensive – depends on the amount of matter in the sample - Mass, volume, length are examples 2) Intensive – depends on the type of matter, not the amount present - Hardness, Density, Boiling Point

Properties are… Words that describe matter (adjectives) Physical Properties- a property that can be observed and measured without changing the material’s composition. Examples- color, hardness, m. p. , b. p. Chemical Properties- a property that can only be observed by changing the composition of the material. Examples- ability to burn, decompose, ferment, react with, etc.

States of matter Solid- matter that can not flow (definite shape) and has definite volume. 2) Liquid- definite volume but takes the shape of its container (flows). 3) Gas- a substance without definite volume or shape and can flow. 1) Vapor- a substance that is currently a gas, but normally is a liquid or solid at room temperature. (Which is correct: “water gas”, or “water vapor”? )

4 th state: Plasma - formed at high temperatures; ionized phase of matter as found in the sun

Copper Phases - Solid

Copper Phases - Liquid

Copper Phases – Vapor (gas)

Physical vs. Chemical Change Physical change will change the visible appearance, without changing the composition of the material. Boil, melt, cut, bend, split, crack Is boiled water still water? Can be reversible, or irreversible Chemical change - a change where a new form of matter is formed. Rust, burn, decompose, ferment

MIXTURES Mixtures are a physical blend of at least two substances; have variable composition. They can be either: 1) Heterogeneous – the mixture is not uniform in composition • Chocolate chip cookie, gravel, soil. 2) Homogeneous - same composition throughout; called “solutions” • Kool-aid, air, salt water Every part keeps it’s own properties.

Solutions are homogeneous mixtures Mixed molecule by molecule, thus too small to see the different parts Can occur between any state of matter: gas in gas; liquid in gas; gas in liquid; solid in solid (alloys), etc. Thus, based on the distribution of their components, mixtures are called homogeneous or heterogeneous.

Phase? The term “phase” is used to describe any part of a sample with uniform composition of properties. A homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases. Note Figure 2. 6, page 45

Section 2. 3 Elements and Compounds OBJECTIVES: Explain the differences between an element and a compound.

Section 2. 3 Elements and Compounds OBJECTIVES: Distinguish between a substance and a mixture.

Section 2. 3 Elements and Compounds OBJECTIVES: Identify the chemical symbols of elements, and name elements given their symbols.

Properties of Compounds Quite different properties than their component elements. Due to a CHEMICAL CHANGE, the resulting compound has new and different properties: • Table sugar – carbon, hydrogen, oxygen • Sodium chloride – sodium, chlorine • Water – hydrogen, oxygen

Symbols & Formulas Currently, there are 117 elements Elements have a 1 or two letter symbol, and compounds have a formula. An element’s first letter always capitalized; if there is a second letter, it is written lowercase: B, Ba, C, Ca, H, He Start learning the elements names and symbols listed in Table B. 7 on page R 53 Some names come from Latin or other languages; note Table 2. 2, page 52

Section 2. 4 Chemical Reactions OBJECTIVES: Describe what happens during a chemical change.
 Phân độ lown
Phân độ lown Block xoang nhĩ ecg
Block xoang nhĩ ecg Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm vết của đường thẳng
Tìm vết của đường thẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Anything that has mass and takes up space
Anything that has mass and takes up space Anything that has mass
Anything that has mass Anything that has mass and takes up space
Anything that has mass and takes up space Anything which occupies space and has mass
Anything which occupies space and has mass Matter is defined as anything that
Matter is defined as anything that Compare and contrast template
Compare and contrast template Matter anything that takes up space
Matter anything that takes up space What are the 7 diatomic elements
What are the 7 diatomic elements Matter is anything that has and occupies
Matter is anything that has and occupies Matter is anything that has mass
Matter is anything that has mass Matter anything that
Matter anything that Matter is anything that...
Matter is anything that... Matter is anything that has both
Matter is anything that has both Matter is anything that occupies space
Matter is anything that occupies space Ionized matter
Ionized matter What is the opposite of sublimation?
What is the opposite of sublimation? No matter anything
No matter anything Matter is anything that
Matter is anything that No matter anything
No matter anything Melting point defintion
Melting point defintion Matter anything that
Matter anything that Matter anything that
Matter anything that Matter anything that
Matter anything that Matter anything that
Matter anything that Properties of matter section 2
Properties of matter section 2 Chapter 2 properties of matter answer key
Chapter 2 properties of matter answer key Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Extensive examples
Extensive examples Physical and chemical properties
Physical and chemical properties What are the 3 properties of a liquid
What are the 3 properties of a liquid Properties of matter vocabulary
Properties of matter vocabulary Classifying matter concept map
Classifying matter concept map Objectives of properties of matter
Objectives of properties of matter General properties of matter
General properties of matter Classification and properties of matter
Classification and properties of matter Properties and changes of matter worksheet
Properties and changes of matter worksheet Is odor observable or measurable
Is odor observable or measurable States of matter jeopardy
States of matter jeopardy Matter jeopardy
Matter jeopardy Volume properties of matter
Volume properties of matter Matter and its properties
Matter and its properties