Chapter 2 Measurements and Calculations 2 1 Scientific








































- Slides: 46

Chapter 2 Measurements and Calculations

2. 1 Scientific Notation Ø Scientific notation is a method for making very large or very small numbers more compact and easy to write. Ø Scientific notation simply expresses a number as a product of a number between 1 and 10, and the appropriate power of 10. Ø Example: 93, 000, 000 = 9. 3 x 107

Ø To determine the power of ten you must determine how many places the decimal must be moved to make the first number a number between 1 and 10. Example: 0. 000167 = 1. 67 x 10 -4 Ø In the above example the decimal point is moved four times, so the exponent is four. Ø When the number is smaller than one the exponent is negative.

Practice Represent the following numbers in scientific notation 1. 238, 000 2. 1, 500, 000 3. 0. 00043 4. 0. 089 5. 357 6. 0. 0055

Calculations in Scientific Notation To multiply numbers in scientific notation multiply the coefficients and add the exponents Ø To divide numbers in scientific notation divide the coefficients and subtract the exponent in the denominator from the exponent in the numerator Ø Before adding or subtracting numbers in scientific notation you must make the exponents the same by moving the decimal point. Once the exponents are the same align the decimal points and add the numbers. Ø

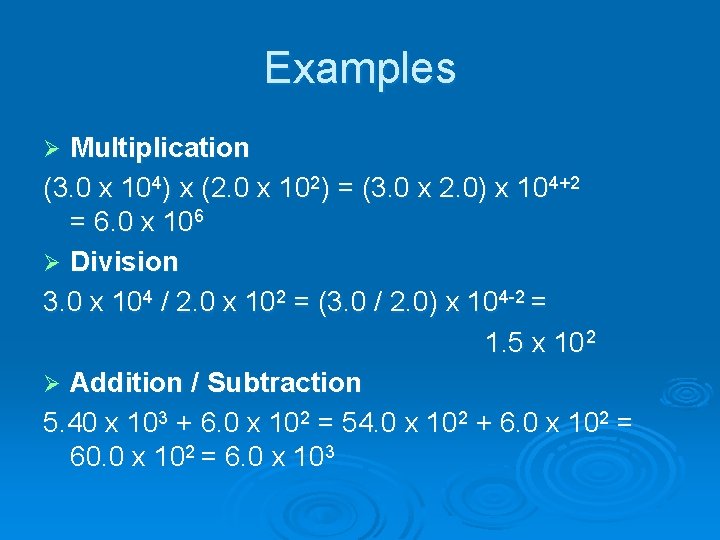
Examples Multiplication (3. 0 x 104) x (2. 0 x 102) = (3. 0 x 2. 0) x 104+2 = 6. 0 x 106 Ø Division 3. 0 x 104 / 2. 0 x 102 = (3. 0 / 2. 0) x 104 -2 = 1. 5 x 10 2 Ø Addition / Subtraction 5. 40 x 103 + 6. 0 x 102 = 54. 0 x 102 + 6. 0 x 102 = 60. 0 x 102 = 6. 0 x 103 Ø

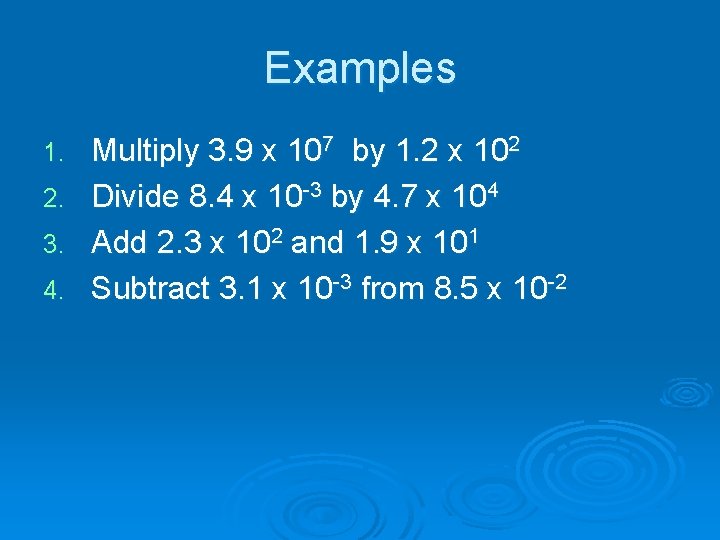
Examples 1. 2. 3. 4. Multiply 3. 9 x 107 by 1. 2 x 102 Divide 8. 4 x 10 -3 by 4. 7 x 104 Add 2. 3 x 102 and 1. 9 x 101 Subtract 3. 1 x 10 -3 from 8. 5 x 10 -2

2. 2 Units Ø The units part of a measurement tells what scale or standard is being used to represent the results of the measurement. Ø There are many different systems of measurement, but the two most common are the English system and the metric system. Ø The metric system is used in science.

Ø In 1960 scientists had a conference to agree on which units to use. The system adopted at the conference is the International System (le Systeme International), or the SI system. Ø The SI system uses the units of the metric system. In the SI system uses prefixes to change the size of the unit.

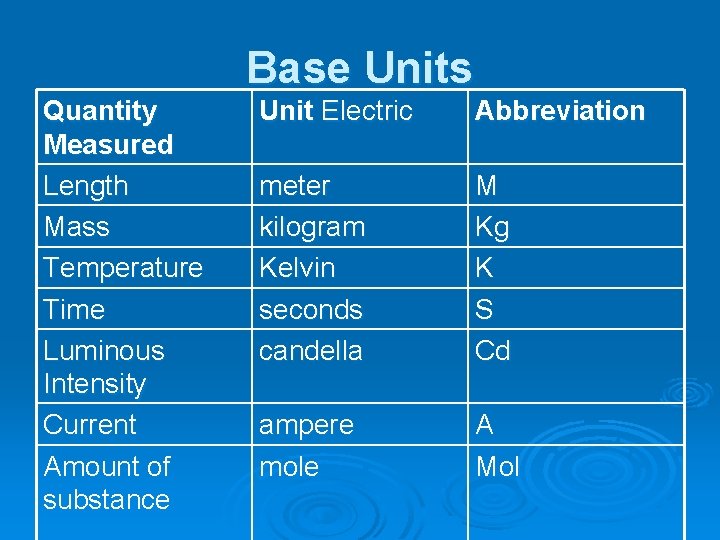
2. 3 Measurements of Length, Volume, and Mass Ø In the SI system there are seven base units from which all other units are derived. Ø Derived units are used for measurements such as volume, which come from the units for length.

Base Units Quantity Measured Length Mass Temperature Time Luminous Intensity Current Amount of substance Unit Electric Abbreviation meter kilogram Kelvin seconds candella M Kg K S Cd ampere mole A Mol

Units of Volume Ø Ø Ø Ø The space occupied by a sample of matter is called its volume. To calculate the volume of any cubic or rectangular object you multiply length, width, and height. The unit for volume therefore comes from the meter which is used to measure length, width, and height. The liter is the most common unit used to measure volume. A liter is one cubic decimeter (1 L = 1 dm 3). A decimeter is equal to 10 centimeters. A smaller unit of volume is the milliliter. A milliliter is equal to one cubic centimeter (1 m. L = 1 cm 3). The volume of any substance will change with temperature.

Units of Mass Ø Weight is a force that measures the pull of gravity on an object. Ø Mass is different from weight. Mass measures the amount of matter in an object. Ø The weight of an object can change with location, but the mass does not. Ø An object can become weightless, but not massless.

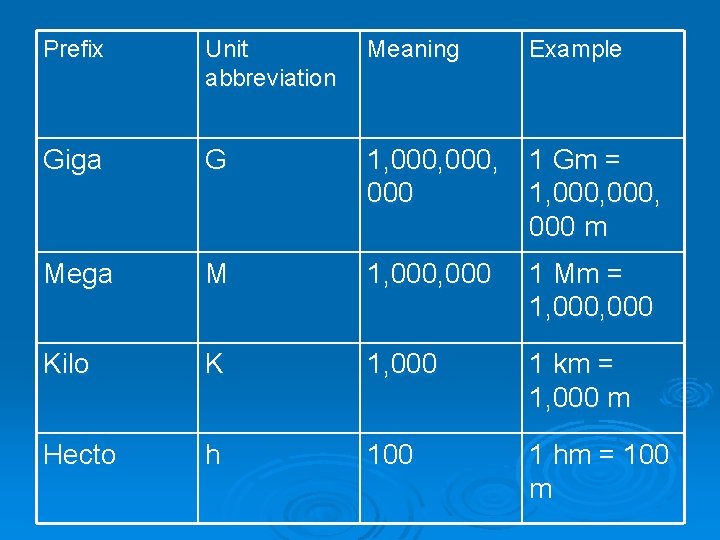
Prefix Unit abbreviation Meaning Example Giga G 1, 000, 000 1 Gm = 1, 000, 000 m Mega M 1, 000 1 Mm = 1, 000 Kilo K 1, 000 1 km = 1, 000 m Hecto h 100 1 hm = 100 m

2. 4 Uncertainty in Measurement Ø Whenever a measurement is made an estimate is required. Ø The numbers that are known because the measuring device has graduations are called certain numbers. Ø The last digit is estimated, and is called an uncertain number.

In lab, when measurements are made, they should be made with all of the numbers known with certainty plus one digit that is estimated. Ø A measurement always has some degree of uncertainty. Ø The amount of uncertainty depends on the device used to make the measurement. Ø The numbers recorded in a measurement are called significant figures. Ø The number of significant figures is determined by the uncertainty in the measuring device. Ø

2. 5 Significant Figures Ø Chemistry often requires many types of calculations. Ø Uncertainty accumulates as calculations are performed on numbers with estimation, for this reason there are rules for working with significant figures.

Rules for Counting Significant Figures 1. 2. 3. Nonzero integers always count as significant figures. Zeros l Leading zeros are zeros that precede all of the nonzero digits. They never count as significant figures. l Captive zeros are zeros that fall between nonzero digits. Captive zeros always count as significant figures. l Trailing zeros are the right end of a number. They are significant only if the number is written with a decimal point. Exact numbers. Often calculations involve numbers that were not obtained using measuring devices but were determined by counting. They can be assumed to have an unlimited number of significant figures. Exact number can also arise from definitions.

Sig Figs & Scientific Notation Ø Rules for counting significant figures also apply to numbers in scientific notation. Ø Scientific notation offers two major advantages: the number of significant figures can be easily determined and fewer zeros are needed to write a very large or very small number.

Example 2. 3 1. Give the number of significant figures for each of the following measurements. a. A sample of orange juice contains 0. 0108 g of vitamin C. b. A forensic chemist in a crime lab weighs a single hair and records its mass as 0. 0050060 g. c. The distance between two points was found to be 5. 030 x 103 ft. d. In yesterdays bicycle race, 110 riders started but only 60 finished

Self-Check Exercise 2. 2 2. Give the number of significant figures for each of the following measurements. a. 0. 00100 m b. b. 2. 0800 x 102 L c. 480 Corvettes

Rounding Off Numbers Ø When you perform a calculation on your calculator, the number of digits displayed is usually greater than the number of significant figures that the result should possess. Ø Therefore you must “round off” your answer. Ø The rules for rounding off are on the next slide.

Rules for Rounding Off If the digit to be removed 1. l l Is less than five, the preceding digit stays the same. Is equal to or greater than 5, the preceding digit is increased by 1. In a series of calculations, carry the extra digits through to the final result and then round off. When rounding off, use only the first number to the right of the last significant digit. 2.

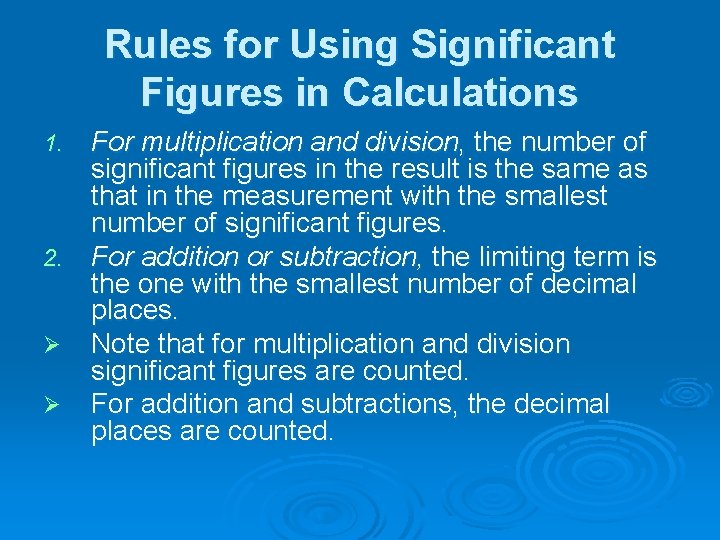
Rules for Using Significant Figures in Calculations For multiplication and division, the number of significant figures in the result is the same as that in the measurement with the smallest number of significant figures. 2. For addition or subtraction, the limiting term is the one with the smallest number of decimal places. Ø Note that for multiplication and division significant figures are counted. Ø For addition and subtractions, the decimal places are counted. 1.

Example 2. 4 Ø a. b. c. d. Without performing the calculations, tell how many significant figures each answer should contain. 5. 19 + 1. 9 +0. 842 1081 – 7. 25 2. 3 x 3. 14 the total cost of 3 boxes of candy as $2. 50 a box

Example 2. 5 Ø a. b. c. d. e. Carry out the following mathematical operations and give each result to the correct number of significant figures. 5. 18 x 0. 0208 (3. 60 x 10 -3) x (8. 123) / 4. 3 21 +13. 8 +130. 36 116. 8 – 0. 33 (1. 33 x 2. 8) + 8. 41

Self- Check Exercise 2. 3 Ø a. b. c. Give the answer for each calculation to the correct number of significant figures. 12. 6 x 0. 53 (12. 6 x 0. 53) – 4. 59 (25. 36 – 4. 15) / 2. 317

2. 6 Problem Solving and Dimensional Analysis Ø Converting from one unit to another is encountered frequently in daily life. Ø Dimensional analysis can be used to help convert from one unit to another. Ø To change from one unit to another a conversion factor is used. Ø A conversion factor is a ratio that relates two units to each other.

An equivalence statement is a statement that gives two different units that are equal to each other. Example: 100 cm = 1. 00 m Ø When using dimensional analysis units cancel. Choose a conversion factor that cancels the units you want to discard and leaves the units we want in the result. Ø Always check to make sure your answer makes sense. This is a good rule for any problem you are completing. Ø

Ø When using dimensional analysis units cancel. Choose a conversion factor that cancels the units you want to discard and leaves the units we want in the result. Ø Always check to make sure your answer makes sense. This is a good rule for any problem you are completing

Example 2. 6 Ø An Italian bicycle has its frame size given as 62 cm. What is the frame size in inches?

Example 2. 7 Ø The length of a marathon race is approximately 26. 2 mi. What is the distance in kilometers?

Self-Check Exercise 2. 5 Ø Racing cars at the Indianapolis Motor Speedway now routinely travel around the track at an average speed of 225 mi/h. What is this speed in kilometers per hour?

2. 7 Temperature Conversions: An Approach to Problem Solving Ø The Fahrenheit scale is widely used in the United States and Great Britain, and is the scale employed in the engineering sciences. Ø The Celsius scale is a second temperature scale used in Canada, Europe and in the physical and life sciences.

Ø On both the Fahrenheit and Celsius scale the unit of temperature is called the degree, and the symbol for it is followed by the capital letter representing the scale on which the units are measured. Ø A third temperature scale is the absolute scale, or the Kelvin scale. The unit of temperature is called the Kelvin and is symbolized by K.

Some important facts about the three temperature scales The size of each temperature unit is the same on the Kelvin and Celsius scales. Ø The Fahrenheit degree is smaller that the Celsius and Kelvin unit. Ø The zero points are different on all three scales. Ø

Converting between the Kelvin and Celsius Scales Ø Converting from Celsius to Kelvin involves add 273 to the Celsius temperature. Ø In order to convert from Kelvin to Celsius, simply subtract 273 from the Kelvin temperature.

Example 2. 8 Ø The boiling point of water at the top of Mt. Everest is 70. ºC. Convert the temperature to the Kevin scale

Example 2. 9 Ø Liquid nitrogen boils at 77 K. What is the boiling point of nitrogen on the Celsius scale?

Self-Check Exercise 2. 6 Ø Which temperature is colder, 172 K or - 75ºC?

Converting Between the Fahrenheit and Celsius Scale Ø The conversion between Fahrenheit and Celsius requires adjusting for the different size units and the different zero points. Ø The factor 1. 80 is multiplied by the Celsius temperature to convert between the different sized units, and then 32 is added to the account for the different zeros.

Example 2. 10 Ø On a summer day the temperature in the laboratory is 28ºC. Express this using the Fahrenheit scale.

Example 2. 11 Ø Express the temperature -40. ºC on the Fahrenheit scale.

Self-Check Exercise 2. 7 Ø Hot tubs are often maintained at 41ºC. What is this temperature in Fahrenheit?

Example 2. 12 Ø On of the body’s responses to injuries is to elevate its temperature. A certain flu victim has a body temperature of 101ºF. What is this temperature on the Celsius scale?

Self-Check Ø An anti-freeze solution in a car’s radiator boils at 239ºF. What is this temperature on the Celsius scale?